Running Head: Responsiveness of the E-RS:COPD in Clinical Trials
Funding Source: This research was sponsored by Novartis. The funder of this study collaborated in the inception, design, data collection, data analysis, interpretation, and writing of the manuscript.
Date of acceptance: September 22, 2021 │ Published online: October 4, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; Evaluating Respiratory Symptoms™ in Chronic Obstructive Pulmonary Disease, E-RS™:COPD; randomized controlled trials, RCTs; St George’s Respiratory Questionnaire, SGRQ; forced expiratory volume in 1 second, FEV1; patient-reported outcome, PRO; Food and Drug Administration, FDA; EXAcerbations of COPD Tool, EXACT; European Medicines Agency, EMA; population, intervention, comparator, and setting, PICOS; standard deviation, SD; long-acting muscarinic antagonists, LAMAs; long-acting beta2-agonists, LABAs; phosphodiesterase-4, PDE4; inhaled corticosteroid, ICS; CXC chemokine receptor 2, CXCR2; Transition Dyspnea Index, TDI; least squares, LS; minimal clinically important difference, MCID
Citation: Bushnell DM, Wilson R, Gutzwiller FS, et al. Use of the Evaluating Respiratory Symptoms™ in COPD as an outcome measure in clinical trials: a rapid systematic review. Chronic Obstr Pulm Dis. 2021; 8(4): 551-571. doi: http://doi.org/10.15326/jcopdf.2021.0235
Online Supplemental Material: Read Online Supplemental Material (446KB)
Introduction
Although respiratory symptoms during stable (non-exacerbating) states are a burden to patients with chronic obstructive pulmonary disease (COPD) and a primary reason for clinic visits,1 relatively little is known about how this outcome is affected by treatments. Precise measurement of respiratory symptoms is important for testing bronchodilators, and even more so for testing new treatments that provide symptomatic relief with benefits more directly associated with symptomatic relief than airflow limitation. The Evaluating Respiratory Symptoms™ in Chronic Obstructive Pulmonary Disease (E-RS™:COPD) is a patient-reported outcome (PRO) measure developed to quantify the severity of respiratory symptoms and test the effects of treatment in clinical trials of stable COPD.
Development of the E-RS:COPD was consistent with standards in the field and United States Food and Drug Administration (FDA) PRO guidance.2 The total score represents overall respiratory symptom severity, with 3 sub-scales capturing breathlessness (5 items), cough and sputum (3 items), and chest symptoms (3 items). The 11 items comprising this instrument are part of an existing measure, the 14-item EXAcerbations of COPD Tool (EXACT).3 Content validity of the E-RS:COPD was addressed through primary and secondary analyses of qualitative data.4 Quantitative, secondary analyses of observational and clinical trial data showed the E-RS:COPD to be reliable with total and subscale scores possessing high levels of internal consistency and reproducibility.4,5 Validity was supported by consistent relationships between the E-RS:COPD and measures of health status (St George’s Respiratory Questionnaire [SGRQ]), pulmonary function (forced expiratory volume in 1 second [FEV1] % predicted), and symptom questionnaires (SGRQ Symptoms, modified Medical Research Council dyspnea status) and known-groups analyses, including smoking status and rescue medication use.4,5 Tests of E-RS:COPD responsiveness were conducted in data from 3 Phase 2 clinical trials.5 Because these trials did not show significant treatment effects in the primary or secondary endpoints, data were stratified into sub-groups experiencing improvement/no improvement from baseline to week 12 using published responder definitions for 4 criterion variables, including the SGRQ (>4 point change) and 6-minute walk test (>26 meters). Results showed E-RS:COPD scores were sensitive to change. Criterion- and distribution-based methods were used to estimate responder thresholds for interpretation (total score≥2-unit decrease (improvement); subscales: breathlessness score ≥ 1-unit; cough and sputum and chest symptom≥0.7-unit).5
Detailed reports on the psychometric properties of the E-RS:COPD were provided to regulatory health authorities during a multi-year, multi-review process culminating in the qualification of the instrument by the European Medicines Agency (EMA)6 in 2015 and the FDA7 in 2016 as an exploratory endpoint in drug development trials. However, little is known about how the E-RS:COPD has been used as an endpoint in clinical trials and how the E-RS:COPD has performed since its qualification. Together, this information is of interest to health authorities and researchers because it will help inform its optimal use in future clinical trials. This review study aimed to identify published pharmaceutical clinical trials that have used the E-RS:COPD measure as an endpoint and summarize these trials, including trial design, endpoint position, and treatment effects, alone and relative to other endpoints.
Methods
This rapid systematic review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and employed several methods that adhere to the scientific rigor, transparency, reproducibility principles of a systematic review, including screening and extracting conducted by 2 independent reviewers, and risk of bias assessment conducted by 2 independent reviewers.8-12 To accelerate the review process, constraints were applied to year of publication, publication type, study design, language, and number of data sources, as well as producing a structured narrative synthesis.
Search Strategy and Selection Criteria
Detailed search criteria are summarized in Table E1 (in the online data supplement). MEDLINE, Embase, and the Cochrane Central Register of Clinical Trials were searched via Ovid for full-text publications published between January 1, 2010 and October 31, 2020 (Table E2 in the online data supplement). The 2010 start date was selected based on the initial availability of the measure to sponsors for exploratory use in their trials before publication of the E-RS:COPD, qualification, and widespread availability. To identify ongoing trials, Embase was searched for conference abstracts published between January 1, 2019 and October 31, 2020 (Table E3 in the online data supplement).
Screening of records, assessment for risk of bias, data extraction, and quality control was conducted by 2 independent reviewers using Covidence software (Veritas Health Innovation, Melbourne, Australia),13 recommended by the Cochrane Effective Practice and Organization of Care.14 After duplicates were removed, titles and abstracts were screened by 2 trained independent reviewers, using the pre-defined screening tool (see Table E4 in the online data supplement), and classified as include, exclude, or unsure. Next, full texts of records were assessed for study eligibility using the study’s pre-defined eligibility criteria (Table E5 in the online data supplement) by the same independent reviewers. Any discrepancies were resolved by consensus, with disputes resolved by a third investigator (first author: DMB).
