Running Head: Pulmonary Rehabilitation and Readmission Rates
Funding: This work was funded by an Agency of Healthcare Research and Quality award (R01HS023305) held by Carlos Camargo.
Date of Acceptance: July 9, 2021. Published Online: July 16, 2021
Abbreviations: pulmonary rehabilitation, PR; chronic obstructive pulmonary disease, COPD; interquartile range, IQR; International Classification of Diseases—9th Edition, ICD-9; stabilized inverse probability weights, SIPW; Centers for Medicare and Medicaid Services, CMS; confidence interval, CI
Citation: Myers LC, Faridi MK, Hasegawa K, Camargo CA. Pulmonary rehabilitation and readmission rates for Medicare beneficiaries with acute exacerbation of chronic obstructive pulmonary disease Chronic Obstr Pulm Dis. 2021; 8(4): 427-440. doi: http://doi.org/10.15326/jcopdf.2020.0193
Online Supplemental Material: Read Online Supplemental Material (222kb)
Introduction
Hospitalizations for acute exacerbation of chronic obstructive pulmonary disease (COPD) are common and costly.1,2 Based on Medicare data, approximately 20% of index hospitalizations for COPD result in a readmission within 30 days.3-5 Strategies to reduce readmissions are important, not only because readmissions are associated with a higher long-term risk of death,6 but also because of more recent financial penalties for institutions with patients readmitted within 30 days of discharge for COPD.7-9
Pulmonary rehabilitation (PR) is a supervised program that includes exercise training, health education, and breathing techniques for patients with lung conditions.10 It has been shown to decrease hospitalizations for COPD in some prospective trials11-14 but not all trials.15-16 Several international guidelines have recommended that PR be started within 3–4 weeks of hospital discharge after an exacerbation.17-18 A recent retrospective analysis using Medicare data showed improved survival at 1-year if PR was initiated within 90 days of a hospitalization for a COPD exacerbation.19
Since 2010, Medicare has covered the cost of PR for elderly Americans with moderate to severe COPD, although most prospective PR trials have been performed abroad.20 A gap exists as to whether PR is associated with lower risk of hospital readmission in the Medicare population in the United States, given the known differences in health care delivery and PR programs between countries.21 We hypothesized that patients receiving at least 1 PR session after a COPD hospitalization would be less likely to be readmitted within 30 days.
Methods
Study Design
This is a retrospective cohort study using a random sample of 5 million fee-for-service Medicare beneficiaries. The Partners Institutional Review Board approved this study (2015P001947).
Data Source
We used Medicare Research Identifiable Files from the Centers for Medicare and Medicaid Services (CMS) between 2010–2012. This time period is after Medicare began covering PR but before CMS’ enactment of financial penalties for 30-day all-cause COPD readmissions. We chose this time period because post-discharge care might have changed after the penalty was enacted in a way that we could not account for using administrative data. To identify all Medicare beneficiaries hospitalized for COPD, we used fee-for service inpatient files that contain institutional claims covered under Medicare Part A and encrypted beneficiary identifiers, admission and discharge dates, and International Classification of Disease-9th edition (ICD-9) diagnosis and procedure codes. The Master Beneficiary Summary Files include encrypted beneficiary identifiers, dates of birth and death, sex, race/ethnicity, and information about program eligibility and enrollment. The encrypted beneficiary identifier was used to link inpatient files to the following data sources: Master Beneficiary Summary files, Institutional Outpatient files, Carrier or Non-Institutional Outpatient files, Durable Medical Equipment files and Chronic Conditions Files, the latter of which includes 27 Chronic Condition Data Warehouse flags.22
Study Sample
We identified patients(aged ≥65 years) who were hospitalized with a primary discharge diagnosisof COPD using the ICD-9 Clinical Modification diagnostic codes. Patients could have: (1) a primary diagnosis of COPD or, (2) a primary diagnosis of respiratory failure with a secondary diagnosis of COPD (Table 1 in the online supplement). We used these codes specifically because they were used in CMS’ Hospital Readmissions Reduction Program.7 The Medicare population is appropriate to study COPD readmissions because COPD is more common in older adults.23 We created the following 2 cohorts: one that was a 30-day readmission cohort and the other that was a 1-year readmission cohort. To be eligible to enter each cohort, patients had to have had the appropriate follow-up time, either through active Medicare enrollment or a known death. We report results of the 2 patient cohorts separately. If multiple encounters for a single patient met the inclusion/exclusion criteria, all encounters were considered eligible.
