Running Head: CMV Seropositivity and Airflow Limitation
Funding support: This work was funded in part by support from the National Institutes of Health’s HL119538 grant to MTB and the Veteran’s Administration’s I01BX002347 and I01 CX001891 grants to MTB.
Date of acceptance: July 19, 2021 │ Published online: July 30, 2021
Abbreviations: cytomegalovirus, CMV; chronic obstructive pulmonary disease, COPD; airflow limitation, AFL; acute exacerbation of COPD, AECOPD; Veterans Affairs, VA; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; body mass index, BMI; inhaled corticosteroid, ICS; odds ratio, OR; confidence interval, CI; incidence rate ratio, IRR; natural killer, NK
Citation: Burkes R, Osterburg A, Hwalek T, Lach L, Panos RJ, Borchers MT. Cytomegalovirus seropositivity is associated with airflow limitation in a cohort of veterans with a high prevalence of smoking. Chronic Obstr Pulm Dis. 2021; 8(4): 441-449. doi: http://doi.org/10.15326/jcopdf.2021.0221
Online Supplemental Material: Read Online Supplemental Material (110KB)
Introduction
The development of airflow limitation (AFL) in chronic obstructive pulmonary disease (COPD) is a hallmark of the condition.1 Although COPD is thought mainly to be due to exposure to noxious particles from combustible tobacco products or biomass fuels,2 altered innate and secondary immunity, extracellular matrix changes, workplace exposures, and genetic predisposition potentiate the effect of environmental exposures in the development of AFL.3-7 The role of chronic herpesvirus infections, specifically cytomegalovirus (CMV), in the pathobiology and epidemiology of AFL is of particular interest due to the putative impact of chronic infections on immune cells and the paucity of clinical literature on this subject. CMV infections have been associated with all-cause mortality in persons who have AFL,8 but not associated directly with the development of AFL. Chronic CMV infection is thought to lead to a proliferation of T cell populations with an altered, pro-inflammatory activity which may synergize with environmental exposures to enhance deleterious effects.9,10 Understanding the associations between CMV+ serostatus, AFL prevalence, and acute exacerbation of chronic obstructive pulmonary disease (AECOPD) events will further elucidate relationships between CMV+ serostatus and presence of AFL in COPD.
To examine the relationship between CMV infection, AFL, and AECOPD, we studied a cohort of current and former smokers at the Cincinnati Veterans Affairs (VA) hospital with available spirometry testing and CMV serostatus and antibody avidity. We hypothesized that CMV+ serostatus and stronger CMV avidity (suggesting a longer-standing chronic infection and/or frequent CMV reactivation due to infectious or environmental insult) would be associated with higher odds of prevalent AFL and increased historical AECOPD events in a cohort of veterans who smoke.
Methods
Study Population
The study cohort was 172 current and former smokers recruited from the Cincinnati Veterans Affairs Hospital system in Cincinnati, Ohio and included 84 participants with and 88 without AFL (defined as a post-bronchodilator forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio <0.70) who had stored blood samples for CMV serology. Participants were recruited from the pulmonary clinic and were included if their reason for referral was listed as “COPD,” “chronic bronchitis,” or “emphysema,” or they were thought to be at risk due to the presence of respiratory symptoms and smoking without the presence of another chronic lung disease. Only those with a smoking history (never smokers with the abovementioned referral diagnoses were excluded), available pulmonary function tests, and no other underlying lung disease were included in the study. Study procedures were performed after obtaining written informed consent from patients. The study design was reviewed and approved by the VA Research and Development Committee and the University of Cincinnati Institutional Review Board (IRB# 2014-2354). All methods were performed in accordance with the relevant guidelines and regulations. Data from patients, such as questionnaires, spirometry results, and all specimens were assigned a unique study number to de-identify patient personal information.