Data Extraction
Two independent reviewers extracted key data elements within the population, intervention, comparator, and setting (PICOS) framework from all published full-text publications and assessed each publication's risk of bias using the criteria outlined by the Cochrane Risk of Bias tool15,16 and guidance from the Cochrane Effective Practice and Organization of Care group.17 Data extraction and quality assessment were conducted using the Covidence software. The PICOS framework and the objectives of this review were used to organize the data extraction tool. A full list of the detailed data extracted is provided in Table E6 in the online data supplement. Discrepancies were resolved by a consensus discussion, with disputes resolved by a third investigator (first author: DMB).
Synthesis
Descriptive statistics were used to summarize the key data elements extracted from all publications included in the review. To avoid trial duplication, publications that reported data from unique clinical trials were included in the narrative synthesis. Publications were classified by E-RS:COPD endpoint positioning (i.e., primary, secondary, exploratory), by the reported primary outcome measure, and by the main treatment intervention drug class. Within this classification framework, treatment effects for the E-RS:COPD and other relevant outcome measures were examined, with a focus on publications that included the E:RS:COPD as a primary or secondary endpoint. Correspondence between treatment effects observed with the E-RS:COPD and other outcomes were summarized. Finally, publications that reported a responder analysis were identified and summarized.
Results
Publication Selection
The literature search identified a total of 225 records (Figure 1). After the removal of duplicates (n=6), 219 titles and abstracts were screened for relevance, with 61 full-text publications screened for study eligibility. Figure 1 details the identification, screening process, and eligibility evaluation, as well as reasons for exclusion at each stage. Of the records screened for eligibility, 34 met the inclusion criteria (28-full text publications; 6 conference abstracts). Conference abstracts were eligible in this rapid review but only for the purpose of identifying recent or ongoing trials including the E-RS:COPD. Therefore, 28 full-text publications were included in the review.
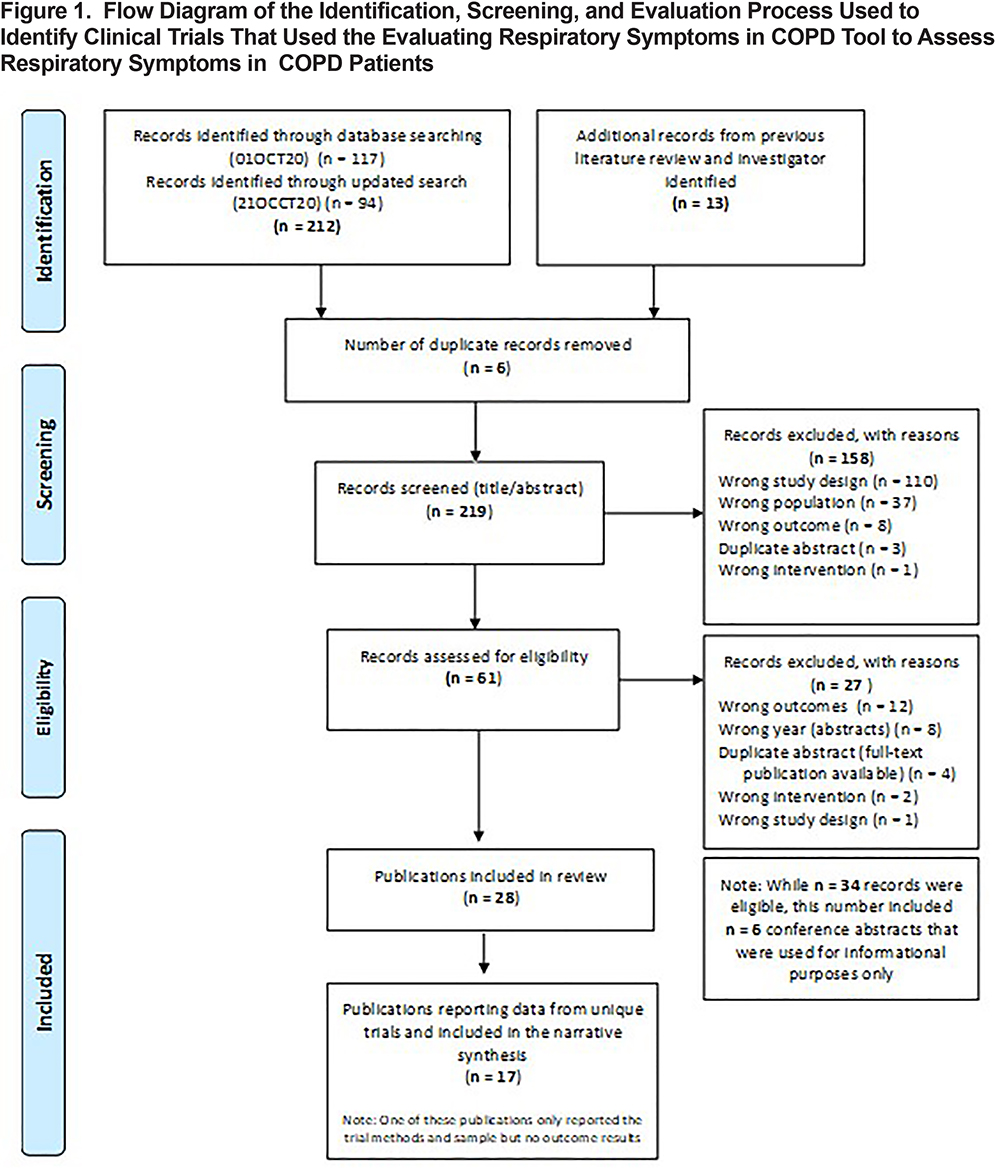
Overview of Included Publications
All 28 publications reported results from double-blind randomized controlled trials (RCTs):
- Seventeen publications reported data from 20 unique trials (3 publications included data from multiple trials).
- Five publications reported different findings from trials previously identified as unique.
- Six publications reported findings from pooled data that included 2 or more of the unique trials.
Overall, 12 publications (43%) reported main trial findings, 6 publications (21%) reported additional pre-specified trial findings, and 10 publications (36%) reported post-hoc or pooled analysis of trial data. Of the 17 publications reporting unique trial data, 1 was a design paper18 with data limited to sample characteristics only. Because PICOS data elements were available, this paper is included in the narrative synthesis, with the sample size dropping to 16 when outcomes data are presented. Additional results for the 28 full-text publications are available in the online data supplement (Table E7 and Figure E1).
