Running Head: Home-based Pulmonary Rehabilitation in COPD
Funding support: Sarah Gephine was supported by doctoral salary from the Fonds de Recherche du Québec–Santé (FRQS). The delivery of home-based PR was financially supported by Adair, Aeris Santé, Bastide, France Oxygène, Homeperf, LVL, Medopale, NorOx, Santélys, SOS Oxygène, Sysmed, VitalAire, and ARS Hauts-de-France. François Maltais holds a GSK COPD Research Chair at Laval University. The funders played no role in the design, conduct, or reporting of this study.
Date of Acceptance: November 2, 2021 │ Published Online: November 9, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; baseline assessment, MO; 8 weeks assessment, M2; 8 months assessment, M8; pulmonary rehabilitation, PR; Global initiative for chronic Obstructive Lung Disease, GOLD; long-term oxygen therapy, LTOT; non-invasive ventilation, NIV; short physical performance battery test, SPPB; timed-up and go test, TUG; 4-meter gait speed, 4MGS; 5-repetition sit-to-stand test, 5STS; 6-minute stepper test, 6MST; modified Medical Research Council, mMRC; clinical COPD questionnaire, CCQ; Hospital Anxiety and Depression scale, HAD; fatigue assessment scale, FAS; body mass index, BMI; standard deviation, SD; minimal clinically important difference, MCID
Citation: Gephine S, Saey D, Grosbois J-M, Maltais F, Mucci P. Home-based pulmonary rehabilitation is effective in frail COPD patients with chronic respiratory failure. Chronic Obstr Pulm Dis. 2022; 9(1): 15-25. doi: http://doi.org/10.15326/jcopdf.2021.0250
Online Supplemental Material: Read Online Supplemental Material (143KB)
Introduction
In addition to exercise intolerance and a sedentary lifestyle, functional capacity, referring to an individual’s balance, mobility, and transfer abilities, is particularly altered in end-stage disease,1 conferring a high risk for disability, hospitalization, and mortality.2,3 The situation may even be worse in individuals who develop hypoxemic or hypercapnic chronic respiratory failure, that could lead to a stage of vulnerability and physical dependence called frailty.4 Frailty syndrome affects approximatively 1 in 5 patients with chronic obstructive pulmonary disease (COPD),5 with an increasing proportion of frail individuals in the most severe stages of the disease. 6,7 Frail patients with COPD are at higher risk of disability and falls, exercise intolerance, anxiety and depressive symptoms, hospitalizations, and death compared to their non-frail counterparts.6,7
Pulmonary rehabilitation (PR) is highly effective at improving dyspnea, exercise tolerance, and health status in patients with moderate to severe COPD.8-10 Although less documented, center-based PR is feasible and has shown similar benefits in COPD patients with chronic respiratory failure.11-13 However, PR attendance and completion are likely compromised in frail individuals with chronic respiratory failure,6,14 to whom a home-based intervention could help in eliminating barriers that affect PR attendance.
The design of an effective PR program to prevent or delay functional decline and disability in older persons is a public health priority15 that has been addressed in multiple reviews.16,17 This question has received little attention in patients with COPD, although a few studies reported short-term effectiveness of center-based PR programs for improving balance, gait speed, and frailty syndrome in patients with moderate to severe COPD.18-20 Whether similar benefits of PR could be obtained in COPD patients with chronic respiratory failure who are at risk of altered functional status and physical frailty is uncertain. Home-based PR, using exercise and self-managing interventions tailored to each patient’s individual abilities and needs, is an attractive approach for these individuals that could further increase the program completion.21,22 Furthermore, focusing on functional status and autonomy in daily life activities and individualizing physical exercises in frail COPD patients with chronic respiratory disease could enhance the benefits of PR on functional capacity and frailty syndrome.
The main objective of this prospective interventional study was to evaluate the effectiveness of an 8-week, home-based PR program with COPD patients with chronic respiratory failure. A secondary objective was to specifically evaluate the impact of frailty status on the efficacy of PR in improving functional capacity, exercise tolerance, health-related quality of life, and anxiety and depressive symptoms.
