Running Head: Roflumilast and Azithromycin for COPD
Funding support:This work was funded through the VA Merit award (Kenyon, I01 BX004965-01A1) and the UC Davis Dean’s Fellow Award to Brooks Kuhn.
Date of Acceptance: July 26, 2021│ Published Online Date: August 11, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; Veterans Health Administration, VHA; interquartile range, IQR; hazard ratio, HR; confidence interval, CI; phosphodiesterase-4, PDE-4; Food and Drug Administration, FDA; Patient-Centered Outcomes Research Institute, PCORI; health care resource utilization, HCRU; VHA Corporate Data Warehouse CDW; long-acting beta2-agonists, LABAs; long-acting muscarinic antagonists, LAMAs; body mass index, BMI; short-acting muscarinic antagonists, SAMAs; standard deviation, SD; subdistribution hazard ratio, SHR; oral corticosteroid, OCS; forced expiratory volume in 1 second, FEV1; short-acting beta2-agonists, SABAs; inhaled corticosteroids, ICSs;
Citation: Lam J, Tonnu-Mihara I, Kenyon NJ, Kuhn BT. Comparative effectiveness of roflumilast and azithromycin for the treatment of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2021; 8(4): 450-463. doi: http://doi.org/10.15326/jcopdf.2021.0224
Online Supplemental Material: Read Online Supplemental Material (174KB)
Introduction
Chronic obstructive pulmonary disease (COPD) exacerbations reduce quality of life, accelerate the rate of irreversible deterioration of lung function, and increase mortality risk.1-6 The cost for treating an exacerbation increases with the severity of baseline disease and the severity of the exacerbation.7,8 Not surprisingly, a majority of the morbidity, mortality, and costs of COPD are attributable to exacerbations in patients with frequent exacerbations.9 Preventing exacerbations—especially the more severe exacerbations that lead to hospitalizations—is imperative in improving patient outcomes and reducing costs.10
For patients prone to frequent and severe exacerbations on appropriate bronchodilator and/or inhaled corticosteroid therapy, Global Initiative for Chronic Obstructive Lung Disease report11 and European Respiratory Society/American Thoracic Society 2017 COPD guidelines on preventing COPD exacerbations12 recommend consideration of chronic azithromycin or roflumilast. Roflumilast is recommended in severe COPD associated with chronic bronchitis, but treatment escalation with azithromycin is not limited to patients with chronic bronchitis with most benefit in non-active smokers. Each of these medications carry significant risks (most notably gastrointestinal symptoms and weight loss with roflumilast and hearing loss, antibiotic resistance, and QTc prolongation with azithromycin).
Roflumilast is an oral, selective inhibitor of phosphodiesterase-4 (PDE-4) that received Food and Drug Administration (FDA) approval13 in 2011. The selective inhibition of PDE-4 increases intracellular levels of cyclic adenosine monophosphate, which then leads a multitude of downstream anti-inflammatory effects, including the decreased release of inflammatory mediators and cytokines. This anti-inflammatory effect can mitigate the chronic inflammation that is thought to contribute to the pathogenesis of COPD exacerbations. Similarly, azithromycin has anti-inflammatory and immune-modulating effects (in addition to its antimicrobial properties). Albert et al showed 250 mg azithromycin daily for 1 year decreased the rate of acute COPD exacerbations,14 echoing the findings from a randomized controlled study of erythromycin.15 While roflumilast and chronic azithromycin have independently been shown to reduce acute exacerbations of COPD in appropriately selected patients, no head-to-head trials have assessed comparative efficacy or outcomes.
The comparative effectiveness between roflumilast and azithromycin remains unclear. The Patient-Centered Outcomes Research Institute (PCORI) is sponsoring an ongoing randomized controlled trial (the RELIANCE study, NCT04069312) that aims to compare as its primary objective the time-to-first all-cause hospitalization or death for roflumilast and azithromycin with a follow-up of up to 36 months.16 The prevalence of COPD is higher in the Veterans’ Health Administration (VHA) population than in the general population.17 The VHA patient population tends to be older, have more comorbidities, and more tobacco abuse, and has a higher health care resource utilization (HCRU), including hospitalizations, than the general population.18 It is, therefore, a potentially higher risk cohort than is being studied in RELIANCE. Furthermore, there are different factors influencing prescribing in the VA population (cost is less of a concern than formulary listing).
