Running Head: Increased HRU and Costs for COPD+HZ vs COPD
Funding Support: GlaxoSmithKline Biologicals SA funded this study (GSK study identifiers: HO-19-19749/VxHO-000050) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also paid all costs associated with the development and publication of this manuscript.
Date of Acceptance: September 23, 2021 │ Published Online: October 1, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; herpes zoster, HZ; health care resource utilization, HRU; patients with chronic obstructive pulmonary disease and herpes zoster, COPD+/HZ+; patients with chronic obstructive pulmonary disease but without herpes zoster, COPD+/HZ–; adjusted incidence rate ratio, aIRR; confidence interval, CI; per person per month, PPPM; post-herpetic neuralgia, PHN; herpes zoster ophthalmicus, HZO; Advisory Committee on Immunization Practices, ACIP; coronavirus disease 2019. COVID-19; transient ischemic attack, TIA; Optum’s de-identified Clinformatics Data Mart Database, Optum CDM; International Classification of Diseases, ICD; Charlson-Quan Comorbidity Index, CCI; emergency department, ED; United States dollars, USD; myocardial infarction, MI; standard deviation, SD; per person per year, PPPY; per person per thousand years, PPPTY; incidence rate ratio, IRR; human immunodeficiency virus, HIV; bone marrow or stem cell transplant, BMSCT
Citation: Ghaswalla P, Thompson-Leduc P, Cheng WY, et al. Increased health care resource utilization and costs associated with herpes zoster among patients aged ≥50 years with chronic obstructive pulmonary disease in the United States. Chronic Obstr Pulm Dis. 2021; 8(4): 502-516. doi: http://doi.org/10.15326/jcopdf.2021.0222
Online Supplemental Material: Read Online Supplemental Material (1350KB)
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by shortness of breath, chronic cough, and sputum production, encompasses a group of progressive respiratory conditions.1 Globally in 2017, COPD was estimated to be the sixth (women) and ninth (men) leading cause of years lived with disability,2 and the seventh (both sexes) leading cause of years of life lost.3 In the United States in 2017, COPD affected an estimated 6.2% of adults overall (2.7%, 6.3%, 10.6%, and 12.8% of those aged 18–44, 45–54, 55–64, and ≥65 years, respectively).4 Annual medical costs for COPD in the United States have been estimated at $32 billion in 2010, with a potential for reaching $49 billion in 2020.5 Some of this cost burden is attributable to acute worsening of respiratory symptoms (COPD exacerbations), for which some patients require hospital care.6 Patients with COPD also appear to be at a 1.1–1.7-fold increased risk of herpes zoster (HZ) (shingles),7-14 commonly reported as a painful rash due to reactivation of latent varicella zoster virus.15 This increased risk is thought to be due to immune dysregulation/impairment in patients with COPD.7,11 The incidence rate of HZ increases with age, doubling from approximately 7 per 1000 person years among those aged 50–54 years to around 14 per 1000 person years among those aged ≥80 years in the United States.16 Up to a third of patients with HZ subsequently suffer from post-herpetic neuralgia (PHN), a neuropathic pain syndrome.15 HZ patients can also develop HZ ophthalmicus (HZO), which involves the ophthalmic division of the trigeminal cranial nerve.17 Overall, HZ and its complications were estimated to incur annual costs (direct medical costs plus productivity losses) of $2.4 billion in 2015 in the United States.18
Routine vaccination against HZ is recommended for immunocompetent adults aged ≥50 years in the United States by the Advisory Committee on Immunization Practices (ACIP).19 Additionally, adults with chronic medical conditions, including chronic pulmonary disease, are recommended by the ACIP to receive HZ vaccination.20 However, HZ vaccination coverage in 2017 in the United States was only estimated to be 35% among adults aged ≥60 years,21 and large declines in weekly HZ vaccination rates (up to 89%) were observed following the coronavirus disease 2019 (COVID-19) national emergency declaration in mid-March 2020 compared to the same period in 2019.22 Coverage among populations with chronic medical conditions is unknown. Despite the increased risk of HZ among patients with COPD,7-14 little is known about the impact of an acute HZ episode on health care resource utilization (HRU) and costs among patients with COPD in the United States, or on COPD-related outcomes such as COPD exacerbations. An increased risk of vascular events such as stroke and transient ischemic attack (TIA) following an acute HZ episode has been reported previously,23 but not specifically for a COPD population.
This study assessed the economic burden, in terms of all-cause and COPD-related HRU and costs, of acute HZ episodes in adults aged ≥50 years with COPD in the United States (for a Graphical Abstract click here). The study also evaluated HZ complications, the incidence rates of COPD exacerbations before and after an acute HZ episode in an exploratory analysis, and the incidence rates of vascular events in patients with COPD, with versus without HZ. Figure 1 summarizes the context, outcomes, and impact of this study for health care professionals.