Methods
Participants and Study Design
This was a prospective interventional study conducted in the north of France from December 2018 to October 2020. Participants were referred to the home-based PR program by their pulmonologist who was responsible for providing the clinical assessment and certifying the presence of COPD according to the Global initiative for chronic Obstructive Lung Disease (GOLD) classification system.23 Eligible patients were aged 40 years or above with a diagnosis of COPD as a main disease, and had chronic respiratory failure, defined as the requirement for either long-term oxygen therapy (LTOT) and/or non-invasive ventilation (NIV). Exclusion criteria included recent participation in PR (<12 months), poorly controlled psychiatric illness, neurological sequelae, or any bone and joint diseases preventing physical activity.
The study was approved by the observational research protocol evaluation committee of the Société de pneumologie de langue Française (CEPRO, number: 2017-007) and all participants signed a written informed consent prior to the start of the program. While baseline participants’ data have been published elsewhere,7 the effects of PR reported in the present study are original.
Home-based Pulmonary Rehabilitation Program
All participants received a home-based PR program tailored to their individual needs as previously described.24 Briefly, the PR program consisted of a weekly supervised 90-minute home session, for 8 weeks. Physical activity training, educational, motivational, and self-management plans were designed and implemented through a collaborative process between the PR team, the patient, and his/her caregiver. The rehabilitation team was composed of 1 pulmonologist, 2 nurses, 1 dietitian, 1 physiotherapist, 2 adapted physical activity instructors and 1 sociomedical beautician. The health care team received the same standardized therapeutic education training from a licensed instructor. Details about the interventions performed during the PR program can be found in the online supplement. Apart from the weekly visit of the team member who supervised the sessions, participants were expected to perform, on their own, personalized physical training (at least 4 sessions/week) and a self-management plan the rest of the week and during the long-term follow-up period.
Assessments
Lung function, assessed by spirometry according to standard guidelines,25 medication, and comorbidity data were collected from the individual’s medical record provided by the pulmonologist. The burden of comorbidity was assessed using the Charlson Index calculated without adjusting for age and without including COPD in the individual score, as previously suggested.26 Participants were entirely evaluated at home at the beginning of the 8-week PR program (M0), at the end of the program (M2, short-term), and at 8 months (M8, medium-term) following study inclusion.
Functional Capacity: Functional capacity was assessed with the short physical performance battery (SPPB) and timed-up and go (TUG) tests. The SPPB is composed of 3 standing balance tests, the 4-meter gait speed test (4MGS), and the 5-sit-to-stand repetitions test (5STS), which were performed according to the National Institute on Aging protocol.27 The sum of the 3 components determined the final SPPB score, with a possible range from 0 (functional impairment) to 12 (maximal functional capacity). TUG required the participant to rise from a seated position, walk 3 meters as quickly and safely as possible, turn around, walk back, and sit down in the shortest time possible as previously described.28
Physical Frailty: Physical frailty was defined using the Fried phenotype model,29 including 5 criteria: unintentional body mass loss history ≥4.5kg, self-reported exhaustion, low weekly self-reported energy expenditure using the modified Minnesota Leisure-Time Physical Activity Questionnaire, slowness measured during the 4MGS, and weakness measured with handgrip dynamometry. Patients with ≥3 criteria present were considered frail; those with 1 or 2 criteria were defined pre-frail, and those with no criteria were considered as robust.
Exercise Tolerance: The 6-minute stepper test (6MST) was used to evaluate exercise tolerance at home, as previously described.30 Participants were all familiarized with the stepper prior to the test. Standardized instructions were given, advising the participant to make the maximum number of steps (defined as a single complete movement of raising one foot and putting it down) possible over a 6-minute period. No encouragement was given during the test.
Symptom Burden, Health-related Quality of Life, and Anxiety and Depressive Symptoms: COPD symptom burden was assessed with the modified Medical Research Council (mMRC) breathlessness scale31 and with the COPD Assessment Test (CAT).32 Health-related quality of life was evaluated with the Clinical COPD Questionnaire (CCQ) which consists of 10 questions related to symptoms, mental state, and functional state (lower score indicates a greater health status).33 The Hospital Anxiety and Depression scale (7 items for anxiety and 7 items for depressive symptoms; lower scores indicate fewer symptoms)34 and the Fatigue Assessment Scale (FAS) (5 items reflecting physical fatigue and 5 items reflecting mental fatigue; lower score indicates fewer fatigue) were also assessed.35
Data and Statistical Analyses
The primary study variable was functional capacity measured with the 4MGS test. Thus, sample size calculation was based on Kon et al19 to detect a 0.06±0.13 m/s improvement in gait speed 8 months after study inclusion, with a power of 80% and an alpha of 5%. With this method, the sample size was calculated to be 39 participants. Assuming that up to 20 % of patients may drop out during the follow-up period, we aimed at recruiting 47 participants.