Data Collection
Demographic data (age, gender, race), body mass index (BMI), inhaled medication usage, smoking status, and pack years smoked were collected at enrollment. BMI was categorized by American Association of Clinical Endocrinologists/American College of Endocrinology guidelines: underweight (<18.5kg/m2), normal weight (18.5–24.9kg/m2), overweight (25–29.9kg/m2), obesity class I (30–34.9kg/m2), and obesity class II-III (>35kg/m2).11 Pre- and post-bronchodilator spirometry was performed at the time of enrollment according to American Thoracic Society/European Respiratory Society guidelines.12 COPD was defined as a participant with a history of smoking who had AFL as defined above. AECOPD was determined by review of each patient’s VA medical records in the 2 years prior to enrollment. Exacerbations were collected over the 2 years prior to enrollment and classified by the total number of AECOPD and by AECOPD severity: moderate (worsening of baseline COPD symptoms requiring escalation of therapy but not hospitalization) and severe (worsening of COPD symptoms requiring hospitalization or emergency department care). Plasma samples were collected at enrollment and isolated from lithium heparin anticoagulated blood and stored at -80oC until use. Assays to assess the presence and antigen-binding activity of CMV antibodies were performed by ELISA according to the manufacturer’s protocol (VIDITEST anti-CMV IgG and IgG avidity, Vidia, Czechoslovakia). The avidity of CMV antigen binding, which measures the strength which CMV-directed IgG binds to CMV epitopes which starts weak and becomes stronger up to 6 months after initial infection, was also performed.13 High avidity represents long-standing infection, repeat CMV exposure, or frequent CMV reactivation from environmental or infectious exposure, while low represents a more recent CMV infection (roughly within the prior 6 months).14
Statistical Analysis
Descriptive statistics were used to examine the mean with standard deviation (normal distribution) or median with Q1–Q3 ranges (non-normal distribution) for continuous variables and frequency and percentage for categorical variables in the entire cohort. Participants were dichotomized based on a positive CMV affinity test (CMV+) versus negative (CMV-) test. Two-sample Student’s -test or Mann-Whitney U testing and chi-squared testing were used to determine the relationships between CMV serostatus and continuous and categorical variables, respectively. Bivariable and multivariable logistic regression analyses were used to assess the relationships between CMV serostatus (exposure) and AFL (outcome). In multivariable models, covariables were selected based on clinical relevance15 and include age (per 5 years), Black race, female gender, pack years smoked (per 5 years), and BMI classification. The associations between CMV avidity (high as referent; exposure) and AFL (outcome) were assessed by bivariate and multivariable analyses. For multivariable analysis, the same variables were used as above. Sensitivity analyses were performed in statistically significant models using BMI as a continuous rather than categorical variable.
We also assessed the associations between the outcome of medical chart documented AECOPD events in the 2 years prior to enrollment and CMV status in participants with AFL (n=84). AECOPD was modeled as either no AECOPD (0 events) or AECOPD (1+ event) or total number of exacerbations over the 2 years of retrospective data collection. AECOPD events were classified as total, moderate, or severe severity in each analysis based on the criteria above. Bivariable and multivariable logistic regression was used to model AECOPD as no events over the prior 2 years (0) versus 1-or-more events over the prior 2 years (1+) and Poisson regression was used to model AECOPD events as counts (number of exacerbations over 2 years prior to enrollment). Multivariable models included the covariables of age, Black race, female sex, pack years smoked, current smoking, BMI classification, and post-bronchodilator FEV1. Due to the high number of participants reporting no exacerbations in the cohort, zero-inflated negative binomial regression was also used to assess the associations between the number of AECOPDs and CMV status.