Material And Methods
Data Source
This study (GSK study identifiers: HO-19-19749/VxHO-000050) used data from the Optum’s de-identified Clinformatics Data Mart Database(Optum CDM), which includes administrative claims associated with medical services provided by health care professionals and detailed information on prescription fills, from January 1, 2013, to December 31, 2018. Optum CDM includes approximately 13 million annual members aged ≥18 years in all U.S. census regions and includes commercially insured and Medicare Advantage members. Optum CDM contains >36 months of historical data on patient demographics, dates of eligibility, death, claims for inpatient and outpatient visits, costs of services, and laboratory tests and results. Data are de-identified and comply with the patients’ protected health information requirements of the Health Insurance Portability and Accountability Act.
Study Design
In this retrospective cohort study, 2 cohorts were identified (based on the International Classification of Diseases [ICD] codes as defined in Supplemental Table S1 in the online supplement): those with a COPD diagnosis, with or without an HZ diagnosis (COPD+/HZ+ and COPD+/HZ–, respectively).
For the COPD+/HZ+ cohort, the index date was the date of the first claim associated with an HZ diagnosis during January 1, 2014, to June 30, 2018. For the COPD+/HZ– cohort, an index date was assigned such that the distribution of time in the pre-index period was similar to that in the COPD+/HZ+ cohort (Figure 2). The 12-month period before the index date was defined as the baseline period, while the observation period was defined as any time after the index date. Patients were required to have ≥12 months of continuous health insurance coverage before and ≥6 months of continuous health insurance coverage following the index date.
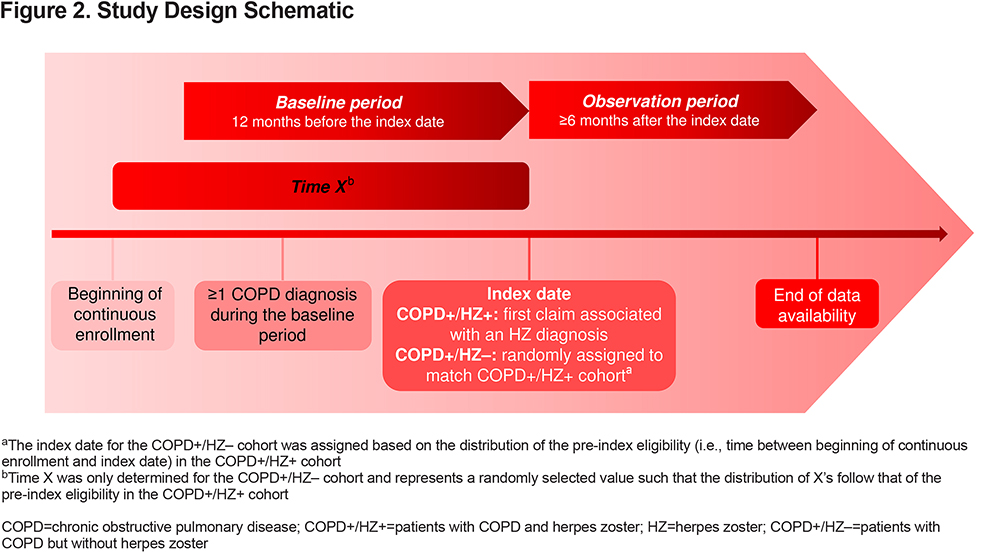
Study Population and Baseline Data
The study population comprised patients aged ≥50 years of known gender on the index date. All patients had ≥1 COPD diagnosis (based on 1 inpatient claim, 2 outpatient claims, or 1 outpatient claim plus a filled prescription for a COPD maintenance treatment; full details of the operational definition modified from the literature24 in Supplemental Table S1) during the baseline period. Patients in the COPD+/HZ+ cohort had ≥1 claim associated with an HZ diagnosis (the first of which was defined as the index date), but no claims associated with HZ complications (PHN or HZO; defined in Supplemental Table S1) during the baseline period and no claims for an HZ vaccine between the beginning of continuous enrollment and the index date. Patients in the COPD+/HZ– cohort had no claims associated with an HZ diagnosis (including HZ complications) and no HZ vaccine between the beginning and end of continuous enrollment.
Data assessed as of the index date were: age, gender, race/ethnicity, geographic region, and type of insurance plan. Data assessed during the baseline period were: asthma, modified Charlson-Quan Comorbidity Index (CCI)25 and its component conditions (listed in Supplemental Table S1), autoimmune comorbidities potentially associated with an increased risk of HZ (listed in Supplemental Table S1), immunosuppression status (based on the immunosuppressive conditions listed in Supplemental Table S1), use of corticosteroids, receipt of influenza vaccine, and all-cause HRU and costs, comprised of medical services (inpatient stays, emergency department [ED] and outpatient visits, and other [skilled nursing facilities, home care services, hospice, vision care, durable medical equipment, services and supplies, and transportation services]) and pharmacy.
Outcomes
The primary objectives were to compare all-cause and COPD-related (defined as a claim where a COPD diagnosis may be listed in any position or order, i.e., as a primary or coexisting condition) HRU and observed paid costs for COPD+/HZ+ versus COPD+/HZ– patients during the first year of the observation period. This was performed overall and stratified by patients’ age (50–64, 65–79, and ≥80 years). Cumulative all-cause and COPD-related cost analyses were also conducted for the first 1-, 2-, 3-, and 6-month follow-up periods. HRU and costs associated with HZ, PHN, and HZO were also estimated.