Cohort Formation
We considered only the first non-elective readmission within 30 days of discharge to be a readmission. The readmission did not have to be at the index hospital. Subsequent readmissions within the same 30-day period were not counted as readmissions or index hospitalizations. However, hospitalizations after the 30-day post-discharge window were counted as index hospitalizations if they met the inclusion criteria, which has been done in previous studies on readmissions.24 The same approach was used to form a subcohort of patients to examine longer term outcome events, such as readmissions occurring in the 1 year after discharge.
We excluded patients who had PR <1 year prior to the index admission to simulate a new user design.25 We excluded patients who left the hospital against medical advice or were transferred between hospitals as evidenced by claims at different hospitals from the same day. We also excluded patients without continuous enrollment in Medicare fee-for-service (part A and B) for 1 year before the date of the index hospitalization.
Exposure
The exposure was receipt of ≥1 outpatient PR session after discharge. Given previous reports of low PR participation rates in the Medicare population,26 we started with a low threshold for inclusion in the exposed group.
The period between hospital discharge and the first receipt of outpatient PR was considered immortal person-time and was not counted as exposed time in the time-to-event analysis. The observation period for the PR group started at the time of the first PR session. The observation period for the non-PR group started at the time of the index discharge date. We identified PR sessions using the codes listed in Table 2 in the online supplement.
We performed 2 sensitivity analyses for the exposure definition and timing. We assessed the association after requiring that the PR group have: (1) at least 2 sessions and (2) at least 3 sessions instead of only 1. We also included the time between index discharge date and first PR session as a fixed effect to further address the issue of immortal time.
Outcomes
The primary outcome was all-cause, non-elective readmission within 30 days of a hospital discharge for COPD.27 This outcome was studied given the focus of COPD in the Hospital Readmissions Reduction Program. The shorter term outcome was chosen to be the primary outcome because the subsequent hospital penalties for higher-than-expected COPD readmissions are based on the 30-day timepoint and this timepoint has been the focus of many studies for comparison. The longer term outcome was examined because of evidence showing more benefit with longer exposure to PR and simply the feasibility of studying longer term outcomes in Medicare claims data, which is not possible in many other data sources.28,29 As secondary outcomes, we examined 30-day COPD-related readmission as well as 1-year all-cause, non-elective and COPD-related readmission.
Covariates Used for Risk Adjustment
Patient demographics (age, sex, race/ethnicity, geographic region) were obtained from Master Beneficiary Summary files. Chronic conditions were identified as described in the CMS COPD Readmissions Methodology Report.30 The codes for the chronic conditions are listed in Table 3 in the online supplement. The following relevant variables were also included as covariates: smoking status and long-term oxygen use in the year prior to admission. Smoking was identified by present on admission flags. Patients were considered to be on long-term oxygen if it was prescribed any time in the year prior to admission. The codes used for identifying long-term oxygen are listed in Table 4 in the online supplement. Patient frailty was also included as part of risk adjustment, because higher frailty has been associated with higher risk of hospitalization in COPD patients.31 We employed an administrative definition of frailty that uses present on admission variables and has been validated in Medicare data that looks back over the year prior to admission.32 Administrative codes used for frailty are listed in Table 5 in the online supplement.