To better understand the patterns of use and associated outcomes, we conducted this retrospective cohort analysis using real-world data from the VHA to compare the effects of roflumilast or chronic azithromycin on subsequent COPD-related hospitalization, all-cause hospitalization, and death.
Methods
Data Source
The main data source of this study is the VHA Corporate Data Warehouse (CDW), a national repository of clinical and administrative data, including demographic data, inpatient and outpatient clinic visit abstracts, and prescription records.18 While robust, this database does have limitations, most notably there is no spirometry or pulmonary function data and no reliable account for active or past tobacco use or the presence of chronic bronchitis. Patients are regularly prescribed chronic azithromycin and roflumilast in the VA, but the latter requires non-formulary drug approval through the pharmacy, introducing an administrative barrier at minimum. To account for hospitalizations outside of the VA, we linked the CDW data with Medicare claims data for the years 2011 to 2017 with the help of the VA Information Resource Center for the patients with Medicare coverage.
Access to the data is through the VA Informatics and Computing Infrastructure, a secure, central analytic platform for performing research.19
This project was approved by the VA Long Beach Healthcare System Institutional Review Board.
Patient Selection
Patients included in this study had to have:
(1) at least 1 inpatient or 2 outpatient visits for COPD exacerbation that were less than 1 year but more than 6 months apart between 2011 to 2017 (Supplement Table 1 in the online supplement);
(2) received concurrent long-acting beta2-agonist (LABA) and long-acting muscarinic antagonist (LAMA) treatment for at least 30 days between 2011 to 2017;
(3) received a prescription for either roflumilast or chronic azithromycin anytime between 2012 to 2017.
The chronic azithromycin cohort was defined by including patients prescribed azithromycin for a minimum of 30 days of continuous supply. Patients were enrolled only at the first instance of roflumilast or chronic azithromycin (i.e., participants on these medications prior to study time were excluded). Patients were excluded if they had a history of confounding conditions or diseases with other indication for macrolides (Supplement Table 1 in online supplement): (1) mycobacteria infections; (2) autoimmune or chronic inflammatory conditions; (3) occupational lung diseases; and (4) bronchiectasis or interstitial lung diseases. Patients were excluded if they initiated roflumilast and azithromycin on the same day, did not have a U.S. mainland-based primary address, or had conflicting birth or death dates.
All possible azithromycin doses and frequency were included. Lastly, the initiation of roflumilast and azithromycin occurred after first COPD diagnosis and after the initiation of concurrent LABA and LAMA therapy.
The initiation of roflumilast and azithromycin was defined as the index event; the date of initiation defined as the index date. Patients were followed until either death or December 31, 2017.
Because many of the patients in our study population were likely to have Medicare coverage and since Medicare and VHA are separate systems that do not bill each other, we conducted a sensitivity analysis that included Medicare claims for the patients who had also had traditional Medicare coverage from the year prior to index date to end of follow-up (i.e., death or December 31, 2017). Because our Medicare dataset only covers up to the end of 2017, we further restricted the patient cohort for this sensitivity analysis to patients who started roflumilast or azithromycin before 2016 to allow for sufficient follow-up time.
Outcomes
Our primary outcomes were time-to-first COPD-related hospitalization, time-to-first all-cause hospitalization, and time-to-all-cause death. COPD-related hospitalizations were defined as hospitalizations with COPD listed as the primary diagnosis.
Baseline patient and treatment characteristics (i.e., demographics, history of tobacco use, body mass index [BMI], comorbidities, medication and treatments received during the year prior to the index event, hospitalization rates during the year prior to the index event, the year and season of treatment initiation, and whether the prescribing physician was a new health care provider to the patient) were included in this study as covariates. A patient was considered to have a given comorbidity if they had at least 1 inpatient record or at least 2 outpatient visits that were more than 6 months, but less than 12 months, apart at any time in the 5 years before the index date (Supplement Table 1 in the online supplement). For history of tobacco use, we relied on International Classification of Diseases-9th and 10th revisions’ diagnoses codes, Current Procedural Terminology codes for smoking counseling, and National Drug Codes for nicotine replacement therapy at any time prior to the index date. The CDW does not have accurate data on active tobacco use.
We further described the duration of treatment for the initial treatment course and treatment cross-over rates as additional insights to the real-world use of roflumilast and azithromycin. Duration of treatment for the initial treatment course was defined as the number of days from the initiation of treatment to either the last day of supply for the last prescription in that course, death, or end of follow-up, whichever occurred first. A patient was considered to have discontinued their initial course of treatment if there was a greater than 30-day gap between their last day of supply and the day of treatment re-initiation.