HRU during the observation period (all-cause, COPD-related, and HZ-related [defined as a claim associated with an HZ diagnosis in any position]) included inpatient stays, ED and outpatient visits, and other (as defined above). Total costs (adjusted to 2018 U.S. dollars [USD] using the medical care component of the Consumer Price Index) during the observation period (all-cause, COPD-related, and HZ-related) comprised medical costs (inpatient, ED, outpatient, and other) and pharmacy costs.
Exploratory objectives included: comparison of the incidence rates of COPD exacerbations (see Supplemental Table S1) before and after an acute HZ episode (using a pre-/post-index study design) in the COPD+/HZ+ cohort; and comparison of the incidence rates of vascular events (stroke, TIA, and myocardial infarction [MI]; see Supplemental Table S1) between the COPD+/HZ+ and COPD+/HZ– cohorts.
Statistical Analyses
All statistical analyses were conducted using the statistical software SAS 9.4 or SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, North Carolina).
Patient Baseline Characteristics:
Baseline demographic and clinical characteristics are described for the COPD+/HZ+ and COPD+/HZ– cohorts. Means and standard deviations (SDs) are reported for continuous variables, and frequencies and percentages for categorical variables. Due to the large sample size, differences between cohorts were assessed using standardized mean differences instead of P-values. Standardized differences were calculated by dividing the difference in the means by the standard deviation for continuous variables, and by dividing the difference in proportions by √p(1-p) (where p is half of the sum of the proportions of the 2 cohorts) for categorical variables. Standardized differences of 20%, 50%, and 80% suggest small, medium, and large differences between cohorts, respectively.26
Primary Analyses:
The primary outcomes (all-cause and COPD-related HRU and costs) were compared between the COPD+/HZ+ and COPD+/HZ– cohorts during the first year following the index date (observation periods lasted a minimum of 6 months after the index date and were truncated at 1 year).
The proportions of patients who used each type of medical service (inpatient, ED, outpatient, and other) – all-cause and COPD-related – are reported (overall and stratified by patients’ age [50–64, 65–79, and ≥80 years]). To account for differing lengths of observation periods, incidence rates of each HRU encounter type are reported on a per person per year (PPPY) basis. To compare HRU encounters (all-cause and COPD-related; overall and in the 3 age groups) between the COPD+/HZ+ and COPD+/HZ– cohorts, adjusted incidence rate ratios (aIRRs) were calculated using negative binomial regressions, as over-dispersion was detected. All multivariable models included the patients’ propensity score and relevant baseline characteristics to account for potential baseline differences between the 2 cohorts. The propensity score was calculated using a logistic regression to model a patient’s propensity of being in their cohort, based on their demographics on the index date (i.e., age, gender, geographic region, and insurance type), clinical characteristics (i.e., diagnosis of asthma, CCI, autoimmune comorbidities potentially associated with an increased risk of HZ, immunosuppression status, and use of oral or inhaled corticosteroids), and costs during the baseline period. Propensity score adjustment was selected over propensity score matching to limit the exclusion of patients resulting from the matching exercise. Ninety-five percent confidence intervals (CIs) and corresponding P-values are reported.
Mean costs (total, medical services [inpatient, ED, outpatient, and other], and pharmacy; all-cause and COPD-related) during the first year of the observation period were calculated on a per person per month (PPPM) basis. Mean total all-cause and COPD-related costs in the 3 different age categories are also reported. All-cause and COPD-related costs were also analyzed during the first 1, 2, 3, and 6 months after the index date.
Adjusted differences in costs between the COPD+/HZ+ and COPD+/HZ– cohorts were estimated using the 2-part modelling approach, which is considered the best approach to model outcomes such as costs, with a skewed distribution for positive costs and a significant proportion of zero values, in the health econometrics literature.27 This approach has been commonly applied to estimate differences in health care expenditures in prior studies.28,29 In the first part, the probability of observing a positive cost was modelled using logistic regression. In the second part, a generalized linear model with a gamma distribution and log link was used to predict costs among patients with positive costs. A recycled predictions approach30 was used to assess the differences in costs between the 2 cohorts by predicting the costs for all patients according to their HZ and COPD statuses using each patient’s own values. Each patient’s data were input back into the estimated regression model twice, each time setting the HZ and COPD variables as follows, regardless of their actual HZ and COPD statuses: (1) HZ=1 and COPD=1, and (2) HZ=0 and COPD=1. Both models included the patients’ propensity score and relevant baseline characteristics. The 95% CIs and approximated P-values were estimated from nonparametric bootstrap procedures with 499 replications. Additionally, among the COPD+/HZ+ cohort, costs associated with a diagnosis of HZ and HZ complications (i.e., PHN and HZO, separately) were summarized on a PPPM basis using means and SDs.
Exploratory Analyses:
In the COPD+/HZ+ cohort, the incidence rates of COPD exacerbations are reported on a per person per thousand years (PPPTY) basis during each of the 12 months immediately before and the 12 months immediately after the index date. Because patients in the COPD+/HZ+ cohort had different COPD risk profiles from those in the COPD+/HZ– cohort, a pre-post design was implemented to minimize inter-subject variation. The incidence rate ratio (IRR), 95% CI, and corresponding P-value were calculated using conditional Poisson regression models, to account for correlations between the pre- and post-index periods within the same patient. In a post-hoc sensitivity analysis conducted to assess the potential impact of the prodromal period (i.e., the month before the recorded onset of HZ), COPD exacerbations were assessed during 1 month before to 2 months after the index date versus the 3 months before that.