Risk of Bias:
Of the 28 full-text publications included in this review, 4 publications were rated as having a low risk of bias across all 7 domains on the Cochrane Risk of Bias tool (14%). In the remaining 24 publications (86%), the majority of domains were rated as low risk: random sequence generation (86%), blinding of participants (89%), incomplete outcome (96%), selective reporting (89%), and other sources of bias (89%). The allocation of concealment and blinding of assessment outcomes domains were mostly rated as unsure risk of bias (75%; 57%, respectively).
Publications Included in the Narrative Synthesis (n=17)
Overall Characteristics:
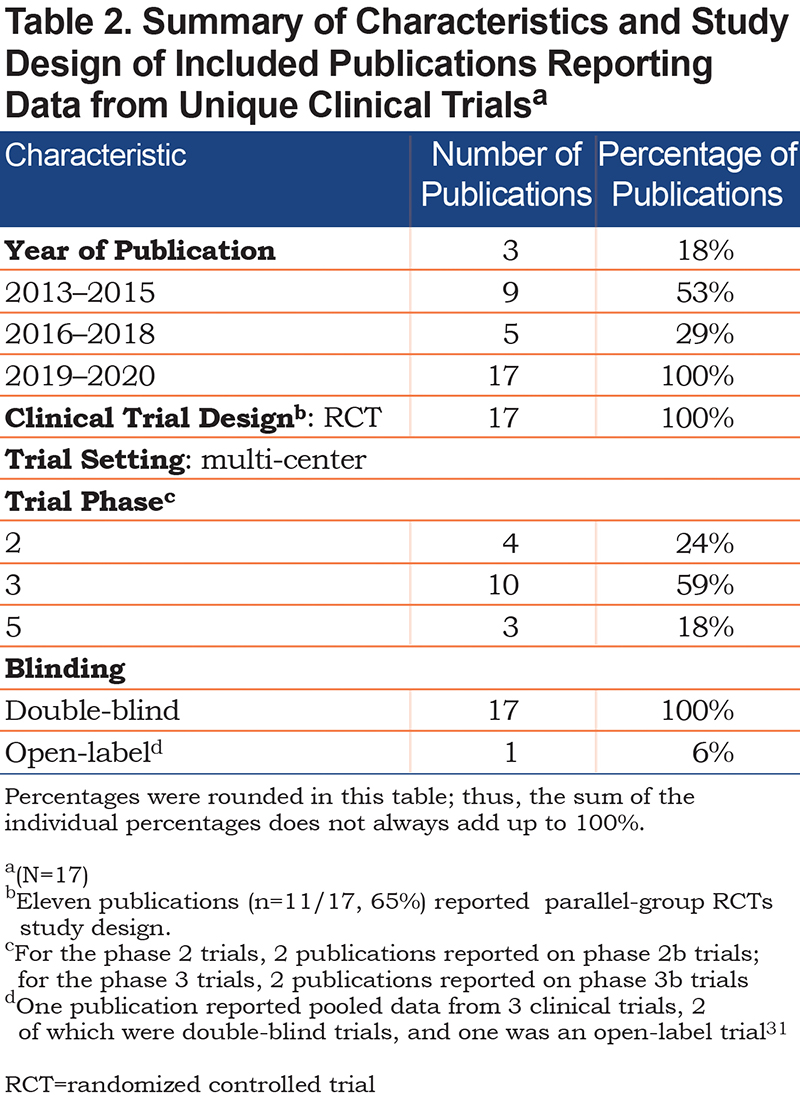
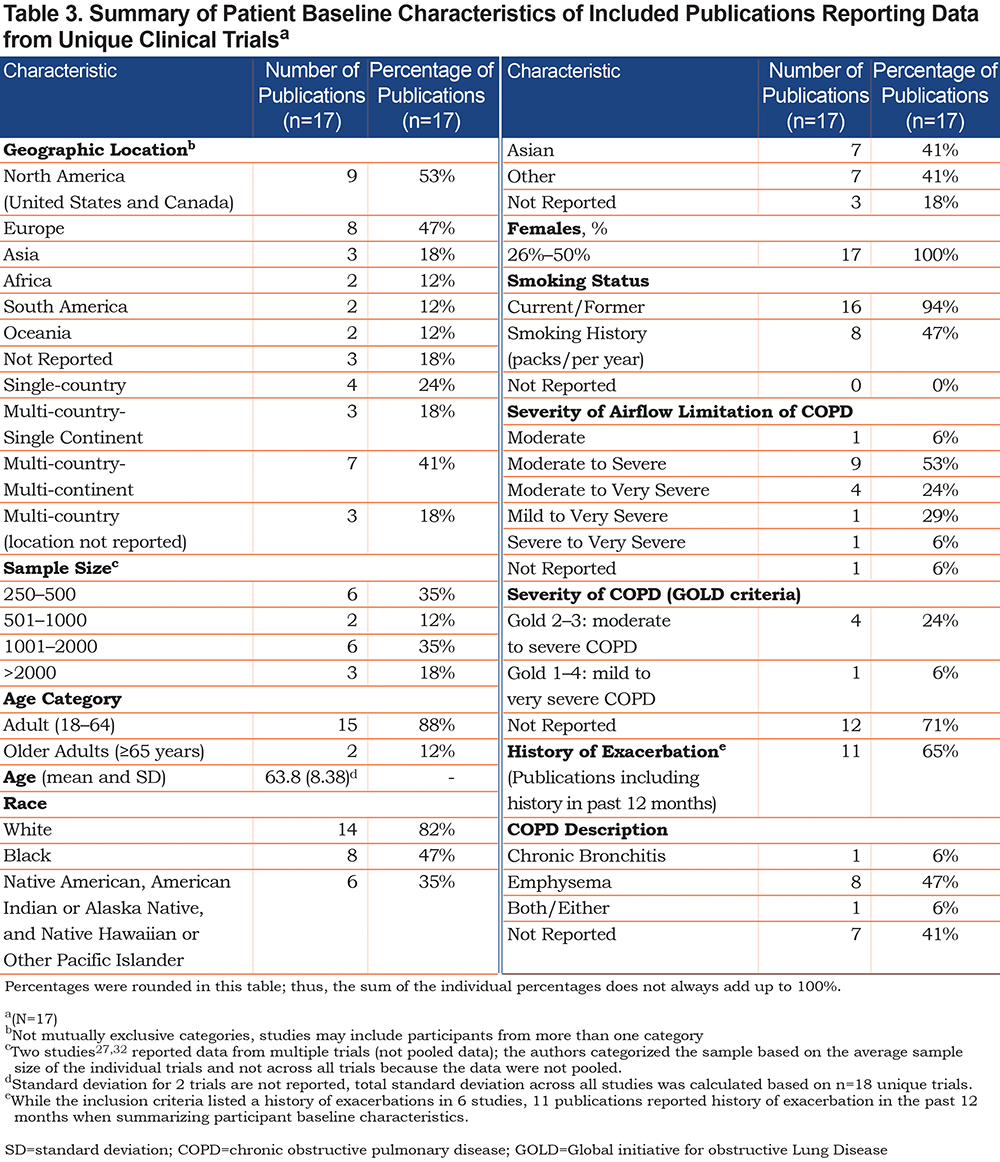
To avoid trial duplication, publications reporting data from unique trials18-34 were included in the narrative synthesis (n=17). Trial characteristics are summarized in Tables 1-5. Most were multi-center international (n=10, 59%), phase 3 (n=10, 59%; Table 2) trials. Sample sizes ranged from 269 to 2431, with half (53%) including over 1000 participants. Study participants averaged 63.8 years of age (average range: 57 to 66 years) and were current or former smokers (Table 3) with moderate-to-severe (53%) or moderate-to-very-severe (24%) airflow limitation.