Statistical Analyses
To evaluate the balance between the 2 treatment groups, descriptive statistics for baseline patient and treatment characteristics are presented alongside their standardized differences.20
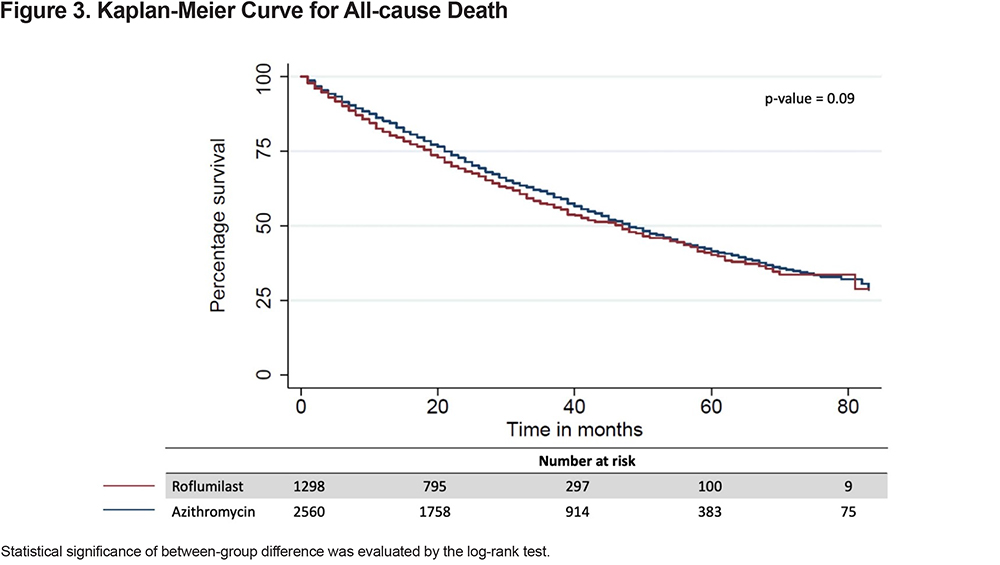
Statistical difference in cumulative incidence of first hospitalization, with death as the competing risk, was analyzed using the Pepe-Mori test,21,22 while median overall survival was estimated by Kaplan-Meier and evaluated by the log-rank test. A 2-sided P value of 0.05 was considered statistically significant for all analyses.
In the adjusted models that controlled for baseline patient and treatment characteristics, cumulative incidence of first hospitalization was analyzed with Fine-Gray models where death was treated as the competing risk. Time-to-all-cause death was analyzed with a Cox proportional hazards model. The proportional hazards assumption of the Cox and Fine-Gray models was checked by testing the statistical significance of the interactions between each covariate and the log of time.23 The final models incorporated any statistically significant time-varying effects of the baseline covariates.24,25
We also performed a sensitivity analysis on the cumulative incidence of first hospitalization for the subgroup of patients who also had traditional Medicare coverage. The number of hospitalizations in the year prior to the index date and the time-to-first hospitalization were updated to include any additional relevant Medicare claims that were not captured in the main VHA dataset.
The dataset was prepared and analyzed using SAS 9.04.01 and Stata/MP 15.1.