Statistical Analyses
Baseline characteristics of the 30-day and 1-year cohorts were described using frequencies with percentages for categorical variables and medians with interquartile range (IQR) for continuous variables. We compared exposure groups using standardized differences, calculated as a difference in means or proportions divided by standard error. Compared to traditional significance testing, standardized differences are not as sensitive to sample size. Imbalance is defined33 as absolute value >0.10. Given the potential for confounding, in which younger, healthier patients attended PR, we used stabilized, inverse probability weighting. Weights are estimated from a treatment selection model, using logistic regression with receipt of PR as the dependent variable without covariates, divided by a model with baseline characteristics as independent variables. Stabilized weights are preferred over regular weights as they produce robust estimates with smaller variance. We show the balance of the baseline characteristics between exposure groups before and after weighting (Table 1 for 30-day cohort and Table 2 for 1-year cohort).


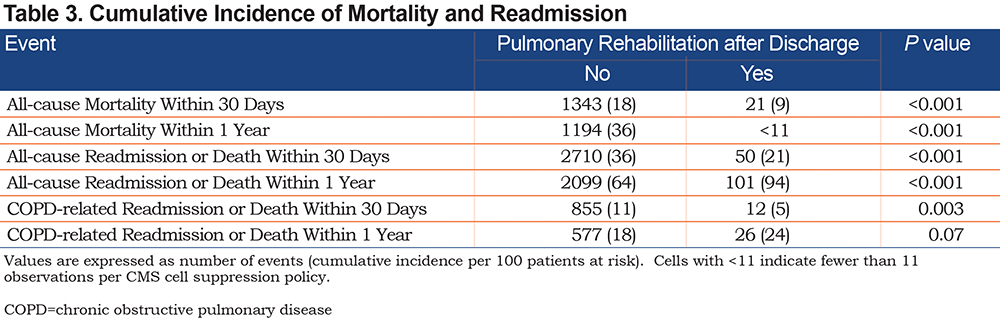
We described the outcomes by comparing the unweighted cumulative incidence of each outcome at 30 days and 1 year, including death as the competing event. Gray tests were used to assess differences between groups by PR status (Table 3). For mortality, the Kaplan-Meier method was used to estimate cumulative incidence with log-rank tests to evaluate differences between groups.
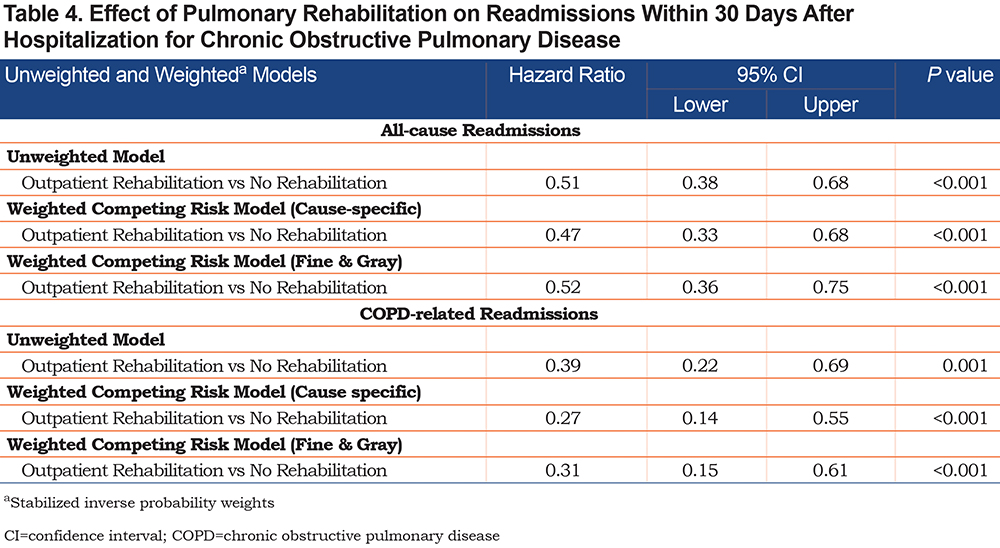
To estimate the association of PR with each outcome, we developed unweighted and weighted cause-specific and Fine-Gray regression34 models in both 30-day and 1-year cohorts accounting for death as a competing risk (Table 4 and Table 5). We reported both cause-specific and Fine-Gray competing risk regression model results, because the 2 methods calculate the risk set differently and produce complementary results.35 Fine-Gray regression produces subdistribution hazard ratios, which are less interpretable than cause-specific hazard ratios. We tested the assumption of proportional hazards, and there was no violation.