Comparisons of baseline variables between frail and non-frail individuals were performed using t-tests for the normally distributed quantitative variables while non-parametric Mann–Whitney tests were used for the non-normally distributed variables. To provide information on the impact of physical frailty on PR non-completion, a qualitative analysis was performed.
To handle missing values for some variables with less than 50% missing data, a multiple imputations procedure (mianalyse) was used to evaluate the changes in study outcomes over time (at M2 and M8). This approach was performed to avoid the observation exclusion for statistical analyses. A monotone missing pattern regression method was performed for continuous variable. Classification variables were imputed using a logistic regression approach. We tested the adequacy of the iterations (convergence) by visual inspection of trace plots. The imputation procedure and the subsequent analyses were performed according to the Rubin’s protocol.36 Variables were expressed as mean±standard deviation or as frequencies and percentages, and were tested using Shapiro-Wilk for normality. The results were considered significant with P-values ≤0.05. All analyses were conducted using the statistical package SAS, Version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
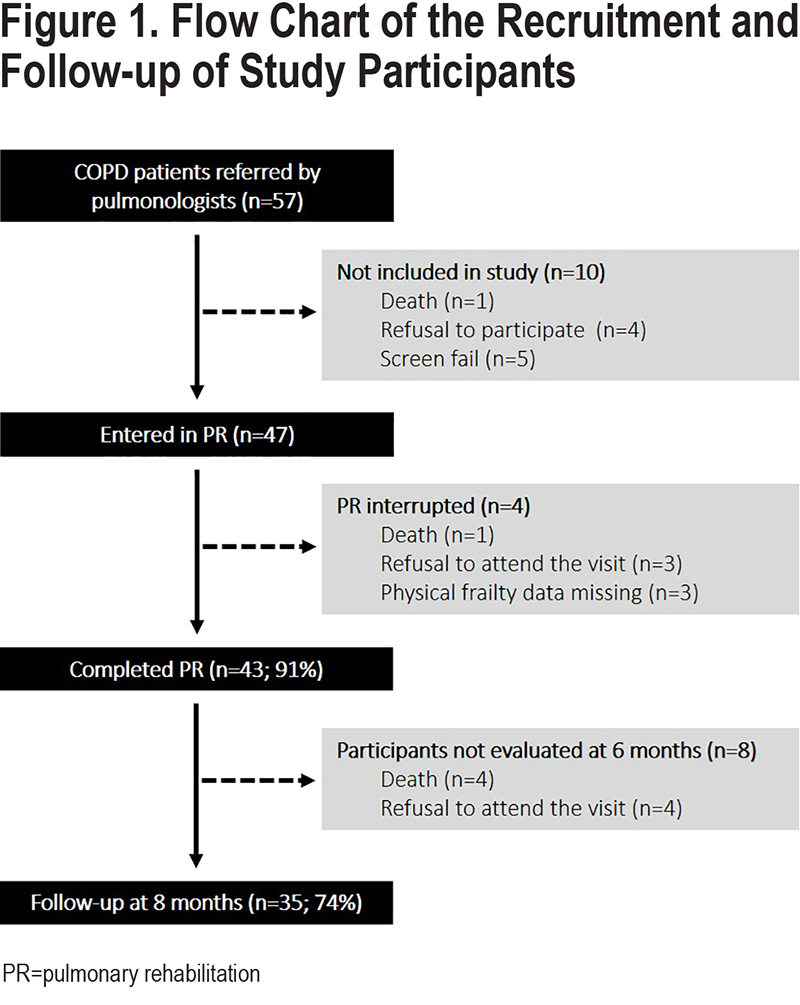
A flow chart of the study participants is presented in Figure 1. From December 2018 to October 2020, a total of 57 patients were contacted. Among them, 1 died before the first visit at home, 4 declined participation and 5 did not meet the inclusion criteria. Among the 47 included patients, 4 did not complete PR, and 8 participants did not attend the 8-month follow-up.