Results
Baseline Characteristics
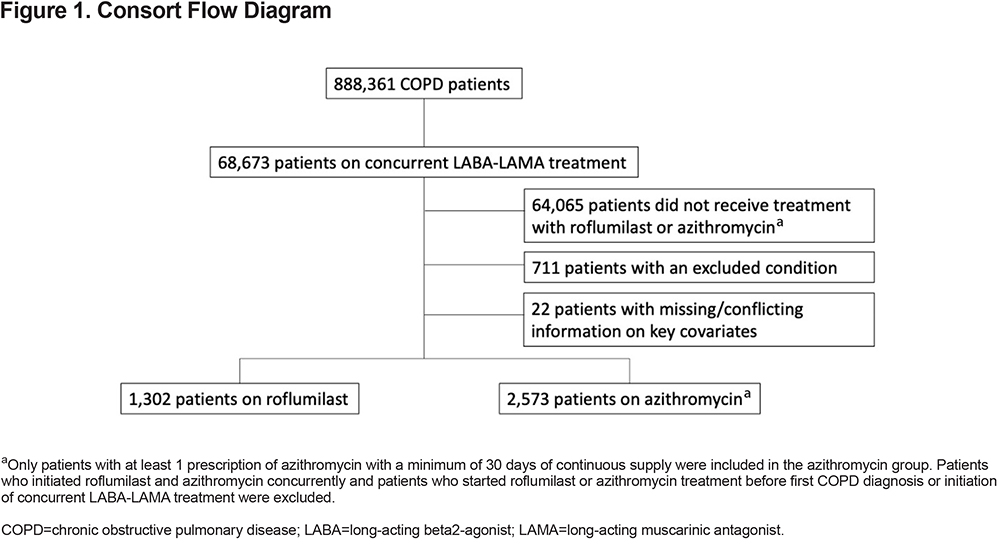
In the final study population, there were 1302 patients treated with roflumilast and 2573 patients treated with chronic azithromycin (Figure 1) There were small but significant differences in race, region, age, and BMI (Table 1). The 2 treatment groups were relatively balanced on baseline comorbidities (Table 1); only chronic liver disease and mood were significantly of higher prevalence in the azithromycin group. There was no significant difference in the baseline COPD-related hospitalization rate, but the azithromycin group did have a statistically significant higher baseline all-cause hospitalization rate. There was also a significant difference in baseline short-acting muscarinic antagonist (SAMA) use with 52% of roflumilast patients versus 57% of azithromycin patients having received at least 1 prescription of SAMA during the prior year (Table 1).

Treatment Characteristics
Baseline treatment characteristics, including the year and season in which treatment was initiated and whether the prescribing physician was a new health care provider to the patient (i.e., have they prescribed anything to the patient before), are presented in Table 1.
The mean treatment durations for the initial treatment course were 273 days (standard deviation [SD] 370) and 243 days (SD 301) for roflumilast and azithromycin, respectively. The treatment crossover rate for the entire study population was 12%, with 11% of patients who were initially treated with roflumilast switching over to azithromycin and 13% of patients who were initially treated with azithromycin switching over to roflumilast.
Hospitalizations