The incidence rates (PPPTY) of vascular events (stroke, TIA, and MI) were compared between the COPD+/HZ+ and COPD+/HZ– cohorts during the entire observation period and monthly during the first 6 months. As the observed data displayed over-dispersion, the aIRRs for stroke and TIA were individually estimated using negative binomial regressions adjusting for the patients’ propensity score and relevant baseline characteristics. Since no over-dispersion of the data was detected for MI, the aIRR for MI was estimated using a Poisson regression adjusting for the patients’ propensity score and relevant baseline characteristics. The 95% CIs and the corresponding P-values from each model are reported.
Ethics Approval and Informed Consent
The study employed a retrospective cohort study design using a large administrative claims database. Data were de-identified and comply with the requirements of the Health Insurance Portability and Accountability Act. Institutional review board review and approval was, therefore, not required, as per the U.S. Department of Health and Human Services regulation for the protection of human participants in research31 The study has been conducted in accordance with the guiding principles of the Declaration of Helsinki. As only existing de-identified data has been analyzed and as patients have not been contacted during the course of this study, the informed consent process is not applicable.
Results
Baseline Characteristics
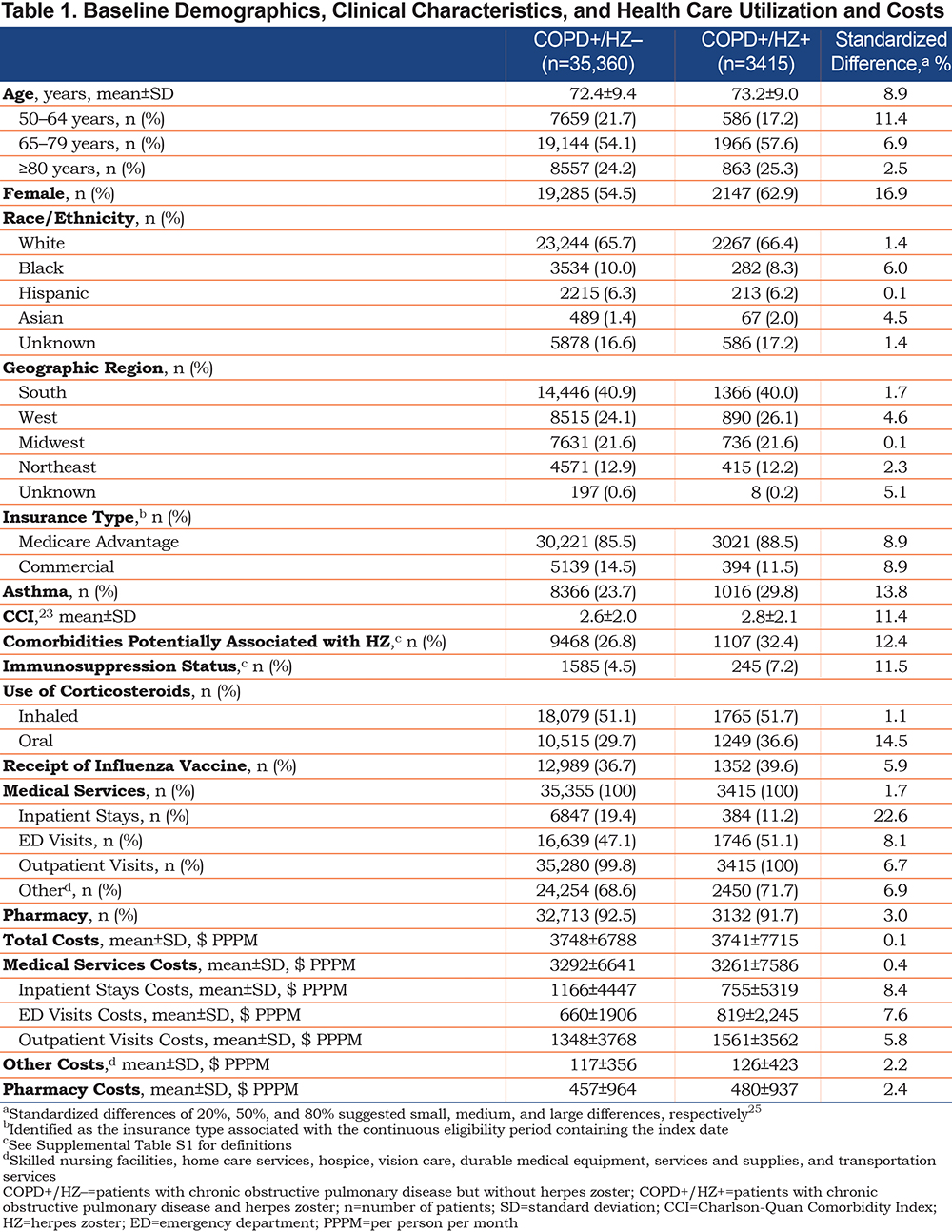
Among a total population of 48,983,801 patients, the COPD+/HZ+ and the COPD+/HZ– cohorts comprised 3415 and 35,360 patients aged ≥50 years, respectively (Supplemental Figure S1). The mean ages of the 2 cohorts were 72.4±9.4 and 73.2±9.0 years, respectively, with 54.5% and 62.9% female (Table 1). The standardized differences were <20% (i.e., small), apart from inpatient stays, which were more common and more costly in the COPD+/HZ– cohort (Table 1).