Baseline Characteristics
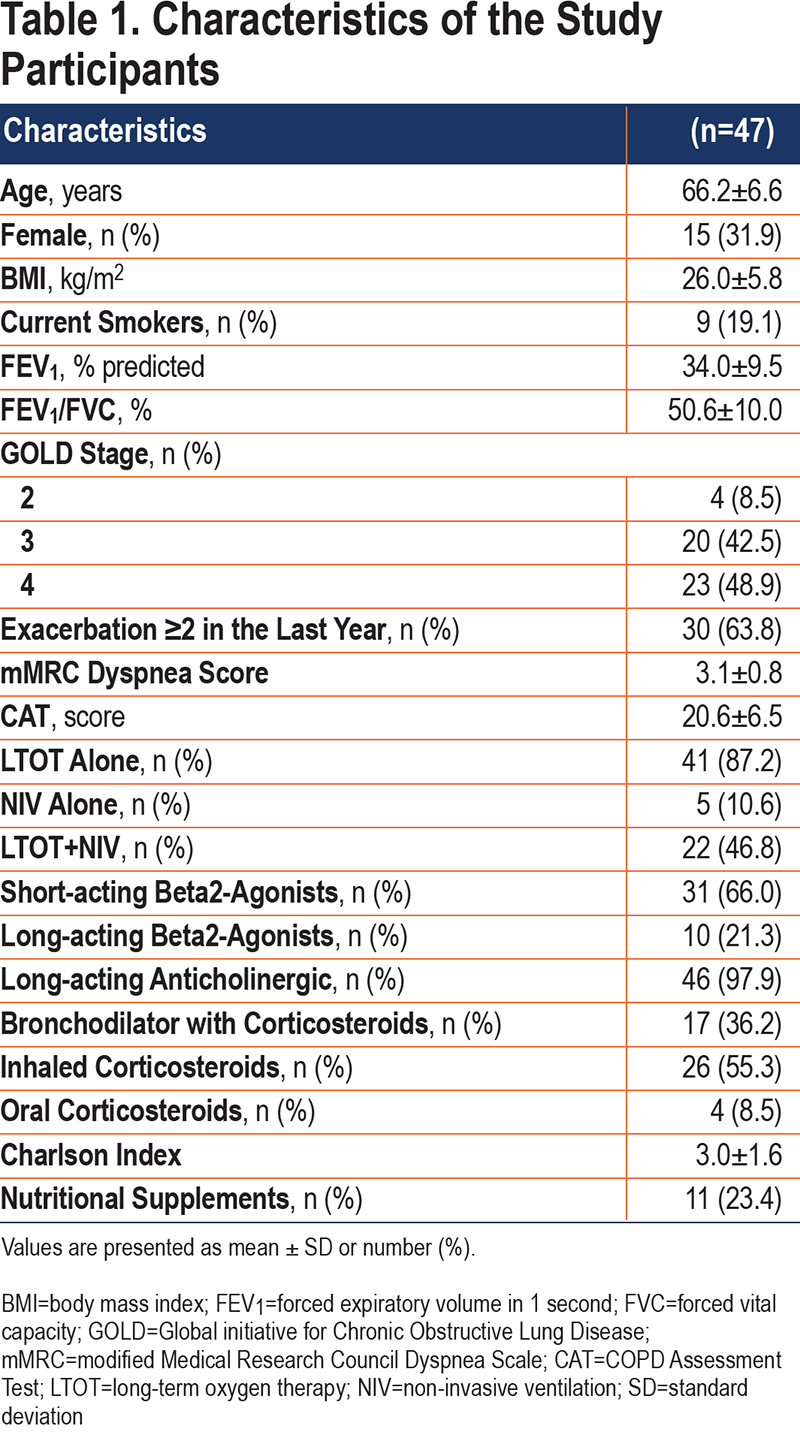
Two-thirds of study participants were men, most were ex-smokers, and, on average, they were overweight and had severe airflow limitation and frequent exacerbations (Table 1). Participants were treated with LTOT alone (41, 87.2%), NIV alone (5, 10.6%), or both therapies (22, 46.8%).