Over a median follow-up of 25 months (interquartile range [IQR] 14–38) and 29 months (IQR 17-48) for the roflumilast and azithromycin groups, respectively, there were 411 (32%) patients in the roflumilast group and 892 (35%) patients in the azithromycin group who had at least 1 COPD-related hospitalization. For these patients, the median times-to-first COPD-related hospitalization were 6 months (IQR 2–14) for the roflumilast group and 8 months (IQR 2–20) for the azithromycin group. The unadjusted cumulative incidence of first COPD-related hospitalization is shown in Figure 2A. In the adjusted model, which controlled for all the baseline variables listed in Table 1, roflumilast had no significant difference compared to azithromycin (subdistribution hazard ratio [SHR], 1.14; 95% confidence intervals (CI), 1.00–1.29) (Table 2)
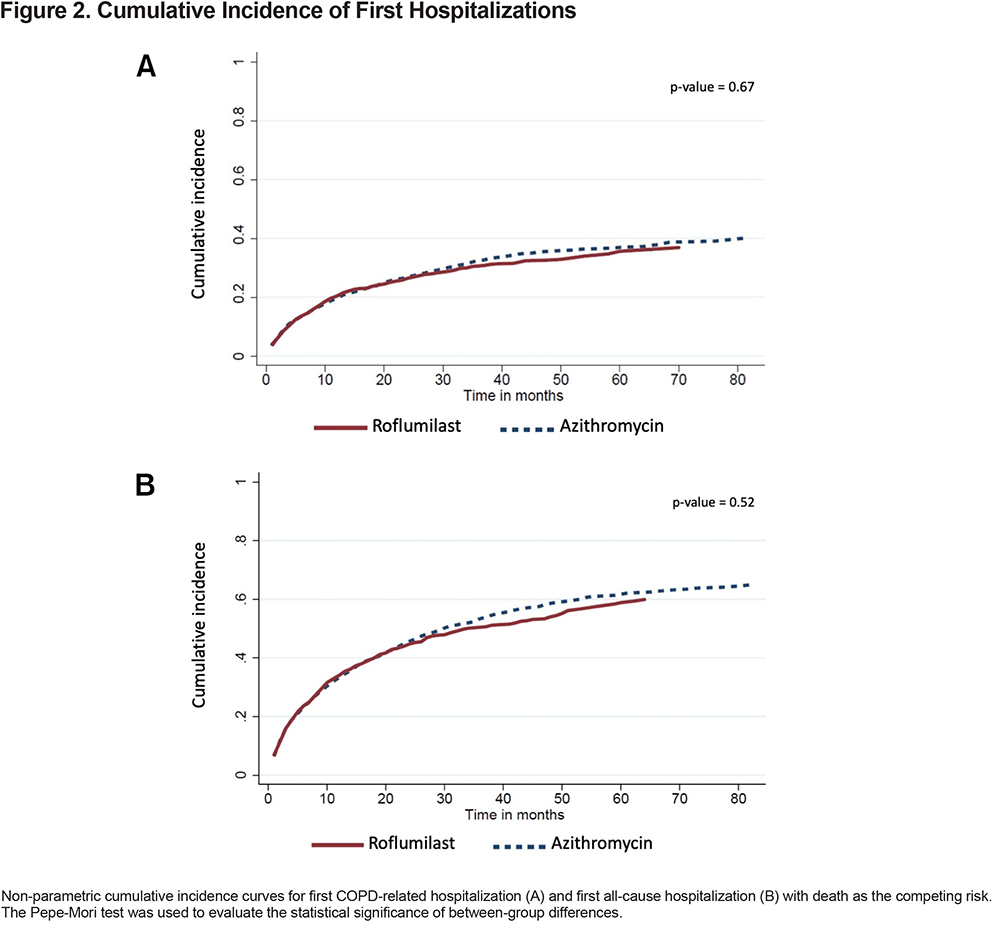
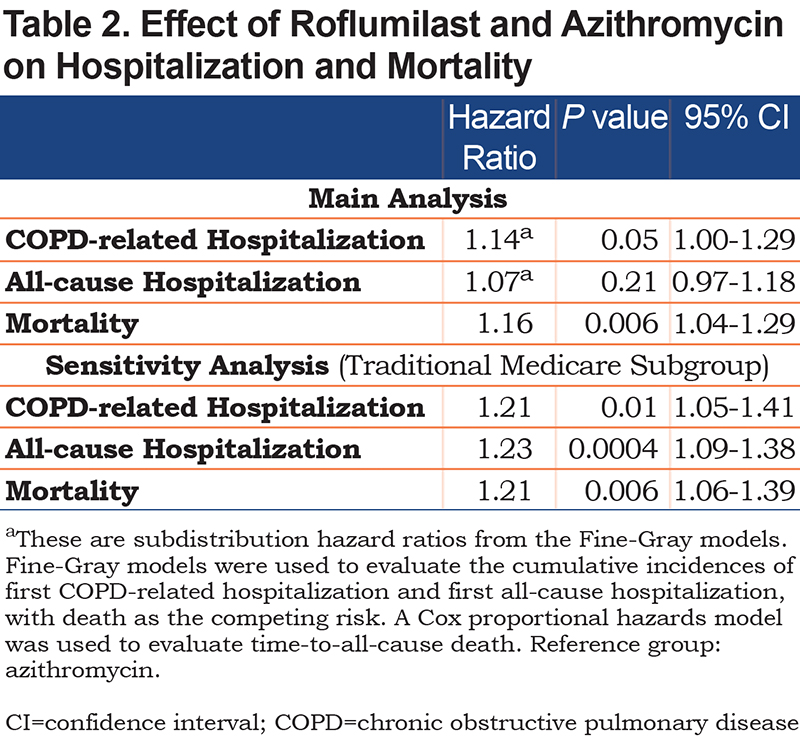
There were 676 (52%) patients in the roflumilast group and 1459 (57%) patients in the azithromycin group who had at least 1 all-cause hospitalization (Figure 2B). For these patients, the median times-to-first all-cause hospitalization were 6 months (IQR 2–15) in the roflumilast group and 7 months (IQR 2–19) in the azithromycin group. In the adjusted model, roflumilast was not significantly different (HR 1.07, 95% CI, 0.97–1.18) (Table 2).
All-Cause Mortality
The all-cause mortality rate in the first year following treatment initiation was 16% in the entire study population and more specifically, 19% for the roflumilast group and 15% for the azithromycin group. The median time-to-all-cause death was 47 months (IQR 19–83) for the entire study population, 47 months (IQR 1681) for the roflumilast group and 48 months (IQR 20–83) for the azithromycin group (Figure 3). Roflumilast had a higher hazard than azithromycin after controlling for baseline characteristics (hazard ratio [HR], 1.16; 95% CI, 1.04–1.29) (Table 2).