All analyses were conducted using SAS9.4 (SAS Institute, Cary, North Carolina). Significance tests and confidence intervals for model estimates were based on robust standard errors to account for clustering within participants. A 2-tailed P<0.05 was considered statistically significant.
Results
Of 1,839,827 hospitalizations, we identified 78,074 for COPD. The 30-day cohort contained 7825 index hospitalizations that met inclusion criteria, of which 235 (3%) had ≥1 PR session and 7590 (97%) who did not (Table 1). The 1-year cohort, which was a subcohort of the 30-day cohort, contained 3401 index hospitalizations, of which 108 (3%) received ≥1 PR session and 3293 (97%) did not (Table 2). The median number of PR sessions was 3 (IQR 1–11) in both cohorts.
Patients who received PR were older in the 30-day cohort (median 81, IQR 74–85 versus 79, IQR 73–85, standardized difference=0.17) but not in the 1-year cohort (median 81, IQR 75–85 versus 80, IQR 73–85, standardized difference=0.10). We did not observe differences in sex by PR status in either cohort. There were few statistically significant differences in the comorbidities between groups, such as percentage of patients who did and did not receive PR with congestive heart failure, vascular disease, diabetes, or renal failure in either cohort even prior to weighting (Table 1 and Table 2). There was no difference in length of hospital stay by PR status in the 30-day cohort, but there was a shorter length of stay in the PR group (median 4, IQR 3–7) compared to the group that did not receive PR (median 5, IQR 3–7, standardized difference =-0.15) in the 1-year cohort.
In the 30-day cohort, there were fewer patients (53%) on long-term oxygen who received PR compared to patients who did not receive PR (60%, standardized difference=-0.16). There were also fewer current/former smokers (38%) who received PR compared to those who did not receive PR (46%, standardized difference=-0.17). Median frailty score was higher in the PR group (7, IQR 5–9 versus 6, IQR 4–8, standardized difference=0.30). The same pattern of baseline characteristics was also seen in the 1-year cohort. For example, there were fewer patients (46%) on long-term oxygen who received PR compared to patients who did not receive PR (56%, standardized difference=-0.20). There were also fewer current/former smokers (35%) who received PR compared to those who did not receive PR (44%, standardized difference=-0.18). Median frailty score was higher in the PR group (7, IQR 5–8 versus 6, IQR 4–8, standardized difference=0.26). Importantly, there were fewer statistically significant differences in baseline characteristics after application of stabilized inverse probability weights.
There were fewer deaths in the PR group in both the 30-day cohort (9 versus 18 per 100 patients at risk, P<0.001) and 1-year cohort (<11 versus 36 per 100 patients at risk, P<0.001). The 30-day, all-cause readmission rate accounting for death as a competing risk was 21 per 100 patients at risk in the PR group compared to 36 per 100 patients at risk (P<0.001, Table 3). The 1-year, all-cause readmission rate accounting for death as a competing risk was 94 per 100 patients at risk in the PR group compared to 64 per 100 patients at risk (P<0.001).
We report the hazard ratios and 95% CIs of the unweighted and weighted survival models for the 30-day cohort in Table 4. The hazard ratio for the weighted cause-specific 30-day readmission cohort was 0.47 (95% CI 0.33–0.68, P<0.001) for all-cause readmission and 0.27 (95% CI 0.14–0.55, P<0.001) for COPD-related readmission. Figure 1 in the online supplement shows the event probability curve over the 30-day period depending on PR exposure. Panel A shows the curve for all-cause, non-elective readmission, while Panel B shows the curve for COPD-related readmissions. The Fine-Gray model, which produces the subdistribution hazard function, showed results similar to the cause-specific regression model for the 30-day cohort; Figure 2 in the online supplement shows the cumulative incidence curve over the 30-day period derived from the Fine-Gray analysis. Panel A shows the curve for all-cause, non-elective readmissions, while Panel B shows the curve for COPD-related readmissions.