Results
Description of Cohort
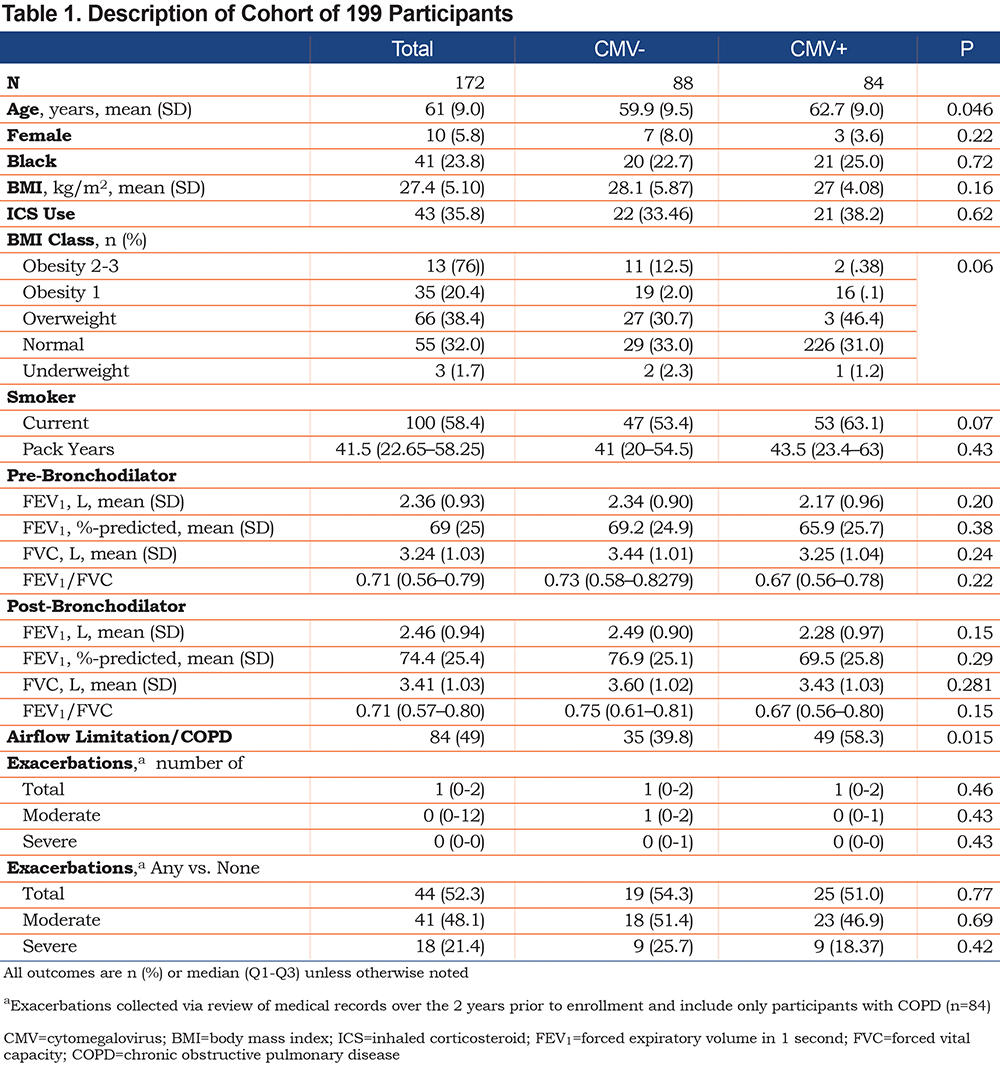
In the overall cohort, 49% of participants were CMV+ (Table 1), 87% of whom had either high or intermediate avidity tests suggesting long-standing and/or repeat CMV exposure. Females composed 6% of the cohort and 24% reported Black race. The mean BMI was 27kg/m2 and 36% reported inhaled corticosteroid use. Mean post-bronchodilator FEV1 was 74%-predicted and FEV1/FVC was 0.71. Post-bronchodilator AFL was present in 49% of the cohort.
In those with AFL/COPD, 52% reported at least 1 AECOPD in the last 2 years. The median exacerbation count for those with AFL/COPD (n=84) was 1, with a Q1–Q3 of 0–2, and a range of 0–9 AECOPD events.