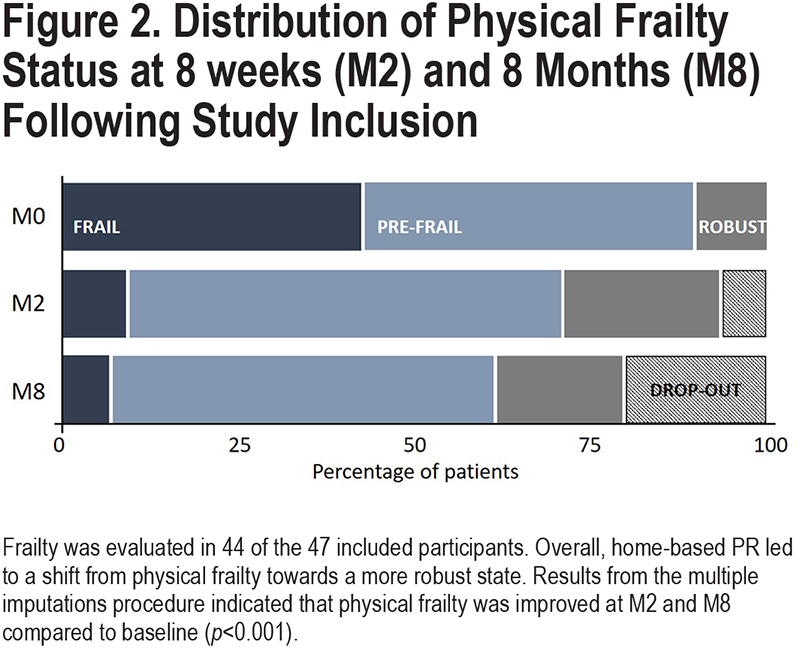
Among the 47 included patients, 3 participants refused physical frailty assessment at baseline. Baseline characteristics of these 3 participants were not different from those of the participants who completed the frailty assessment. Of the remaining 44 participants, 19 (43%) participants were physically frail, 21 (47%) were pre-frail and 4 were robust (10%) (Figure 2). The latter 2 categories of participants were grouped under the term “non-frail” participants. At baseline, frailty individuals had lower 4MGS, balance, SPPB, 6MST scores, and lower health-related quality of life, and higher fatigue and anxiety and depressive symptoms compared to the non-frail patients (p<0.01) (these data are detailed elsewhere).7
Home Pulmonary Rehabilitation Effectiveness
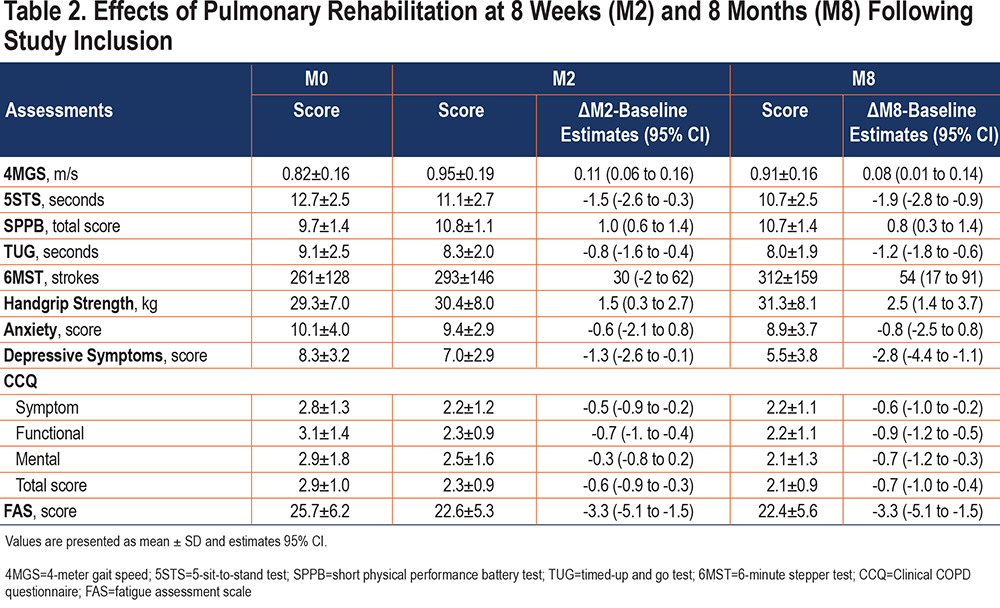
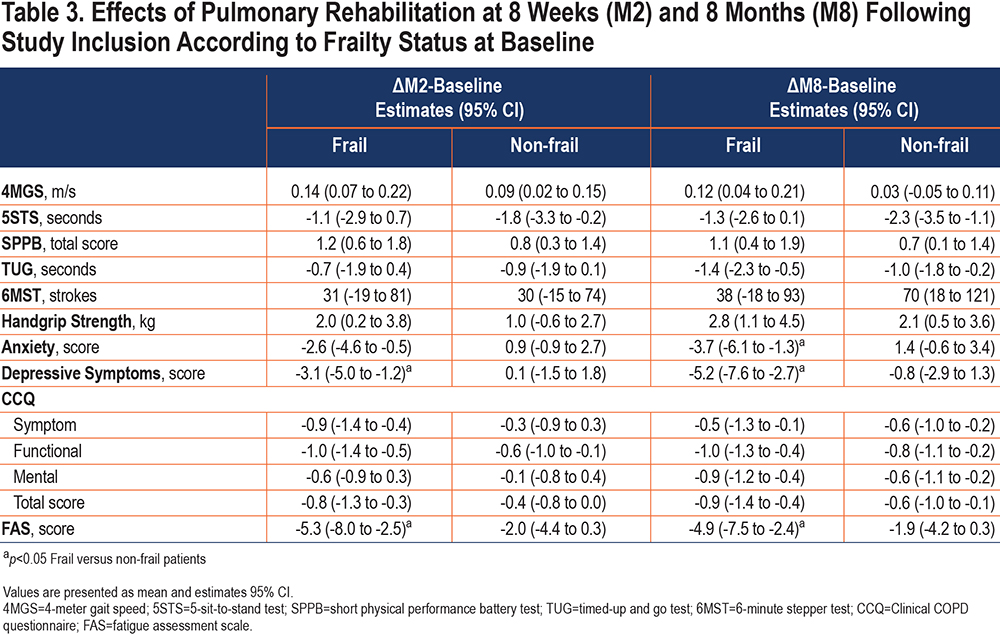
Short- and medium-term effects of PR are shown in Table 2. Compared to baseline, functional status, handgrip strength, health-related quality of life (CCQ total score, symptom, and functional scores), and fatigue scores were all improved at M2 and M8 (p<0.05), while exercise tolerance, depressive symptoms, and CCQ mental score were only improved at M8 (p<0.01). Changes in anxiety symptoms were not significant at either M2 or M8. There was no significant difference between M2 and M8 in any of the clinical assessments.