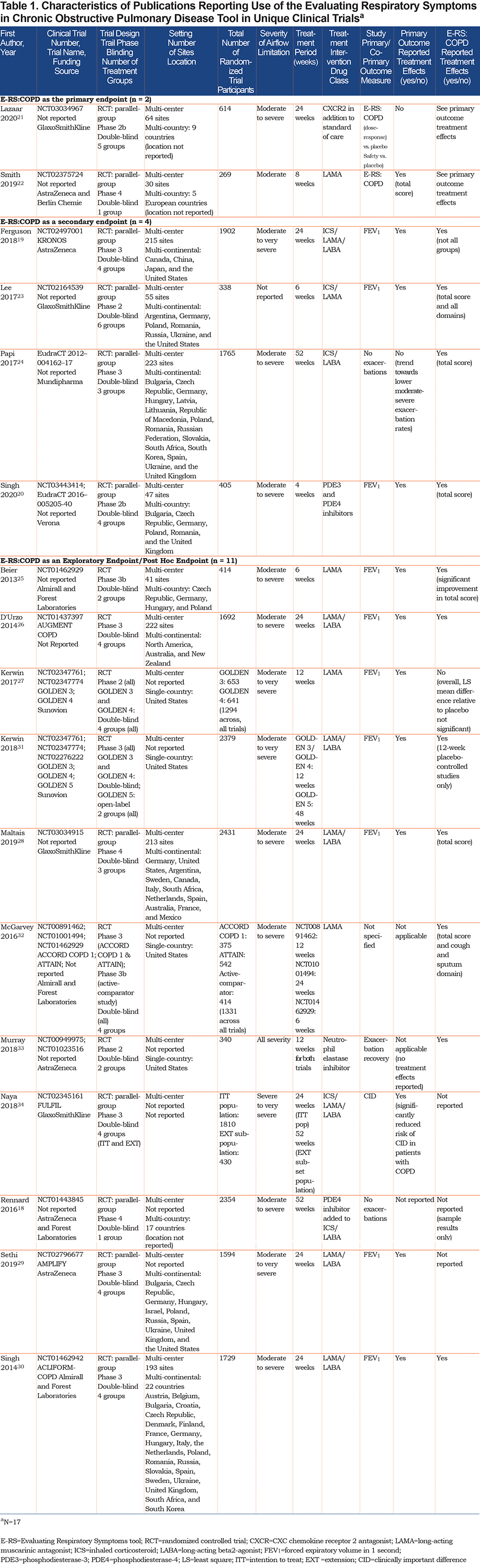
Treatment interventions were categorized as bronchodilator therapy (i.e., long-acting muscarinic antagonists [LAMAs]; long-acting beta2-agonists [LABAs]; phosphodiesterase-4 [PDE4] inhibitors), with or without inhaled corticosteroids (ICSs), and non-bronchodilator therapy (neutrophil elastase inhibitor; CXC chemokine receptor 2 [CXCR2] antagonist). Most publications included a bronchodilator therapy without ICSs (n=9/17, 53%) as the main treatment of interest, followed by LAMAs/LABAs (n=5, 29%; Table 4). Aclidinium bromide alone (n=3), or in combination with formoterol (n=2)/formoterol fumarate (n=1), was the most frequently investigated drug therapy, while only 2 non-bronchodilator drug therapies were investigated (AZD9668; Danirixin).