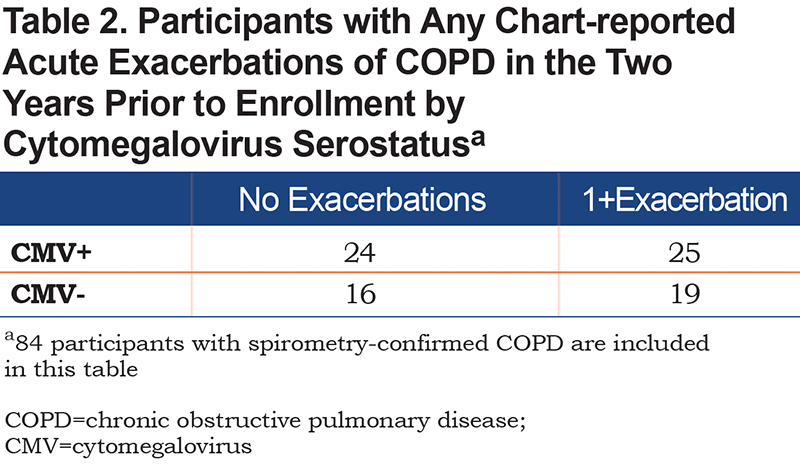
CMV+ participants were significantly older (63 versus 60 years-old). There was no difference in BMI, current versus former smoker, and pack years smoked between the 2 groups. There was no difference in pre- or post-bronchodilator FEV1 %predicted between the 2 groups. There was no association between CMV+ serostatus and exacerbators versus non-exacerbators (Table 2), type of AECOPD events, or number of AECOPD events.
Associations Between Cytomegalovirus Status and Airflow Limitation
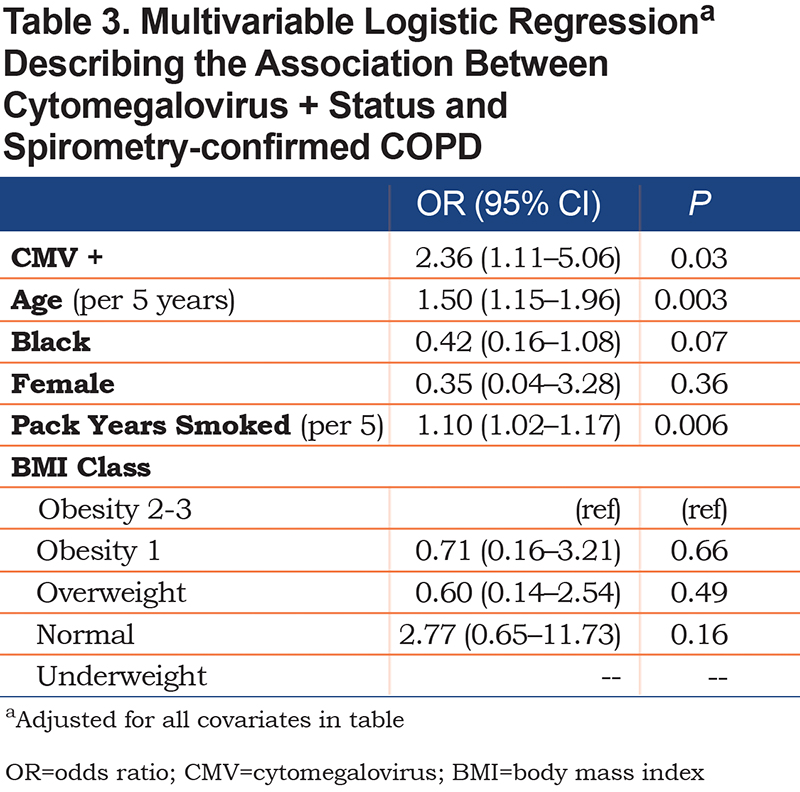
In bivariable logistic regression analysis, CMV+ participants had 112% (odds ratio [OR] 2.12) higher odds of having AFL (95% confidence interval [CI] 1.15–3.88, P=0.002). In multivariable regression controlling for clinically-relevant covariables, CMV+ participants had 136% (OR 2.36) higher odds of AFL (95% CI 1.11–5.06, P=0.01) (Table 3). There were no statistically significant relationships between CMV avidity in bivariate or multivariable analysis. In bivariate regression, neither high binding avidity (OR 3.90 [95% CI 0.76–20.0]; P=0.12) nor intermediate biding avidity (OR 2.00 [95% CI 0.51–7.88]; P=0.32) were associated with AFL. In multivariable regression using the same covariables as above, neither high binding avidity (OR 5.74 [95% CI 0.76–27.0]; P=0.07) nor intermediate binding avidity (OR 4.53 [95% CI 0.67–49.5]; P=0.11) were associated with AFL.