Sensitivity Analysis
Of our main study population, 2030 (659 roflumilast; 1371 azithromycin) patients had concurrent, continuous, traditional Medicare coverage from the year prior to the index date to the end of follow-up. The baseline hospitalization rates during the year prior to index date were higher and the median time-to-first hospitalization were shorter when Medicare-reimbursed hospitalizations were included (Table 3).
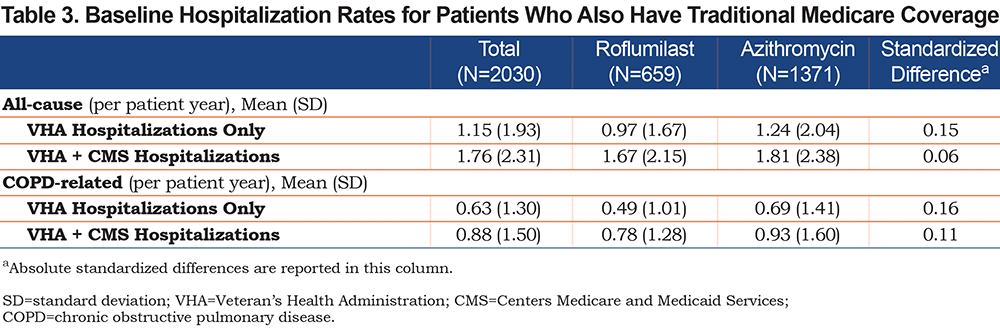
For the 321 patients in the roflumilast group who had at least 1 COPD-related hospitalization, the time-to-event was shortened from a median of 11 months (IQR 4–29) to 6 months (IQR 2–13). For the 673 patients in the azithromycin group who had at least 1 COPD-related hospitalization, the time-to-event dropped from a median of 13 months (IQR 4–31) to 7 months (IQR 2–18). The unadjusted cumulative incidence of first COPD-related hospitalization between the 2 treatment groups is shown in Figure 4A. Roflumilast in the adjusted model had a higher hazard relative to azithromycin (SHR, 1.21; 95% CI, 1.05–1.41) (Table 2).
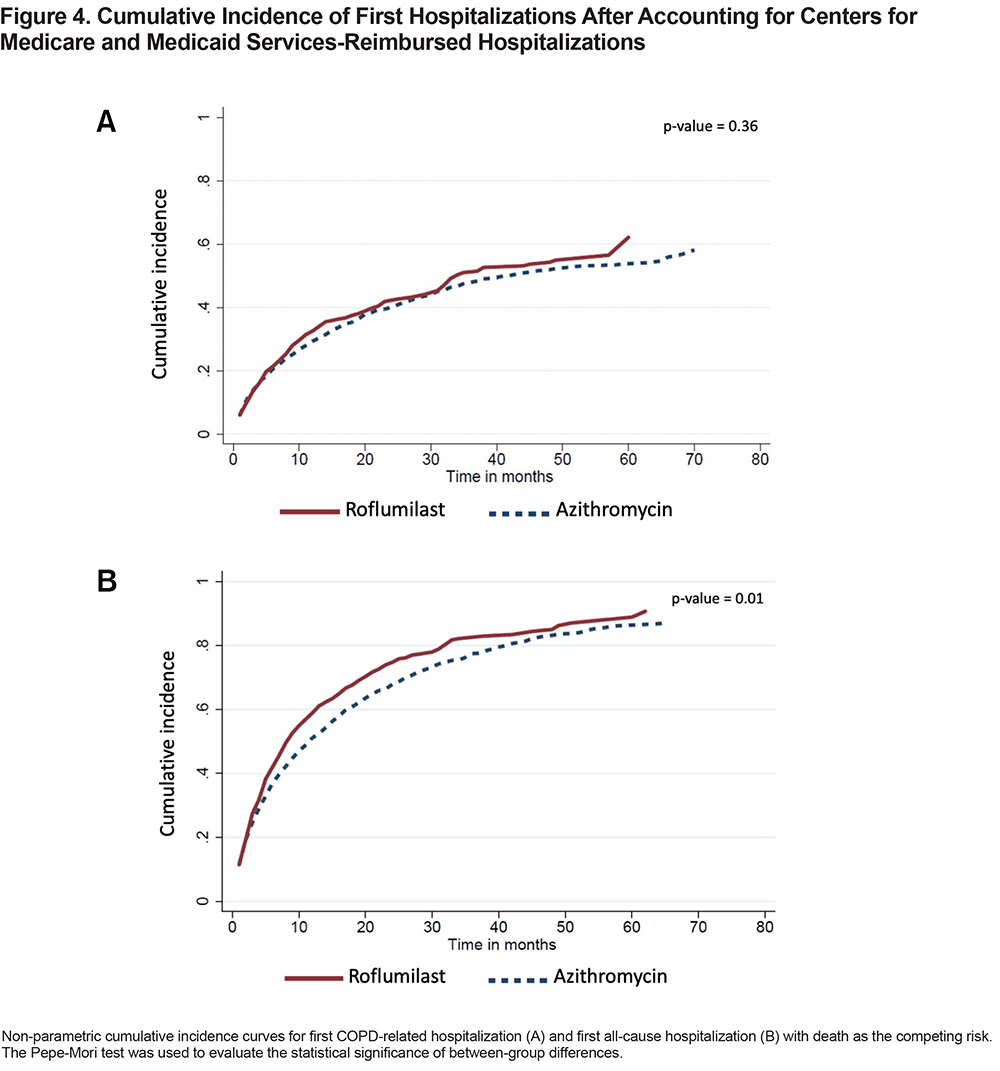
There were 520 patients in the roflumilast group and 1055 patients in the azithromycin group who had at least 1 all-cause hospitalization. The median times-to-first all-cause hospitalization were 5 months (IQR 2–11) and 6 months (IQR 2–15) in the roflumilast and azithromycin groups, respectively. The unadjusted cumulative incidence of first all-cause hospitalization is shown in Figure 4B. Roflumilast again had a higher hazard than azithromycin in the adjusted model (SHR, 1.23; 95% CI, 1.09–1.38) (Table 2).
Discussion
In general, the 2 treatment groups in our study were balanced across most of the baseline characteristics with small differences in race, region, baseline medications, and treatment characteristics. The mean BMI in the roflumilast group was 27.68, and despite the known side effect of weight loss with roflumilast, 5% of the patients were underweight at initiation. In the chronic azithromycin group, the mean BMI was 27.05, with 7% of the patients underweight. The only other comorbidities that were slightly imbalanced between the 2 treatment groups were chronic liver disease and mood disorders, both being higher in prevalence in the azithromycin group. These differences reflect the prescribing information on the FDA label for roflumilast, which recommends against use in patients with moderate-to-severe liver impairment, psychiatric events, including suicidality, and weight loss.26Baseline all-cause hospitalization rates were higher in the azithromycin group.