Health Care Resource Utilization
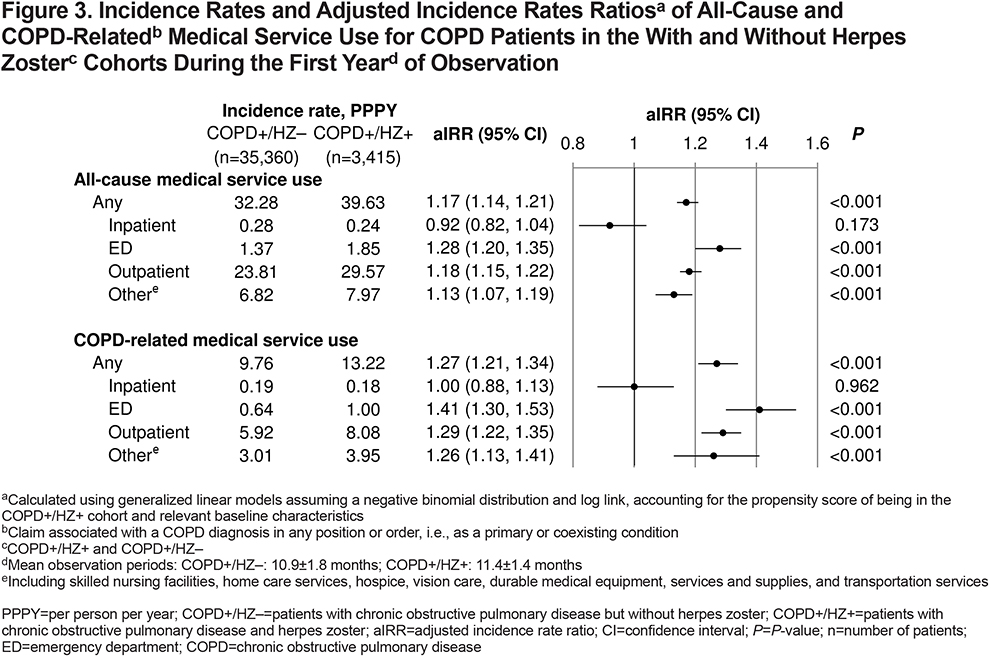
Patients in the COPD+/HZ+ versus COPD+/HZ– cohort had significantly more all-cause (39.63 versus 32.28 PPPY; aIRR 1.17; 95% CI 1.14, 1.21; P<0.001) and COPD-related (13.22 versus 9.76 PPPY; aIRR 1.27; 95% CI 1.21, 1.34; P<0.001) medical service use during the first year of observation (Figure 3). Incidence rates of all-cause and COPD-related ED and outpatient visits, and other medical service use were significantly higher in the COPD+/HZ+ cohort than in the COPD+/HZ– cohort, while inpatient stays were similar (Figure 3). Similar trends were observed in the 2 older age categories (65–79 and ≥80 years), while in the 50–64-year age group, other medical service use was similar in both cohorts (Supplemental Figure S2).
Costs
The mean total all-cause cost during the first year of the observation period was higher in the COPD+/HZ+ cohort than in the COPD+/HZ– cohort ($4140±$7616 versus $3749±$7954 PPPM), and the total cost difference was statistically significant after adjusting for baseline covariates (+$313 PPPM; 95% CI $110, $536; P<0.004) (Supplemental Figure S3A). Adjusted all-cause costs during the first year were significantly higher in the COPD+/HZ+ versus COPD+/HZ– cohort for ED and outpatient visits, and other medical service use costs, but were not significantly different for inpatient or pharmacy costs (Supplemental Figure S3A). Costs among patients with costs >$0 in each category are detailed in Supplemental Table S2.
In the 2 oldest age groups, the adjusted total all-cause cost differences during the first year of observation were significant, while in the youngest age group, the adjusted cost difference was not significant (Supplemental Figure S4A).
The mean total COPD-related cost during the first year of the observation period was also higher in the COPD+/HZ+ cohort than in the COPD+/HZ– cohort ($1541±$3980 versus $1231±$3097 PPPM), and the adjusted total cost difference was statistically significant (+$152 PPPM; 95% CI $57, $249; P<0.004) (Supplemental Figure S3B). Adjusted COPD-related costs during the first year were significantly higher in the COPD+/HZ+ versus COPD+/HZ– cohort for ED and outpatient visits, and other medical service use costs, but were not significantly different for inpatient or pharmacy costs (Supplemental Figure S3B). The adjusted total COPD-related cost difference during the first year of observation was significantly higher in the COPD+/HZ+ versus COPD+/HZ– cohort in the oldest age group only (Supplemental Figure S4B).