Associations Between Cytomegalovirus Status and Acute Exacerbation of COPD
In cohort participants with AFL/COPD (n=84), the association between CMV status and AECOPD and odds and incidence rates were assessed. In bivariable analysis, there were no associations between odds of having 1+ versus 0 of any AECOPD type, moderate AECOPD or severe AECOPD, and CMV+ serostatus. No significant associations were seen in multivariable logistic regression modeling and the results are presented in Supplementary Table 1 in the online supplement.
With AECOPD events modeled as counts in Poisson regression, CMV+ status was associated with a lower incidence of total AECOPD (incidence rate ratio [IRR] 0.68 [95% CI 0.46–0.98], P=0.04) and moderate AECOPD (IRR 0.63 [95% CI 0.40–0.98]; P=0.04), but no relationship was seen with severe AECOPD. The relationships between lower incidence rate and CMV+ status were attenuated in multivariable models controlling for clinically relevant covariates (Supplementary Table 2 in the online supplement). When modeling as counts using zero-inflated negative binomial regression, there were no significant associations observed in bivariate or multivariable analyses between CMV+ status and AECOPD outcomes.
Discussion
In this study of 172 persons at risk for AFL, the presence of serum antibodies to CMV was independently associated with increased odds of AFL when controlled for clinically relevant covariates, including demographic variables, pack years smoked, and BMI. These findings suggest a remote CMV exposure and potential episodes of acute illness leading to CMV reactivation may be associated with prevalent AFL in a cohort of veterans with a high prevalence of smoking. In participants with AFL, there was no correlation between CMV+ status and increased odds or incidence of reported AECOPD in the 2 years prior to enrollment when controlled for clinically relevant covariates.
To date, limited information exists in the literature regarding chronic CMV infection and associations with obstructive lung disease. Ours is the largest cohort to date looking specifically at epidemiologic associations between CMV serostatus and airflow-related outcomes, as well as leveraging CMV antibody avidity to approximate timing of CMV exposure. Another study used proportional hazard modeling to associate CMV infection with COPD-related mortality in both those with and without AFL at the time of enrollment.16 These findings are notable in light of other studies associating chronic CMV infection and all-cause mortality that was believed to be due to chronic low-grade inflammation.8 It has been proposed that this pro-inflammatory state may be driven by proliferation of CD28- T cells caused by continual CMV reactivation in those with chronic airways disease.9 Our findings further this putative relationship by describing the independent association between CMV+ serostatus and prevalent spirometry-defined AFL in a clinical cohort of 199 veterans with a high prevalence of smoking for the development of COPD. Also, assessment of CMV IgG antibody avidity and relationships with COPD in CMV+ participants show that those with low avidity (suggesting more recent CMV infection compared to longer standing infections with more reactivations) have a marginally significant 77% lower odds of having AFL in our cohort, as well. While we cannot completely discern if CMV infection is on the causal pathway to AFL development, these findings suggest a potential relationship between CMV infection and/or frequent CMV reactivations and either the development of airways obstruction or failure to reach full potential lung function due to CMV infection early in life (the timing of which we cannot infer in this analysis).
CMV is rarely cleared after initial infection and can cause a latent, subclinical chronic infection which may promote a pro-inflammatory state with a wide range of adverse consequences.17-20 The effect of CMV serostatus on atherosclerosis development is a well-studied example of a relationship between CMV serostatus and long-term health outcomes as atherosclerosis and smoking-related lung diseases are thought to be pathophysiologically related. Chronic CMV infection is associated with increased levels of inflammatory cytokines associated with the progression of atherosclerosis21,22 with potential associations between CMV-positivity and clinical atherosclerotic outcomes.22 More advanced airways obstruction,23 increased inflammatory markers in those with COPD, 24 and increased products of extracellular matrix breakdown25 are all associated with worse atherosclerosis-related outcomes. The presence of CD28- T-cells seems to play a role in the putative link between CMV infection and poor cardiovascular and pulmonary outcomes.18,26 Similar cellular pathways to adverse clinical events in COPD and atherosclerosis, along with the strong implication of a link between inflammation-driven pathophysiology in these diseases, lend credence to the potential for chronic CMV infection to promote systemic inflammation that may cause airways disease in at-risk individuals. Longitudinal studies, preferentially observing lung function decline over several years, exacerbation frequency and severity, and outcomes in comorbid conditions in smokers or COPD patients with CMV exposure, will add further clarity to this hypothesis.