Overall, home-based PR led to a shift from physical frailty towards a more robust state (Figure 2). Among the 18 completers who were frail prior to PR, 4 (22.2%) were still frail, 11 (61.1%) became pre-frail, and 3 (16.7%) became robust at M2. No participants had moved from a robust or pre-frail state to a frail state after PR. Three initially frail patients did not improve their frailty status at M2 and M8.
Impact of Frailty on Pulmonary Rehabilitation Effectiveness and Attendance to Pulmonary Rehabilitation
Except for the 5STS, TUG, 6MST, and CCQ mental score, physically frail participants improved all their clinical assessments at M2 compared with baseline (p<0.05), while the non-frail participants only improved the SPPB and its components and the CCQ functional score (p<0.05) (Table 3). Overall, these benefits were maintained at M8, and additional benefits were obtained at M8 for the TUG and CCQ mental score in both groups (p<0.01) and for the 6MST, handgrip strength, CCQ symptom, and total scores in the non-frail participants compared with baseline (p<0.05). Only the improvement on depression and fatigue scores at M2 and on anxiety score at M8 was higher in the initially frail participants compared to their non-frail counterparts.
Four of the 5 participants who died during PR or during the 6-month follow-up were frail prior to PR. The other 7 participants who did not attend M2 or M8 were not initially frail.