After adjusting for these differences, our results showed azithromycin was associated with slightly improved survival outcomes than roflumilast. While there were no differences in the hazard ratios for first COPD-related hospitalization and first all-cause hospitalization in the main analysis, our sensitivity analysis showed that the VHA dataset missed hospitalizations that occurred outside of the VHA system. When Centers for Medicare and Medicaid Services (CMS) hospitalization claims were included, the higher hazard ratios for COPD-related hospitalization and all-cause hospitalization with roflumilast treatment became statistically significant.
Randomized clinical trials of roflumilast demonstrated modest improvements to lung function and no clinically meaningful improvement in quality of life over placebo.13 Furthermore, the mean rates of severe exacerbations per patient-year were low for both the roflumilast (0.12) and placebo (0.15) groups and not significantly different (rate ratio [RR], 0.82; 95% CI, 0.63–1.06).26 Mortality in this study was 2%–3% and likewise not different between the 2 groups.27 In a subsequent 2015 study, the inclusion criteria was modified from requiring at least 1 exacerbation27 to requiring at least 2 exacerbations.28 There were also changes as to which concomitant treatments were allowed in the studies. In this 2015 study, the hospitalization rate was 0.238 per patient-year in the roflumilast group, which was statistically significantly lower (RR, 0.761; 95% CI, 0.604–0.960) than the 0.313 in the placebo group. There was however still no difference in mortality.
The results from the azithromycin trial13 were similar to the earlier roflumilast trial (i.e., M2–124 and M2–125) results; the hospitalization rates (mean of 0.34 events per patient-year in the azithromycin group and 0.49 in the placebo group) were not statistically significantly different (HR, 0.82; 95% CI, 0.64–1.07). Mortality rate was likewise low (3% in the azithromycin group and 4% in the placebo group) and not statistically significantly different (p=0.87).