Future studies should focus on the timing of the initial CMV infection or focus on participants at-risk for COPD in adolescence or early mid-life. Generally, chronic airways obstruction is thought to be due to either failure of the lungs to reach maximal capacity due to a variety of early-life factors and/or a more rapid decline in lung function after full lung development.27,28 In immunocompetent individuals infected with CMV, manifestations tend to be subclinical but the presence of the virus elicits continued immune surveillance.29 The presence of CMV may alter particular immune cell structure and function which may potentiate and augment the inflammatory response to noxious stimulants subsequently leading to AFL/COPD. Natural killer (NK) cells are indispensable in the control of CMV infections.30 In the presence of a chronic CMV infection, NK cells have a subtype shift to more cytotoxic and pro-inflammatory entities with an increase in CD57 and NKG2C signaling properties.31-34 A shift to NK cell populations expressing this signaling has been associated with AECOPD previously,31 and further understanding the long-term effects of expressing these NK cell populations in the development of AFL is an important, ongoing endeavor. Due to the need for constant immune control, persons with CMV infection also have alterations of their T-cell population that may, over time, exhaust the T-cell response leading to early immunoscenescence.29,35-37 This mechanism may put active smokers at higher risk of lung function decline. Also, childhood CMV infection potentially may interdict full lung maturation increasing the subsequent risk of AFL should these individuals be exposed to tobacco smoke or other noxious inhalants. CMV infection in early adulthood is associated with relative poverty,36 an already known risk factor for COPD risk as an adult.38 Our findings raise potential questions about the potential role of CMV infections, the subsequent development of AFL, and whether this association more specifically affects children from a low socioeconomic status or represents CMV infection contracted at other life stages. Further study specifically focusing on associations between early and mid-life CMV+ serostatus and maximum lung growth and lung function decline is warranted.
This study has limitations. This study is cross-sectional and, as such, we are only able to show CMV+ serostatus and increased odds of COPD prevalence. Due to the high number of those with AFL we cannot approximate true risk. While this finding is novel, further longitudinal study is needed to more thoroughly assess this relationship. Further, the CMV infected cohort was older, more likely to smoke, and had worse lung function potentially putting these participants at a higher risk of developing a CMV infection rather than a CMV infection being the cause of these findings. Due to these samples being collected in the VA health system, females are under-represented which limits generalizability. Further, using a VA-based cohort may limit generalizability to the overall population. Collection of socioeconomic status would have improved the modeling approach based on associations between poverty, CMV status, and COPD as discussed above. The reported AECOPD odds were determined from events only measured in the VA medical record and non-VA COPD-related health care encounters were not recorded and AECOPDs may have been undercounted. The relatively small sample size may impact our ability to assess some associations due to the effect on statistical power, namely the relationship between avidity and AFL. Also, the use of historical AECOPD events raises the possibility of survivorship bias, which would influence our reported associations.
Conclusion
In conclusion, in a sample of veterans who smoke, CMV+ serostatus is associated with increased odds of AFL when controlled for clinically relevant cofactors. Based upon these findings, longitudinal studies of associations between early- and mid-life CMV exposure and lung function decline in smokers will better elucidate this proposed relationship. Investigation of factors mitigating early- and mid-life CMV exposures and increased risk of the development of AFL would prove beneficial but remain theoretical at the time of this writing.
Acknowledgements
RMB wrote the manuscript which was critically assessed and edited by all other authors. MTB, AO, TH, LL, and RJP collected the data. RMB performed the statistical analysis. All authors were involved in the conceptualization of the manuscript. All authors agreed to submission of the final, submitted manuscript.
Availability of Data and Materials:
Data for this study are from a deidentified cohort. Data sets for this article are stored in the Borchers Lab at the University of Cincinnati and are available by contacting Dr. Borchers at borchemt@ucmail.uc.edu.
Declaration of Interest
RMB, AO, LL, TH have no competing interests. MTB receives grants from the National Institutes of Health (NIH) and the VA. RJP is a site principal investigator on NIH-funded studies and recently on a clinical trial funded by Sanofi.