Discussion
An original aspect of this study was to include patients with COPD requiring either LTOT and/or NIV. Although they were characterized by a severe airway obstruction with frequent exacerbations and several comorbidities, 8 weeks of real-life, home-based PR were effective for improving functional capacity, frailty status, health-related quality of life, and fatigue scores, at short- and medium-term. Physical frailty was also reversed in almost 80% of COPD patients who were frail prior to the program, shifting towards a more robust state that was maintained 6 months after the end of PR.
Physical frailty was not a barrier for benefiting from PR; on the contrary, general health-related quality of life, general fatigue, and anxiety and depressive symptoms of the patients who were physically frail prior to the program were improved by PR, while these significant benefits were not seen in their non-frail counterparts.
Exercise tolerance, daily physical activity, health-related quality of life, and survival are reduced in COPD patients with chronic respiratory failure compared to early stages of the disease.11,22,37,38 The 6MST is an attractive field test to evaluate exercise tolerance outside of a hospital.30 Six (31.6%) of the initially frail participants were unable to perform the 6MST and consequently received the score of zero. In these individuals, we relied on the assessment of the functional status, using a variety of testing procedures such as gait speed, sit-to-stand, or combined functional tests, to document the baseline clinical status and to quantify the effects of PR. The interest for these field tests has recently surged in COPD due to their clinical relevance for predicting disability, hospitalization, or even mortality.2,39
COPD patients with chronic respiratory failure benefited from the home-based PR program by improving their functional status, handgrip strength, health-related quality of life, and general fatigue. These results were consistent with previous studies.11,12,22 Importantly, these benefits were also maintained 6 months after the end of PR. Unlike previous studies in chronic respiratory failure measuring exercise tolerance using the 6-minute walking test,11,12 the change in 6MST at the end of PR was not significant nor clinically important for the patients (+30 strokes, below the minimal clinically important difference [MCID] of 40 strokes).40 However, this change became significant and reached the proposed MCID for this parameter 6 months after PR. This phenomenon was also observed for the depressive symptom score and the mental component of the health-related quality of life score, and even though there was no significant difference between M2 and M8 in any of the clinical assessments, we noticed that the 5STS, TUG, handgrip strength, and CCQ scores kept improving 6 months after PR compared with the end of the program. It could be argued that missing data could have impacted these results, nevertheless, we used a multiple imputations procedure to reduce the statistical bias. Therefore, we can assume that some of the participants maintained their physical training program and good health behaviors after the end of PR, possibly explaining the medium-term improvements. However, this information was not systematically collected, and results need to be interpreted with caution.
PR was effective for reversing the physical frailty status in almost 80% of the participants who were frail prior to the program. The majority of the frail participants (61%) progressed to a prefrail status after PR, and a few patients (17%) even reached a robust state. These results are consistent with those of Maddocks et al 6 who showed an improvement of the frailty status in 60% of the patients who were frail prior to an 8-week outpatient PR program. We extend these findings by showing that benefits on frailty status were maintained 6 months after the end of PR. Maddock et al also reported that being frail was associated with over double the odds of program non-completion.6 In the present study, frailty status did not seem to have an impact on PR non-completion or non-attendance since the dropout rate was similar between the initially frail participants and their non-frail counterparts. Due to the small sample size, caution is warranted when interpreting this result.
Finally, we confirmed that being frail does not prevent the patients with COPD from benefiting from PR.6 Patients who were physically frail prior to the intervention improved their functional capacity, handgrip strength, anxiety and depressive symptoms, health-related quality of life (symptom, functional, and total score), and general fatigue at short- and medium-term after PR. The benefits on health-related quality of life, general fatigue, and anxiety and depressive symptoms were numerically higher in frail participants compared to their non-initially frail counterparts. Although the scores for these variables were initially lower in frail patients compared to the non-frail ones, these results are no less informative about the effectiveness of home-based PR in frail COPD patients with chronic respiratory failure. We observed that exercise tolerance did not improve in physically frail patients compared to the non-frail patients. This observation supports that beyond exercise capacity, other physical capacity domains might be more sensitive to detect PR effectiveness in the most fragile patients. It could be argued that the lack of exercise capacity improvement could be due to a smaller number of physical training sessions in frail participants compared to the non-frail ones. Unfortunately, we cannot confirm this hypothesis as the patients’ recording of their unsupervised training sessions in a physical activity diary was optional, therefore, we do not have valid data for each participant which constitutes a study design limitation. Tracking unsupervised physical training is a challenge for home-based PR that has yet to be addressed. Including the patient’s caregiver in the update of the physical activity diary or using technological tools such as a phone application or connected cycle ergometer could be considered.