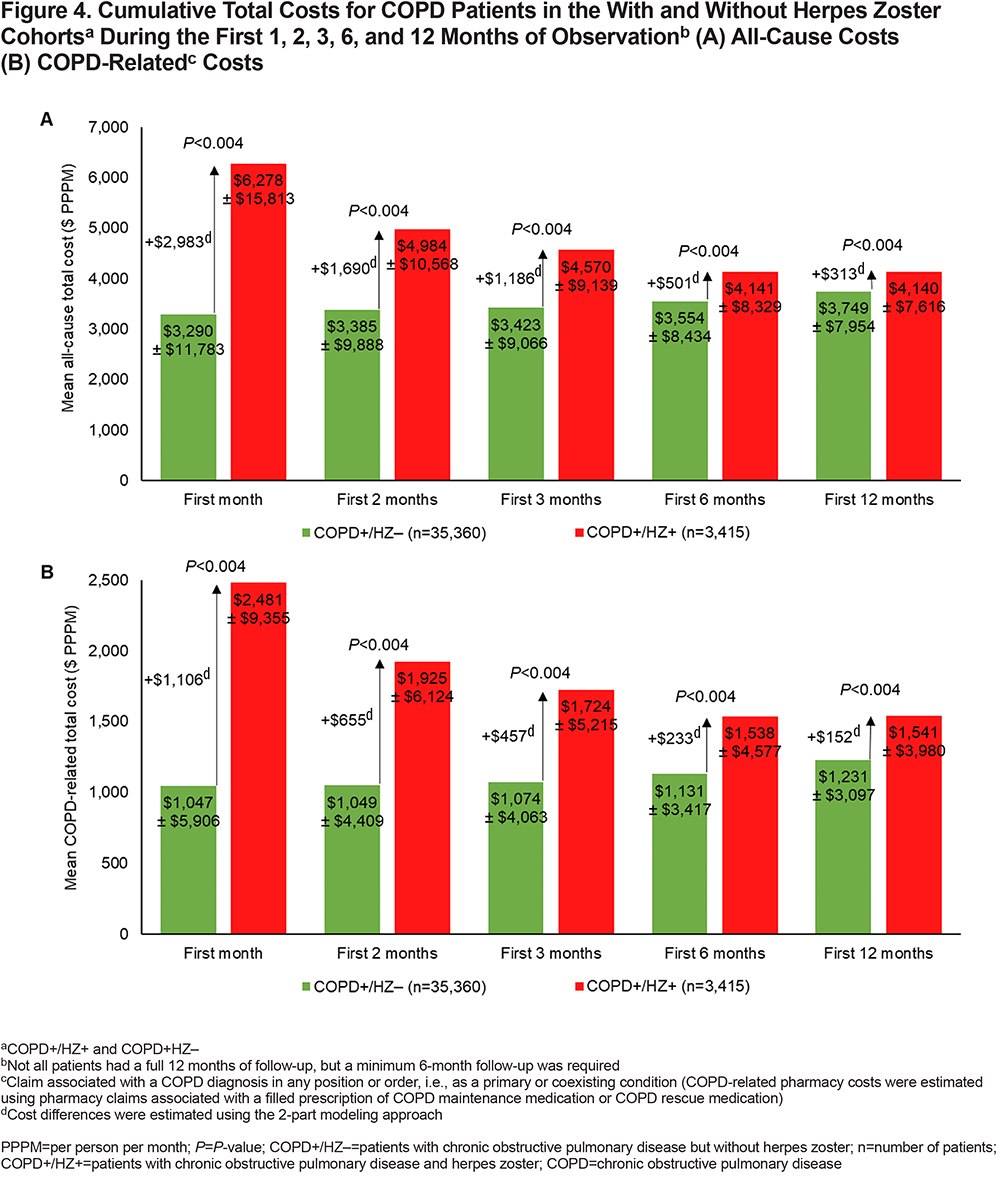
All-cause (Figure 4A) and COPD-related (Figure 4B) PPPM costs were highest in the COPD+/HZ+ cohort during the first month after the index date and cumulative PPPM costs decreased thereafter, as did the adjusted cost differences between the COPD+/HZ+ and COPD+/HZ– cohorts.
Mean HZ-related total cost in the COPD+/HZ+ cohort amounted to $244±$1056 PPPM during the first year of observation, while mean PHN-related medical service cost was $56±$481 PPPM and HZO-related medical service cost was $24±$542 PPPM (Supplemental Table S3). Overall, 574 patients (16.8%) had costs associated with PHN (mean cost among these patients: $332±$1134 PPPM) and 258 (7.6%) had costs associated with HZO (mean cost among these patients: $321±$1952 PPPM) (Supplemental Table S3).
COPD Exacerbations
In the COPD+/HZ+ cohort, the incidence rate of COPD exacerbations (moderate and severe combined) was lower in the 12 months after the HZ index date than in the 12 months before the HZ index date (749 versus 861 PPPTY; IRR 0.87; 95% CI 0.83, 0.92; P<0.001) (Supplemental Figure S5). However, in a post-hoc analysis, the incidence rate of COPD exacerbations in the COPD+/HZ+ cohort was higher during a 3-month period ranging from 1 month before to 2 months after the HZ index date versus the 3 months before that (999 versus 888 PPPTY; IRR 1.13; 95% CI 1.03, 1.23; P=0.010) due to a spike in exacerbations in the month before the HZ index date (Supplemental Figure S5).