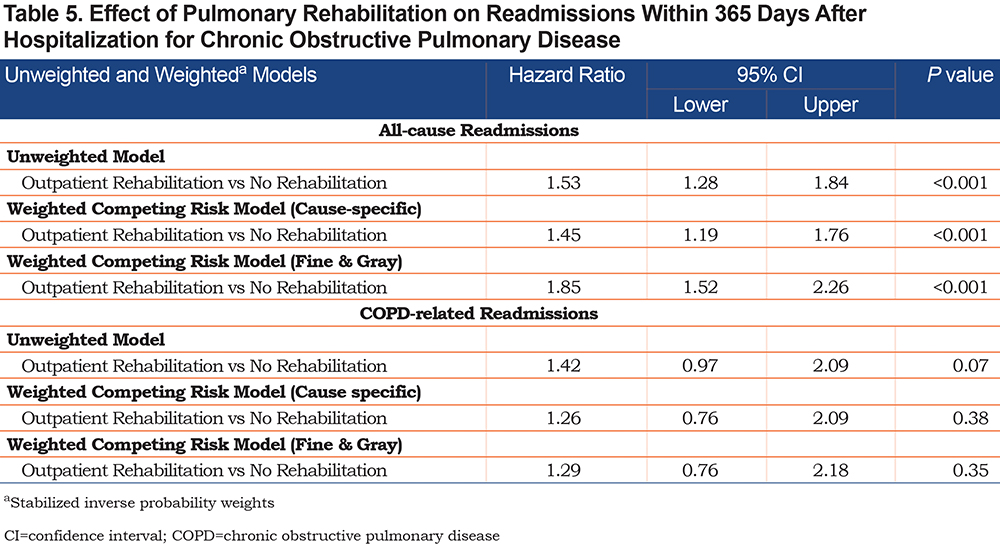
We report the hazard ratios and 95% CIs of the unweighted and weighted survival models for the 1-year cohort in Table 5. The hazard ratio for the weighted cause-specific 1-year readmission cohort was 1.45 (95% CI 1.19–1.76, P<0.001) for all-cause and 1.26 (95% CI 0.76–2.09, P=0.29) for COPD-related readmission. Figure 3 in the online supplement shows the unweighted event probability over the 1-year period depending on PR exposure. Panel A shows the curve for all-cause, non-elective readmissions, while Panel B shows the curve for COPD-related readmissions. The Fine-Gray model showed results similar to the cause-specific regression model for the 1-year cohort; Figure 4 in the online supplement shows the cumulative incidence curve over the 30-day period derived from the Fine-Gray analysis. Panel A shows the curve for all-cause, non-electives readmissions, while Panel B shows the curve for COPD-related readmissions.
Sensitivity Analyses
The results of the 2 sensitivity analyses were similar. When at least 2 or at least 3 PR sessions were required for patients to be counted in the PR group, the hazard ratio for 30-day readmissions was 0.57 (95% CI 0.37–0.88, P=0.01) and 0.42 (95% CI 0.24, 0.72, P=0.002), respectively. Additionally, after including the time between index discharge date and first PR session as a fixed effect to account for immortal time, the 30-day association held (hazard ratio 0.27, 95% CI 0.17–0.42, P<0.0001).