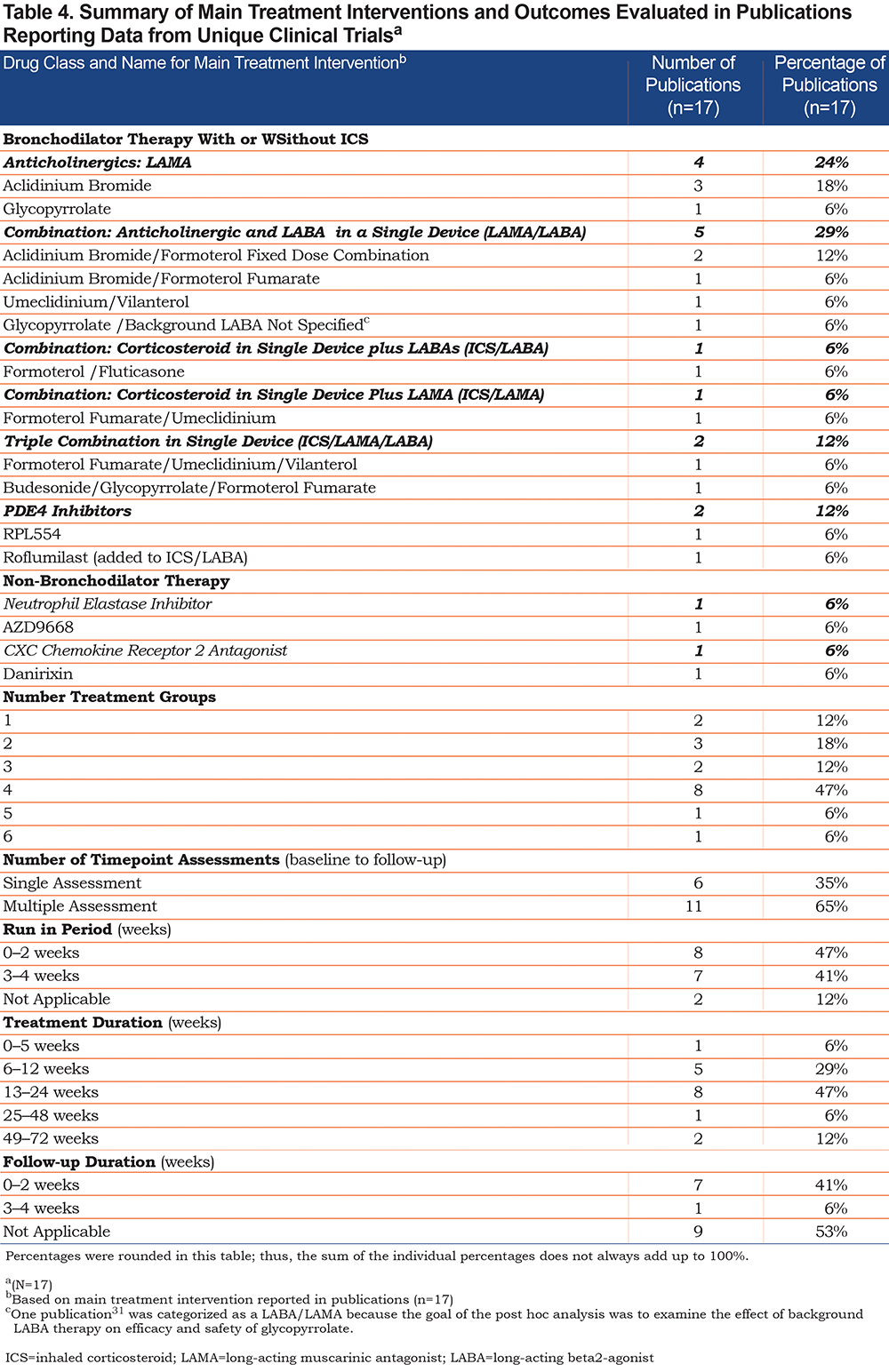
In addition to the E-RS:COPD, 11 different PRO measures were identified, with the majority of publications including 2 or more PRO measures (n=14, 82%; Table 5). Many publications included other PROs as a secondary endpoint (n=10, 59%).
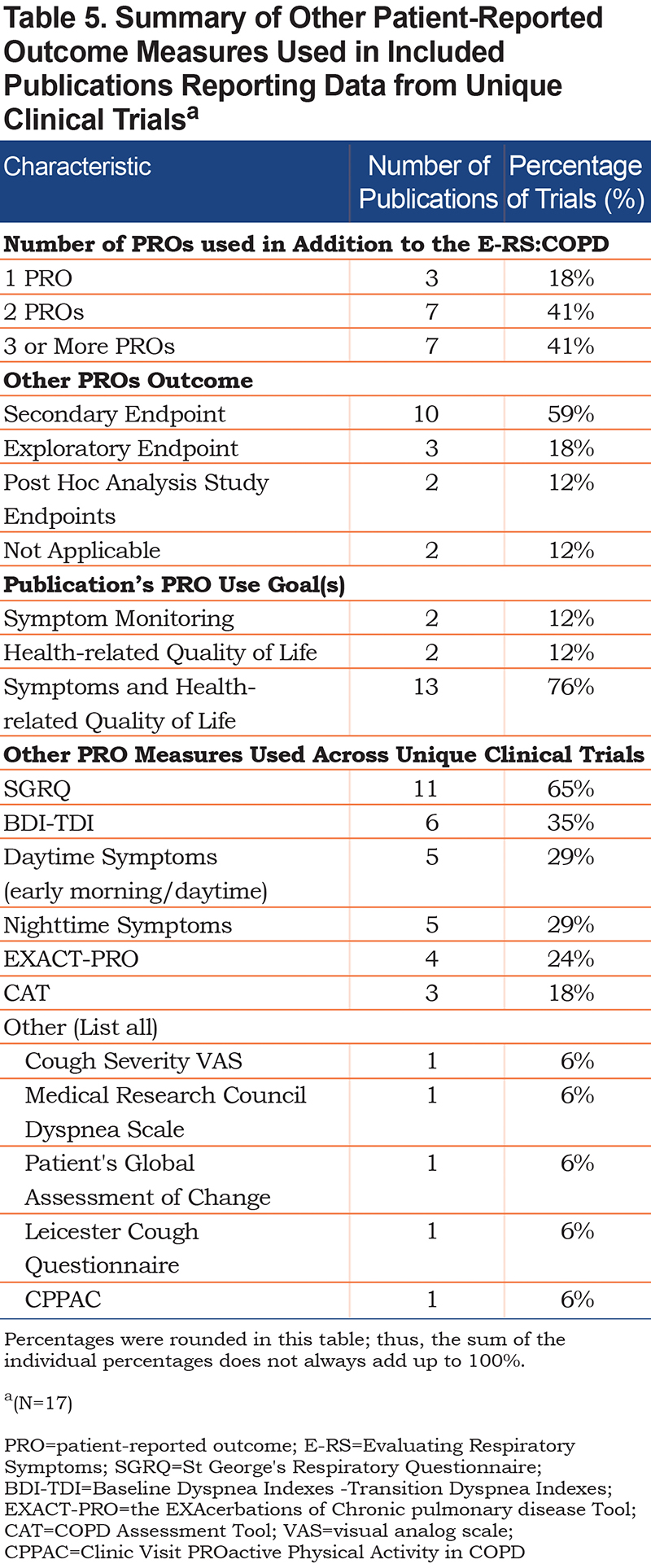
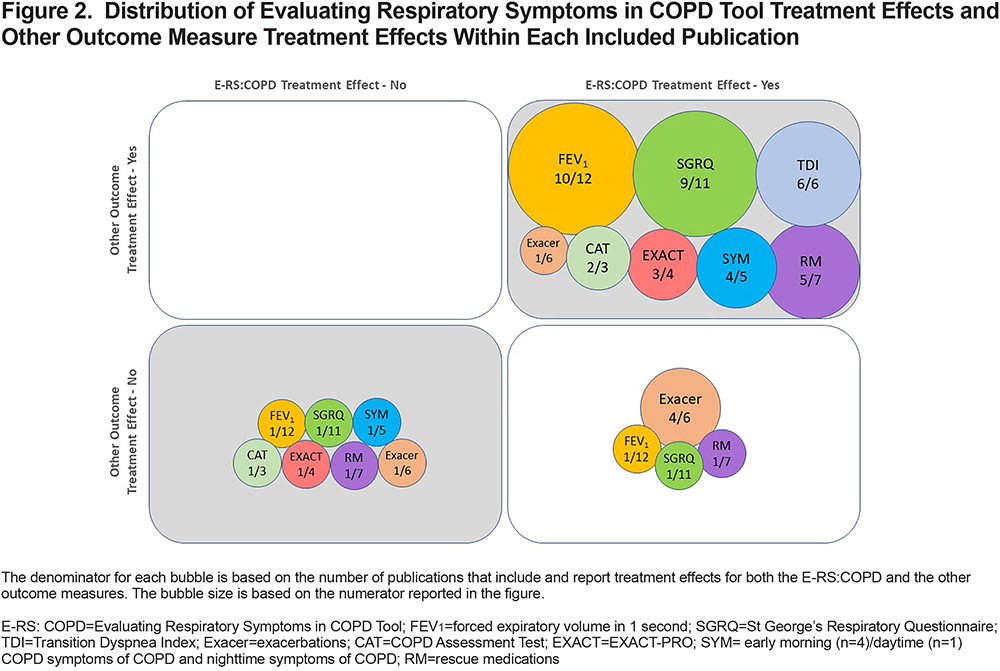
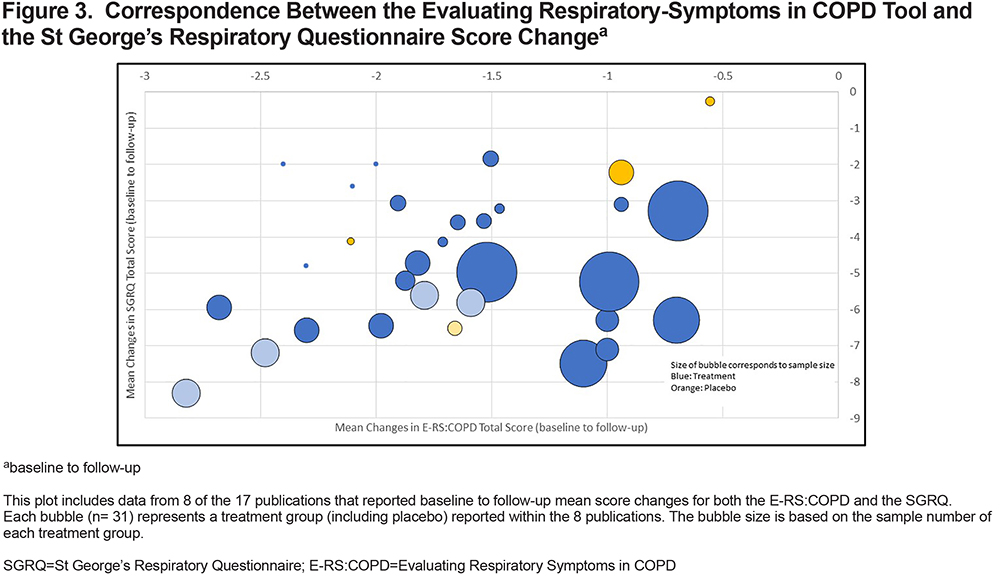
Figure 2 visualizes the correspondence between the E-RS:COPD treatment effects (significant/non-significant) and other trial outcomes (PROs, FEV1, number of exacerbations, rescue medications). Overall, statistically significant E-RS:COPD treatment effects corresponded with significant treatment effects in 8 other outcomes, including FEV1 (59%), SGRQ (53%), and Transition Dyspnea Index (TDI) (35%; see upper right quadrant of Figure 2). Similarly, in instances when there were no E-RS:COPD treatment effects (non-significant), there were no treatment effects with other outcome measures. There were no divergent cases, i.e., non-significant E-RS:COPD effects with significant effects observed in other outcome measures (see upper left quadrant). There was also a clear pattern of correspondence between E-RS:COPD total and SGRQ total mean score changes from baseline to follow-up (treatment periods varied) (Figure 3).
E-RS:COPD as Primary or Secondary Endpoint in Unique Trials (n=6)
Characteristics:
Six publications described trials that positioned the E-RS:COPD as a primary (n=2) or a secondary (n=4) endpoint (Table 6).19-24 These trials involved samples of 269 (1 treatment group) to 1902 (4 treatment groups) patients with moderate-to-severe COPD (Table 1), testing a bronchodilator therapy (n=5) with1 trial testing a non-bronchodilator therapy (CXCR2).21 Treatment duration ranged from 4 to 52 weeks. FEV1 served as the primary endpoint in 3 and exacerbation frequency in 1. For the 2 trials using the E-RS:COPD as a primary endpoint, 1 reported change from baseline in total score over 8 weeks22 while the second (co-primary with safety) reported change from baseline in total and subscale scores at 6 months.21
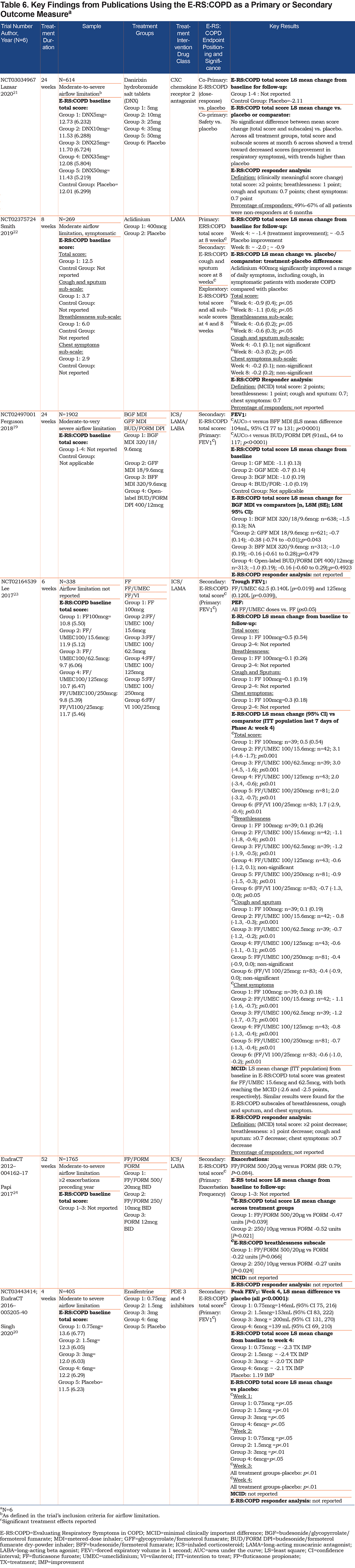
Treatment Effects:
Four publications reported mean baseline E-RS:COPD total scores, with all participants entering the studies at a similar symptom severity level (mean=11.68, SD=0.50; range: 9.7 [6.06]–13.6 [6.77]; Table 6). Four publications reported E-RS:COPD total score least square (LS) mean change from baseline to follow-up,19,20,22,23 while 1 publication reported subscale LS mean change from baseline to follow-up.23 Two of these publications reported a 2-point or greater total score improvement (i.e., decrease in scores) across treatment groups (range: ~2.0 points to ~2.4 points as estimated from figure data).20,22 Ferguson and colleagues 19 reported LS mean total score change for each treatment group, with the mean change scores ranging from 0.7 to 1.1 points.