Vascular Events
The incidence rates of stroke and TIA after the index date were significantly higher in the COPD+/HZ+ cohort than in the COPD+/HZ– cohort, even after adjustment for baseline covariates (stroke: aIRR 1.54, 95% CI 1.18, 2.01; TIA: aIRR 1.39; 95% CI 1.14, 1.69; P=0.001; Supplemental Figure S6), with the largest difference observed during the first month after the index date (stroke: 109.6 versus 47.4 PPPTY; TIA: 92.3 versus 44.1 PPPTY; data not shown). Conversely, the incidence rates of MIs were similar in the 2 cohorts, during the entire follow-up (Supplemental Figure S6) and during the first month after the index date (23.9 versus 22.0 PPPTY; data not shown).
Discussion
The higher HRU observed among the COPD+/HZ+ cohort compared to the COPD+/HZ– cohort translated into significantly higher total all-cause and COPD-related costs in the year following the onset of HZ in U.S. patients aged ≥50 years, with the majority of this difference in the first month.
During the first year after HZ diagnosis, the mean adjusted cost difference between patients in the COPD+/HZ+ versus COPD+/HZ– cohorts was +$313 PPPM, including a mean adjusted COPD-related cost difference of +$152 PPPM and mean HZ-related costs in the COPD+/HZ+ cohort of $244 PPPM. The latter is higher than in an older U.S. study in a general HZ population (aged ≥22 years), which reported mean HZ-attributable costs of $100 PPPM (2006 USD) during the 3 weeks before to the year after an HZ diagnosis,32 likely because the current study population was older.
Previous systematic reviews and meta-analyses on the risk factors for HZ have associated comorbidities such as, asthma, diabetes, chronic kidney disease, and COPD with an increased risk of HZ.9,14 To our knowledge, no previous studies have examined the additional cost of HZ in patients with COPD or in patients with other comorbidities. However, some studies have reported on the additional cost of other conditions among U.S. patients with COPD33 or the additional cost of HZ among immunocompromised patients.34,35 Mannino et al33 reported that, among patients with COPD, those who also had asthma, osteoporosis, diabetes, depression, chronic kidney disease, cardiovascular disease, or anemia had additional costs ranging from $3129 (asthma) to $10,762 (anemia) (2012 USD) in the year following a COPD diagnosis.33 The incremental cost of the COPD+/HZ+ versus COPD+/HZ– cohort in the current study ($3756 in the first year) was similar to the lower end of the additional costs reported by Mannino et al.33
Two studies have reported on the differences in direct medical costs between matched immunocompromised U.S. patients with or without HZ,34,35 with quite different results. Meyers et al35 included a range of immunocompromised patients (mainly identified by immunosuppressant use) aged ≥50 years, while Li et al34 included patients aged 18–64 years with human immunodeficiency virus (HIV), solid organ transplant, bone marrow or stem cell transplant (BMSCT), or cancer, and patients aged ≥65 years with cancer. In the study by Meyers et al,35 the mean unadjusted incremental cost (2013 USD) during the first 3 months after HZ was +$1087, but after adjustment for baseline health care costs, this fell to +$197 and +$722 for uncomplicated and complicated HZ, respectively. In the study by Li et al,34 mean incremental costs (2014 USD) during the first 3 months were much higher, ranging from $2549 to $3108 for patients with cancer, solid organ transplant, or HIV, and reaching $13,332 among those with BMSCT. For comparison, the incremental all-cause adjusted cost difference between the COPD+/HZ+ and COPD+/HZ– cohorts in the current study was +$3558 during the first 3 months after HZ, i.e., considerably higher than the costs in the study by Meyers et al,35 and similar to the non-BMSCT costs in the study by Li et al.34 These differences could be related to the very different patient populations in the 3 studies. Patients in the study by Meyers et al35 had the best health at baseline (means across populations: CCI: 2.2–2.5; total health care costs: $1865–$2245 PPPM), followed by those in the current study (2.6–2.8; $3741–$3748 PPPM), then those in the study by Li et al34 (3.1–7.0; $4503–$40,950 PPPM). Despite these differences, both studies34,35 reported that HZ incurred additional health care costs among immunocompromised patients, and that the largest incremental costs occurred in the early months after HZ. This front-loading of incremental costs was also observed in the current study, in which 79.4% of the first-year all-cause incremental cost in the COPD+/HZ+ versus COPD+/HZ– cohort was accrued in the first month.