Discussion
PR is an intervention that has been tested in several prospective studies in Europe, Asia, and Oceania. However, the association between PR and readmissions has not been studied in a Medicare cohort from the United States. Given that PR regimens vary so much between providers, health systems, and countries,36 we investigated the association in the Medicare population after CMS began reimbursing for PR but before hospitals were penalized for COPD readmissions. Using stabilized inverse probability weighting to limit the effect of confounding, we detected an association between PR and lower risk of short-term readmissions (both all-cause and COPD-related), yet higher risk of long-term all-cause readmissions. The results were consistent using 2 different competing risk regression analyses (cause-specific and Fine-Gray). The benefit observed in the short-term readmission cohort cannot be explained by patients having lower comorbidity burden or frailty in the PR group. The median frailty score for patients receiving PR was actually higher. We were able to detect the potential benefit of PR even with a relatively small sample size (n=7825). These findings support the notion that PR in the month after hospitalization for COPD may be associated with lower risk of readmission.
The later timepoint produced somewhat unexpected results. The association between PR and all-cause readmissions reversed direction at 1 year, with PR associated with higher risk of readmission. We suspect that this is due to patients not continuing to attend PR sessions. The median number of PR sessions of patients in both cohorts was the same, which indicates that PR was occurring early in the post-discharge period without additional sessions over the course of the year. This is consistent with prior literature by Spitzer et al showing that Medicare patients have low PR participation percentages (2.7%),26 which was approximately the participation percentage in both of our cohorts. However, Spitzer et al reported the median number of sessions to be 16, which is considerably higher than ours (approximately 3). An alternative explanation for the higher readmission risk at 1 year is that patients who receive PR are more engaged in their care and have pulse oximeters at home, for example, to trigger calls to their physicians and ultimately rehospitalizations. Given that the rate of death was lower in the PR group, it’s possible that readmission does not necessarily signal a “bad” outcome, which is typically how readmissions are portrayed in the literature. Further analyses using full Medicare samples, not random samples, might be able to tease apart the type of regimen associated with benefit, such as duration of regular attendance and intensity (number of sessions per week).
The differing associations of PR with all-cause versus COPD-related readmission at the later timepoint were curious. Respiratory readmissions are the most common cause of readmission after a COPD hospitalization,37 so we might expect that COPD-related readmissions would also increase if all-cause readmissions increased. However, we attribute this finding to the multimorbidity in COPD that can evolve over the course of a year.38-43 We report comorbidities that were present only on the index admission. A previous study showed that incident congestive heart failure in COPD patients is associated with a 3-fold increase in mortality at 1 year.44 It is possible that patients developed a cardiac comorbidity that required an inpatient stay, thus contributing to all-cause readmissions but not to COPD-related readmissions. We caution overinterpretation of the results at the later timepoint given the relatively small sample size (n=3401), which could also produce spurious results. Further research is needed to understand the optimal timing of PR initiation, the minimal number of sessions needed to observe a decrease in readmissions and what other factors impact all-cause readmissions in the COPD Medicare patient population over the course of a year.
The fact that death rates are lower in the PR groups at both timepoints is reassuring. We observed this phenomenon despite worse frailty scores in the PR group. Previous literature has shown that when patients do participate in PR, they have lower mortality in a dose-response way,19 so this observation about death is generally consistent with previous literature. We used robust methods to address death as a competing risk.
We designed the study to limit the effect of confounding. Previous studies have shown that younger, healthier, and wealthier people receive PR.45 If less ill patients attended PR and were less likely to be readmitted, PR may appear beneficial when the effect is an artifact of the patients who were able to attend, but we showed that patients in the PR group were more frail. Methodologically, we used stabilized inverse probability weighting to first model the probability of receiving PR and then did 2 types of competing risk regression. While no post-hoc analytic method can account for unmeasured confounding, stabilized inverse probability weighting simulates prospective data by addressing measured confounding. Importantly, there were few univariate differences in the groups after application of the weights. Beyond the use of stabilized inverse probability weighting, we also adjusted for as many confounders as available in administrative data, including smoking status, long-term oxygen use, the administrative definition of frailty, and need for mechanical ventilation.
This study extends current work in the field of COPD readmissions and PR.5,11-14,28,46-50 A systematic review by Puhan et al49 examined 8 randomized controlled trials and reported moderate evidence that PR decreases 1-year hospital readmissions with an odds ratio of 0.21 to 0.91. The results were heterogeneous (I2=77%) and all studies were performed abroad.49 Ours is one of the first studies to examine the association of PR and readmission rate of Medicare patients with COPD.