Four of the 6 publications reported a significant primary endpoint treatment effect (FEV1 [n=3]; E-RS:COPD [n=1]), while 2 publications21,24 reported a trend towards a primary endpoint improvement without statistical significance (decrease in respiratory symptom scores [E-RS:COPD] lower exacerbation rate) (Table 6). The publication that included the E-RS:COPD as the primary endpoint21 and investigated a non-bronchodilator therapy, reported a trend toward improvement in respiratory symptoms, but no significant difference between E-RS:COPD LS mean total score change (or subscales) versus placebo.
Three of the 4 publications with the E-RS:COPD as a secondary endpoint presented trials testing bronchodilator therapies,20,23,24 and each of these reported statistically significant E-RS:COPD total score treatment effects (i.e., improvement, or decrease in scores) versus placebo (n=2) or treatment comparator (n=2; Table 6). Ferguson and colleagues19 reported statistically significant E-RS:COPD total score change for 1 treatment group versus comparator (p=0.043) with no significant treatment effects for the other treatment groups (p=0.479; p=0.492).
In terms of the E-RS:COPD subscales, 3 publications22-24 (n=3) reported statistically significant breathlessness subscale treatment effects, 2 publications reported statistically significant cough and sputum treatment effects,22,23 and 1 publication23 reported statistically significant chest symptom treatment effects (Table 6).
Correspondence Between Trial Primary Endpoint Treatment Effects and E-RS:COPD Treatment Effects:
As noted, the 4 articles presenting the E-RS:COPD as a secondary endpoint (i.e., change from baseline in respiratory symptoms) reported statistically significant E-RS:COPD treatment effects. In each case, significant improvements were observed in the primary outcome measure, specifically, lung function19,20,23 and exacerbation frequency.24 Ferguson and colleagues19 reported a primary endpoint treatment effect of improved lung function with a corresponding respiratory symptom improvement for 1 treatment group (not all). Lazaar and colleagues21 found corresponding non-significant treatment effects among the co-primary endpoints: change from baseline in dose-response on respiratory symptom severity (E-RS:COPD) and safety (adverse events, 12-lead electrocardiogram, clinical laboratory, and hematological evaluations).
Responder Analysis:
Three of the 6 publications that included the E-RS:COPD as a primary or secondary endpoint referenced the interpretation guidelines (proposed responder definition or clinically meaningful score change threshold)21-23 (Table 6) for symptomatic improvement proposed by Leidy and colleagues5:
- E-RS:COPD total score ≥ 2.0-point reduction (scale range: 0–40)
- E-RS:COPD breathlessness subscale score ≥ 1.0-point reduction (scale range: 0–17)
- E-RS:COPD cough and sputum subscale score ≥ 0.70-point reduction (scale range 0–11)
- E-RS:COPD chest symptoms subscale score ≥ 0.70-point (scale range: 0–12)
Lee and colleagues23 reported that the mean total score changes of 2 treatment groups exceeded 2 points (-2.6 points; -2.5 points) and reported results for exceeding thresholds for the subscales of breathlessness (≥1.0 point), cough and sputum (≥0.70 points), and chest symptoms (≥0.70 points). Singh and colleagues20 also reported group differences versus placebo for all 4 ensifentrine doses at week 4 that were near or greater than the E-RS:COPD total score 2-point change.
Only 1 of 3 publications referencing interpretation reported the percentage of E-RS:COPD responders, indicating that 49% (treatment) to 67% (placebo) were non-responders.21
E-RS:COPD as an Exploratory Endpoint in Unique Trials (n=11)
The E-RS:COPD tool was included as an exploratory or post hoc endpoint in 11 publications reporting unique trial data (Table 1).18-34 Given that the main focus of this synthesis was on publications that included the E-RS:COPD as a primary or secondary endpoint, we limit the reporting of the exploratory results to the responsiveness of the E-RS:COPD.
E-RS:COPD total score LS mean change from baseline to follow-up was reported in 9 publications, with LS mean score changes ranging from -0.69 points to -3.14 among investigational bronchodilator therapies. Of these publications, 4 reported total score LS mean change from baseline to follow-up among treatment groups that were ≥ a 2 point decrease (improved respiratory symptoms).20,26,32,34 One study28 reported breathlessness LS mean score changes ranging from -0.22 to -0.67 across groups, cough and sputum LS mean score change of -0.32 to -0.45 across groups, and chest symptoms LS mean score changes of -0.15 to -0.39 across groups.
Of the 11 publications, 8 (7 bronchodilator and 1 non-bronchodilator) reported E-RS:COPD total score treatment effects, all statistically significant (Table 1) and demonstrating correspondence with the primary endpoint treatment effects. One publication reported a decline in total score (improved symptoms) from baseline to follow-up versus placebo that was less than 2 points and did not reach statistical significance.31 Only 1 publication reported treatment effects on E-RS:COPD subscale scores for both aclidinium and tiotropium.25
Three publications28,32,33 referenced the guidelines for symptomatic improvement5 proposed by Leidy et al5,28,32,33 in 2014. One reported the number of responders, with the percentage of E-RS:COPD total score responders 36% among the treatment group versus 27% among each of the active comparators.28
Discussion
To our knowledge, this review study is the first to systematically examine and summarize the existing publications that reported on the use of the E-RS:COPD as a symptom outcome measure in pharmaceutical trials since its qualification. While the E-RS:COPD has been qualified by the FDA and EMA as an exploratory endpoint in drug development trials, several sponsors have elected to use it as a primary or secondary endpoint. Overall, the literature confirms that the E-RS:COPD is responsive to change, as shown by its ability to detect symptomatic improvements over time, and between treatment groups.