It is difficult to directly compare any of the roflumilast trials13,27,28 to the azithromycin clinical trial by Albert et al14because the inclusion and exclusion criteria differed. For example, the roflumilast trials required at least a 20 pack-year smoking history and a post-bronchodilator forced expiratory volume in 1 second (FEV1) of ≤50%. In contrast, the Albert et al study required a 10 pack-year history and an FEV1 of < 80%. With regards to COPD exacerbation history, the Albert et al study required patients either be on continuous supplemental oxygen or have had 1 acute exacerbation during the previous year.
It is likewise difficult to compare this real-world study with the aforementioned clinical trials. The clinical trials had extensive exclusion criteria. For example, all the clinical trials excluded patients with asthma or patients who had an exacerbation during the 4-week baseline observation period. In contrast, in the interest of generalizability, we neither excluded patients with asthma nor patients who had a recent exacerbation. Given the more extensive exclusion criteria of the clinical trials, as well as the higher mortality rate in this study, it is likely that our patient population was sicker with more comorbidities at baseline than the clinical trial patient populations.
In the earlier roflumilast studies, LABAs, SAMAs, and short-acting beta2 agonists (SABAs) were allowed in the study, but LAMAs and inhaled corticosteroids (ICSs) were not. In contrast, the 2015 roflumilast study specifically selected for patients on LABA-ICS combination therapy, with or without LAMAs, while excluding chronic systemic corticosteroids, SAMAs and SABAs. The azithromycin study did not have any inclusion and exclusion criteria on medication use, but only 5% and 4% of the patients in the azithromycin and placebo groups, respectively, received LABA-LAMA combination therapy at baseline.
Limitations
There are limitations in our study due to unique aspects of the VHA population and database that may influence generalizability and cause confounding by indication. First, the dataset is predominantly comprised of White men with military service. Secondly, the VA is different from private health care health systems. As these patients are largely capitated, we have robust data on prescribed and filled medications, but lack important information such as spirometry, tobacco use, and presence of chronic bronchitis. In the VA system, roflumilast has a special designation requiring authorization for use, which could bias clinicians towards using chronic azithromycin which requires no such request.
As is true for all observational, non-randomized studies, there are likely unmeasured variables (i.e., variables not included in our regressions) that could potentially bias our results. Spirometry data, clinical phenotype (e.g., chronic bronchitis), active tobacco use, and LAMA/LABA/ICS adherence and appropriate delivery were not available for our analysis. Reliable smoking (both past and active) data is especially important in this population, as it is a significant contributor to exacerbation risk and potentially the response to azithromycin.11 Due to lack of spirometry and exposure data, the diagnosis of COPD was presumed based on discharge diagnosis, which is imprecise. Given the high burden of disease and typical involvement of a pulmonologist when prescribing these medications, inappropriate diagnosis of COPD is likely less prevalent than observed in the general population. These limitations of the dataset introduce the threat of confounding by indication.
Lastly, highlighted in our sensitivity analyses, the VHA administrative data only captures encounters at VA facilities or VHA-reimbursed fee basis encounters, and so for patients who have other sources of health care coverage, their HCRU may not be fully captured. Given that many of our patients are over 65 and would likely have Medicare, we performed a sensitivity analysis to capture any additional Medicare-reimbursed hospitalizations. In the main analysis, roflumilast had higher hazards than azithromycin for COPD-related hospitalizations and for all-cause hospitalizations, but these results were not statistically significant. The hazard ratios, again in favor of azithromycin, were only significant when Medicare-reimbursed hospitalizations were included in the analysis for the patients who have traditional Medicare coverage.
Conclusion
Our study of the nationwide VHA database describes the cohorts of COPD patients treated with chronic azithromycin and roflumilast. With the inherent limitations of retrospective observation studies with largely administrative data, azithromycin was associated with better survival, COPD-related hospitalizations, and all-cause hospitalizations. Further comparative effectiveness research, including clinical trials, is required to further investigate this association.
Acknowledgments
This study is the result of work supported with resources at the VA Long Beach Healthcare System.
Author contributions: Dr. Kuhn takes responsibility for the content of this manuscript including the data, analysis, and the manuscript text. JL and IT were responsible for data acquisition. JL, IT, NK, and BK participated in analysis and interpretation. JL, IT, NK, and BK participated in writing the manuscript.
Declaration of Interests
The authors report no financial conflicts of interest.