The mechanism of pulmonary rehabilitation impacting readmission as an outcome is plausible. Patients hospitalized for COPD lose muscle mass51 and exercise tolerance.52 These parameters can respond to rehabilitation therapy. It is possible that patients learn tangible techniques such as pursed lip breathing, tripoding, or airway clearance during only a few visits, which could produce a therapeutic effect. Research is ongoing to identify the optimal combination of exercise in PR, such as endurance training, interval exercise training, strength training, and breathing training.
Guidelines have been developed by different academic societies to address the question of whether PR should be initiated after hospitalization for COPD exacerbation. A combined consensus statement from the American Thoracic Society/European Respiratory Society published in 2013 described PR that starts within 3 weeks of discharge from the hospital for an exacerbation as safe, feasible, and effective.12,17,20,49,53 However, a later guideline from the same group published in 2016 made only a conditional recommendation for PR to begin as an inpatient or within 3 weeks of discharge.54 A different combined consensus statement from the American College of Chest Physicians/Canadian Thoracic Society published in 2015 recommended PR within 1 month of an exacerbation for patients with moderate to very severe COPD.18 PR remains only a conditional recommendation by the American Thoracic Society17 because of 1 study that showed potential harm if PR was initiated in the hospital.54
The results of this study should be interpreted in the context of the design. Using large scale administrative data does not provide insight into the quality of PR available, the physician referral patterns for PR, or the socioeconomic/geographic barriers in accessing PR. Previous work has shown a positive correlation between density of available PR programs and participation in PR but only for non-Hispanic Whites, not Blacks.55 Our study does not provide insight into referral patterns or why patients did not attend PR even if they obtained a referral – e.g., they were too short of breath to attend the session; they did not anticipate it being useful; or they did not have transportation. Studies using data other than administrative data are necessary to provide these insights. Because we do not have access to pulmonary function test data, we also cannot describe the severity of obstruction of patients in the cohort and use it for additional risk adjustment. Medicare reimbursement rules require that patients have moderate obstruction in order to qualify for PR, so we assume most have forced expiratory volume in 1 second of <50% of predicted. Lastly, the benefit of PR could be heterogeneous across patients, so further work is needed to identify patients who might benefit the most, such as those with multiple exacerbations in a short period of time or patients with severe respiratory symptoms.
Strengths of the current study include strict, rigorous inclusion/exclusion criteria, use of data prior to the CMS readmission penalties were enacted, inclusion of the administrative definition of frailty for risk adjustment, use of stabilized inverse probability weighting to limit measured confounding, inclusion of death as a competing risk in 2 complementary survival analyses, and examination of both all-cause and COPD-related outcomes at 2 time points.
Conclusions
In summary, we demonstrate that exposure to at least 1 PR session is associated with a lower risk of all-cause and COPD-related readmission at 30 days, even in older, more frail patients, but a higher risk of all-cause readmission at 1 year. We suspect the inverse association at 1 year was because patients did not attend additional PR sessions beyond the first 30 days. Given the burden of COPD readmissions across the United States and the lack of studies on PR for COPD in the Medicare population,19,26,55 it is important to better understand interventions, such as PR, that may decrease the likelihood of readmission. Further research is needed about the optimal timing, dose, and patient population that benefit most from PR.
Acknowledgments
The authors wish to thank Ashley Sullivan, MS, MPH, and Janice Espinola, MPH, for their numerous contributions at the EMNet Coordinating Center (Massachusetts General Hospital, Boston, MA).
Author contributions: All authors participated in the design of the study, analysis, interpretation, and creation of the manuscript draft.
Data Availability: Data was made available by the Agency for Healthcare Research and Quality and the Centers for Medicare and Medicaid Services. The data is patient-level and cannot be shared publicly.
Declaration of Interest
The authors report no conflicts of interest.