Clinical Perspectives
Combining physical exercises, nutritional support, self-management strategies, and cognitive therapies provides the best benefits on functional status and physical frailty in older adults without chronic respiratory disease.41 Similarly, the field of pulmonary rehabilitation could benefit from the experiences in geriatric medicine by targeting interventions that improve balance, gait ability, flexibility, muscle strength, and power.17,41 Our study demonstrated the importance of assessing not only exercise tolerance but also functional capacity and physical frailty in COPD patients with chronic respiratory failure. Although the optimal approach to improve these features remains to be determined in COPD, the PR intervention that was entirely delivered at home, including the clinical assessments, in the present study and that targeted functional capacity, confirmed that this important aspect of the disease is amenable to therapy even in the presence of chronic respiratory failure.
Methodological Considerations
The monocentric, non-randomized nature of this study and the absence of a control group may limit the scope of the present results. A cross-over design, in which the control group receives the home-based PR after an 8-week control period could be considered to strengthen the present results. However, data were collected systematically and consistently as an integral part of the home-based PR in COPD patients with chronic respiratory failure in whom the survival prognosis is compromised. In this context, the use of a more robust randomized controlled study design may not be realistic considering the relative urgency to initiate therapy in this population at high risk of deterioration. We also acknowledge that we did not strictly follow the Fried physical frailty categorization by grouping the pre-frail and robust patients together. Nevertheless, results were the same when we ran the statistics removing the 4 robust participants from the non-frail group. The impact of the home-based intervention could have been underestimated in 7 study participants who had their 8-month follow-up evaluation performed during the confinement related to the COVID-19 pandemic, a period during which they were likely more sedentary. However, the conclusion of the study regarding the efficacy of PR at 8 months was not altered when these 7 participants were removed from the analysis.
Conclusion
In COPD patients with chronic respiratory failure, home-based pulmonary rehabilitation may be effective for improving functional capacity, anxiety and depressive symptoms, and health-related quality of life. Physical frailty was also improved in almost 80% of the participants who were frail prior to pulmonary rehabilitation, shifting towards a more robust state that was maintained over 6 months after the intervention.
Acknowledgments
We thank the members of the rehabilitation team: Sophie Duriez, Mathieu Grosbois, Marjorie Lambinet, Gaelle Tywoniuk, Valentine Opsomer, Florence Urbain, and Virginie Wauquier. We also thank Serge Simard (Institut universitaire de cardiologie et de pneumologie de Québec, Université Laval, Québec, Canada) for his assistance with the statistical analysis.
Author contributions: SG was responsible for conceptualization, execution, acquisition of data, formal analysis and interpretation, and writing the original draft. DS was responsible for conceptualization, data interpretation, supervision, and writing – review and editing. JMG was responsible for execution, acquisition of data, writing – review and editing, and funding acquisition. FM was responsible for conceptualization, data interpretation, supervision, and writing – review and editing. PM was responsible for conceptualization, data interpretation, supervision, and writing – review and editing. All authors gave final approval of the version to be published.
Data Availability: Data for this study are from a deidentified cohort. Data are stored in the company FormAction Santé, Pérenchies France and are available by contacting Dr. Jean-Marie Grosbois at jmgrosbois@formactionsante.com.
Declaration of Interest
SG, PM, and DS have no conflicts of interest to disclose. JMG reports personal fees unrelated to the submitted work from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Vitalaire, and CSL Behring. FM reports grants from GlaxoSmithKline, AstraZeneca, Sanofi, Novartis, Boehringer Ingelheim, and Grifols, personal fees from GlaxoSmithKline, Boehringer Ingelheim, Grifols, and Novartis, and reports having a financial participation in Oxynov, a company which is developing an oxygen delivery system outside the submitted work. The funders played no role in the design, conduct or reporting of this study. The authors report no other potential conflicts of interest in this work.