Most publications reported on trials investigating bronchodilator therapies with ICSs (e.g., LAMAs and/or LABAs with ICS). This finding was expected, as the combination of widening the airways, via a bronchodilator, and the anti-inflammatory actions of an ICS are more likely to provide symptomatic relief than a single bronchodilator therapy. One publication that included the E-RS:COPD as a primary endpoint reported results from a non-bronchodilator drug therapy, danirixin, that was administered in addition to standard of care inhaled medications.21 While this study (as well as a previous phase 2 study examining danirixin that was not included in this review because it was published as a letter to the editor35), highlighted a positive trend in respiratory symptom improvements, no significant treatment effects have been reported as a result of this drug therapy. Further, Lazaar and colleagues21 reported a large unexpected placebo effect. The authors attributed this finding to an observed study effect during the 7-day run-in period before treatment, which may have contributed to the lack of treatment effects observed in this clinical trial. Thus, future trials may benefit from a prolonged run-in period to mitigate the potential for a placebo treatment effect.
Statistically significant treatment effects for the E-RS:COPD were consistent with other treatment effects, including FEV1, SGRQ, and TDI. While lung function, typically measured by spirometry, is the most common endpoint in COPD drug trials, it is well known that associations are weak between airflow limitation (FEV1) and PROs, including symptoms and health status.36-38 Further, research investigating COPD treatments is evolving, with an increased interest in new treatments focusing on symptom relief, thus, highlighting the need to include a patient-reported symptom outcome measure as a key or primary endpoint. Symptom-specific measures complement pulmonary function and health status measures to provide a comprehensive evaluation of the effects of treatment on how patients feel and function. Given the essential role of symptomatic distress in the lives of patients with COPD, understanding the effects of various treatments on these symptoms could drive actionable treatment goals in the clinical setting.
This review highlights a gap in the approach used to identify clinically relevant effects in pharmacological interventions. Specifically, a limited number of publications discussed the interpretation of results, and fewer still provided responder analyses, which is a preferred method for determining and communicating clinical relevance. Use or reference to the proposed interpretation guidelines5 was inconsistent. None of the papers discussed retesting the proposed guidelines in the trial. Trial samples and study designs were generally consistent with the E-RS:COPD context of use and the data underlying the proposed interpretation guidelines. This review included studies with participants who were clinically stable with moderate-to-severe airflow limitation. Complementary baseline E-RS:COPD scores were approximately 9 to 14 points, also suggesting moderate symptomatology.5 Although it is reasonable to assume the proposed E-RS:COPD interpretation guidelines would apply to these reviewed studies, investigators should include confirmation in their research plan and make adjustments as needed.
This rapid review highlights that several publications that included the E-RS:COPD as primary or secondary endpoints appeared to follow minimal reporting standards for inclusion of PROs within clinical trials. However, it is evident there is a need for further guidance on how to include and report clinically meaningful treatment effects within clinical trials using instruments such as the E-RS:COPD. Such standards,39,40 in conjunction with the FDA guidance on PROs,2 should be reported consistently to provide the necessary information to make informed decisions when evaluating new drug therapies.
Future trials testing new COPD drug treatments that aim to provide symptomatic relief should enroll patients with moderate-to-severe respiratory symptoms and target those with the greatest unmet need to increase the likelihood of detecting a clinically meaningful effect. Further, in addition to being a valid, reliable, and responsive tool for measuring respiratory symptoms in patients living with moderate-to-severe COPD, the E-RS:COPD may be a useful PRO measure of respiratory symptoms in other populations. For example, a recent post-hoc examination of the psychometric properties and responsiveness of this tool was done among adults living with chronic airflow obstruction and a reversible component known as asthma-COPD overlap (ACO), with results indicating the E-RS:COPD was a suitable measure in this group of ACO patients.41 Also, Bacci and colleagues assessed the E-RS:COPD in an idiopathic pulmonary fibrosis population and found that its items applied to their respiratory symptom experience.42 Again, these new context of uses would need to be tested for validity and reliability, and examine potential new scoring algorithms.
Limitations of this Research
Results should be considered in light of this review’s limitations. While the goal of this rapid review was to produce synthesized knowledge on the use of the E-RS:COPD to support decision making in a timely manner, it is important to acknowledge that the applied constraints may have led to the exclusion of relevant E-RS:COPD data. Specifically, this search only included papers published in English, did not include grey literature, and was limited to 3 databases that may have excluded trials published in non-English countries, or remain unpublished. Results and conclusions are based on information that appeared in the publication itself, with some publications including a comprehensive reporting of E-RS:COPD results (e.g., mean change from baseline to follow-up, responder definitions, treatment effects, responder analysis) and others including fewer of these elements.
Conclusions
Findings from this review demonstrate that the E-RS:COPD has been used in 20 RCTs testing the efficacy of treatment in patients living with moderate-to-severe COPD. Statistically significant E-RS:COPD treatment effects moved in the same direction as the main outcomes. Presentation of trial results should include responder analyses to facilitate interpretation and application of results.
Acknowledgments
Author Contributions:
All authors contributed to the conception and design of the review. RW executed the literature search. All authors analyzed and interpreted the data. DMB and RW contributed equally as co-first authors and all authors participated in the development and critical review of the manuscripts. DMB had full access to all the data and final responsibility for the decision to submit for publication. All authors provided final approval for publication submission and are accountable for the accuracy and integrity of this work.
We also extend our appreciation to Rienne Schinner, Senior Library Services Specialist at Evidera, for her assistance and support in refining the search strategy. We also are grateful for the research support provided by the following Evidera staff during data collection and data synthesis: Saifra Khan Sohail, Ismail Budhiarso, Fanyang Zeng, and Sonja Stringer. We are grateful for the expertise, time, and invaluable feedback provided by our external expert reviewer: Sanjay Sethi, MD. Finally, we thank Shirish Dongare and Harneet Kaur for assisting with the submission of this manuscript.
Declaration of Interest
DMB, RW, and NKL are employed by Evidera|PPD which received funding from Novartis to conduct the systematic literature review. FSG, CH, and CT are employed by Novartis. CFV received consultation remuneration as an expert pulmonologist consultant for Novartis.