In the current study, COPD exacerbations peaked in the month before the first HZ claim. However, in the initial stage of HZ, known as the prodromal phase, symptoms such as headache, pain, photophobia, and malaise may be reported by some patients preceding the typical HZ rash onset.36 Such patients may, therefore, not have received an HZ diagnosis until the typical rash appeared. As COPD exacerbations included outpatient/ED visits with COPD as a primary or secondary diagnosis plus a corticosteroid/antibiotic prescription within 7 days, and corticosteroids are also used to treat HZ,37 visits during the month before the HZ diagnosis could have been primarily due to these prodromal symptoms rather than true COPD exacerbations. Alternatively, corticosteroids – used to treat COPD exacerbations – are known to weaken the immune system and have been associated with a 1.7-fold increased risk of HZ.38 Thus, it may have been that COPD exacerbation treatment resulted in HZ, rather than HZ impacting COPD exacerbations.
In the current study, HZ was associated with 1.4- and 1.5-fold higher incidence rates of TIA and stroke, respectively, during a mean follow-up of 25 months. Many studies and meta-analyses have examined these associations, and these have recently been reviewed by Wu et al.23 In the reviewed meta-analyses, the risk of stroke/TIA was increased 1.6–1.9-fold (all significant) during the first month after HZ, 1.1–1.2-fold during the first year (most significant), and 1.1–1.4-fold (most significant) after the first year.23 Our results are in line with the upper risk estimate after the first year, but higher than the risk estimates from most of the meta-analyses for this time period. This likely reflects the different patient populations, as patients in the current study all had COPD. Interestingly, a recent study has reported a 25-fold higher risk of HZ in the year after stroke.39
We observed no increased risk of MI during a mean follow-up of 26 months after HZ. Again, various studies have examined this association, with a meta-analysis40 published in 2017 which found a 1.3-fold increased risk of cardiac events (MI or acute coronary syndrome) during the first 3 months after HZ (significant by fixed-effect but not by random-effect models), which fell to a 1.1-fold increased risk of cardiac events (MI, acute coronary syndrome, or coronary artery disease) after 1 year (significant in both models). A later study reported no association between HZ and MI during the month either side of the HZ episode nor during the month before to the year after HZ.41
Limitations and Strengths
Various limitations should be considered when interpreting the study findings. Administrative claims databases such as Optum CDM are generated primarily for the payment of health services delivered by health care professionals and facilities. Therefore, the database used for this study does not include some clinical variables, including pulmonary function tests (to assess COPD severity), physician notes, and certain patient characteristics, such as smoking status and body mass index. This may have resulted in residual confounding. Further, the entire COPD population will not have been captured as adults with less severe COPD are likely undiagnosed. When defining COPD exacerbations, temporal restrictions were used to avoid misclassification of medication use associated with exacerbations, where possible. For example, moderate exacerbations were identified as a ≥1 dispensing of a systemic corticosteroid or antibiotic within 7 days of an outpatient or ED visit. However, some parameters used to capture COPD exacerbations (see Supplemental Table S1) could possibly have been due to other conditions. Hence, future studies should utilize a more robust definition of COPD exacerbations to better explore the relationship between exacerbations and HZ.
The calculation of HRU and costs may be subject to coding errors, omissions, or missing data. There is evidence that HZ-related costs can accumulate before HZ diagnosis.42 Thus, if HZ-related HRU occurred prior to the HZ diagnosis, e.g., during the prodromal period, it would not have been attributed to the follow-up period. As a result, HRU and associated costs may have been underestimated as the prodromal period may not have been fully captured.
Finally, although Optum CDM, from which data were derived, covers approximately 13 million annual members aged ≥18 years, the results of this study may not be generalizable to patients not covered by this commercial insurance program.
However, the present study also has strengths such as the fact that, to our knowledge, it is the first to assess the burden of HZ in patients with COPD and that it included a large sample size.
Conclusions
Patients aged ≥50 years with COPD and HZ had higher HRU and costs than those with COPD but without HZ. The results of this study may be used to estimate the potential cost benefits of HZ vaccination in the United States among special populations, such as adults with COPD.
Acknowledgements
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance, manuscript coordination, and design support for the digital illustration, on behalf of GSK. Grégory Leroux coordinated manuscript development and editorial support. The authors also thank Jenny Lloyd (Compass Medical Communications Ltd., on behalf of GSK) for providing medical writing support.
Author contributions:
All authors participated in the design or implementation or analysis, and interpretation of the study; and in the development of this manuscript and in its critical review with important intellectual contributions. All authors had full access to the data and gave approval before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with the recommendations of the International Committee of Medical Journal Editors for conduct, reporting, editing, and publication of scholarly work in medical journals.
Declaration of Interest
PG was an employee of the GSK group of companies at the time of the study conduct and of the manuscript development and holds shares in the GSK group of companies. PG is now employed by, and holds shares in, Moderna. PTL, WYC, CK, MJW, MSD, JW, and SP are employees of Analysis Group, Inc., a consultancy that received funding from the GSK group of companies to conduct this study. MB is an employee of, and holds shares in, the GSK group of companies. BJP was an employee of, and held shares in, the GSK group of companies at the time of the study conduct and of the manuscript development. BPY reports having received personal fees from the GSK group of companies for consultation related to COPD and herpes zoster during the conduct of this study and for unrelated study of COPD and herpes zoster. BPY also reports having received personal fees from the GSK group of companies for participation in advisory boards related to COPD and herpes zoster, outside the present study. The authors declare no other financial and non-financial relationships and activities.