Running Head: Electronic Medical Records and Alpha-1 Detection
Funding Support: This study was supported by a grant from the Alpha-1 Foundation. The Alpha-1 Foundation did not provide any input to the design or conduct of the study.
Date of Acceptance: November 9, 2021 │ Published Online: November 16, 2021
Abbreviations: alpha-1 antitrypsin deficiency, AATD; electronic medical record, EMR; electronic patient messages, EPM; chronic obstructive pulmonary disease, COPD; Alpha-1 Coded Testing, ACT; Food and Drug Administration, FDA; long-acting muscarinic antagonist, LAMA; long-acting beta2-agonist, LABA; Cleveland Clinic Foundation, CFF; respiratory therapists, RTs
Citation: Lam SW, Strange C, Brantly ML, Stoller JK. A novel detection method to identify individuals with alpha-1 antitrypsin deficiency: linking prescription of COPD medications with the patient-facing electronic medical record. Chronic Obstr Pulm Dis. 2022; 9(1): 26-33. doi: http://doi.org/10.15326/jcopdf.2021.0260
Introduction
Alpha-1 antitrypsin deficiency (AATD) remains highly under-recognized worldwide.1,2 Persisting evidence suggests that affected individuals experience long delays between initial symptoms and first diagnosis, with mean estimates of the diagnostic delay interval ranging from 5.6 to 8.3 years.3,4 Furthermore, there is little to suggest that this delay is shortening over time, i.e., between 1994, when first examined, and 2019 when most recently estimated.5,6 Furthermore, affected individuals often see many health care providers with AATD-attributable symptoms before being diagnosed. Stoller et al showed that 43% of PI*ZZ genotype individuals saw > 3 physicians before initial diagnosis.4 Not surprisingly, because AATD is a progressive disease, diagnostic delays are associated with worsened clinical status at the time of initial diagnosis.5,7 Accordingly, treatment guidelines for managing chronic obstructive pulmonary disease (COPD) recommend that all adults with fixed airflow obstruction on pulmonary function tests be tested for AATD.8-10
In the context of this persisting under-recognition and estimates that fewer than 10,000 of the estimated 100,000 PI*ZZ genotype Americans have been diagnosed,11,12 many strategies to enhance recognition of AATD have been undertaken, mostly with modest results.13-15 Such strategies include offering free testing for AATD, including confidential home-based tests; issuing reminders to physicians on pulmonary function test reports when fixed airflow obstruction is detected; providing electronic prompts to test for AATD in the electronic medical record (EMR) of patients with COPD; and enabling respiratory therapists to encourage testing at the point of PFT testing when fixed airflow obstruction is detected. These strategies have led to generally modest rates of detecting AATD among COPD patients, i.e., in the range of 0%–12% for detecting PI*ZZ genotype individuals.13-15
To address persisting under-recognition of AATD and to explore alternative methods to directly engage patients in detection, the current report presents the results of a novel method for detecting AATD. Targeted individuals – adults prescribed an inhaler indicated only for COPD – were approached directly through a patient-facing portal of the EMR with an invitation to undergo free, confidential, home-based AATD (through the Alpha-1 Coded Testing Study, sponsored by the Alpha-1 Foundation).
Methods
This study was reviewed and approved by the institutional review boards of the Cleveland Clinic, Medical University of South Carolina, and University of Florida.
This prospective, observational, proof-of-concept study evaluated the effectiveness of using an integrated EMR system to identify and encourage patients to undergo free, home-based AATD testing. As shown in Figure 1, patients were eligible if they received care at the Cleveland Clinic, were adults (>18 years), opted in to receiving electronic messages from the EMR patient portal (MyChart, Epic, Verona, Wisconsin), and received a prescription for a combination inhalation mediation that was only approved by the Food and Drug Administration (FDA) for treating COPD. These inhalers include inhaled fluticasone/umeclidinium/vilanterol (until October 2020, when the FDA approved its use for asthma); glycopyrrolate / formoterol; tiotropium / olodaterol; and umeclidinium / vilanterol. Patients were excluded if they had undergone prior AATD genetic testing as documented in the Cleveland Clinic EMR.
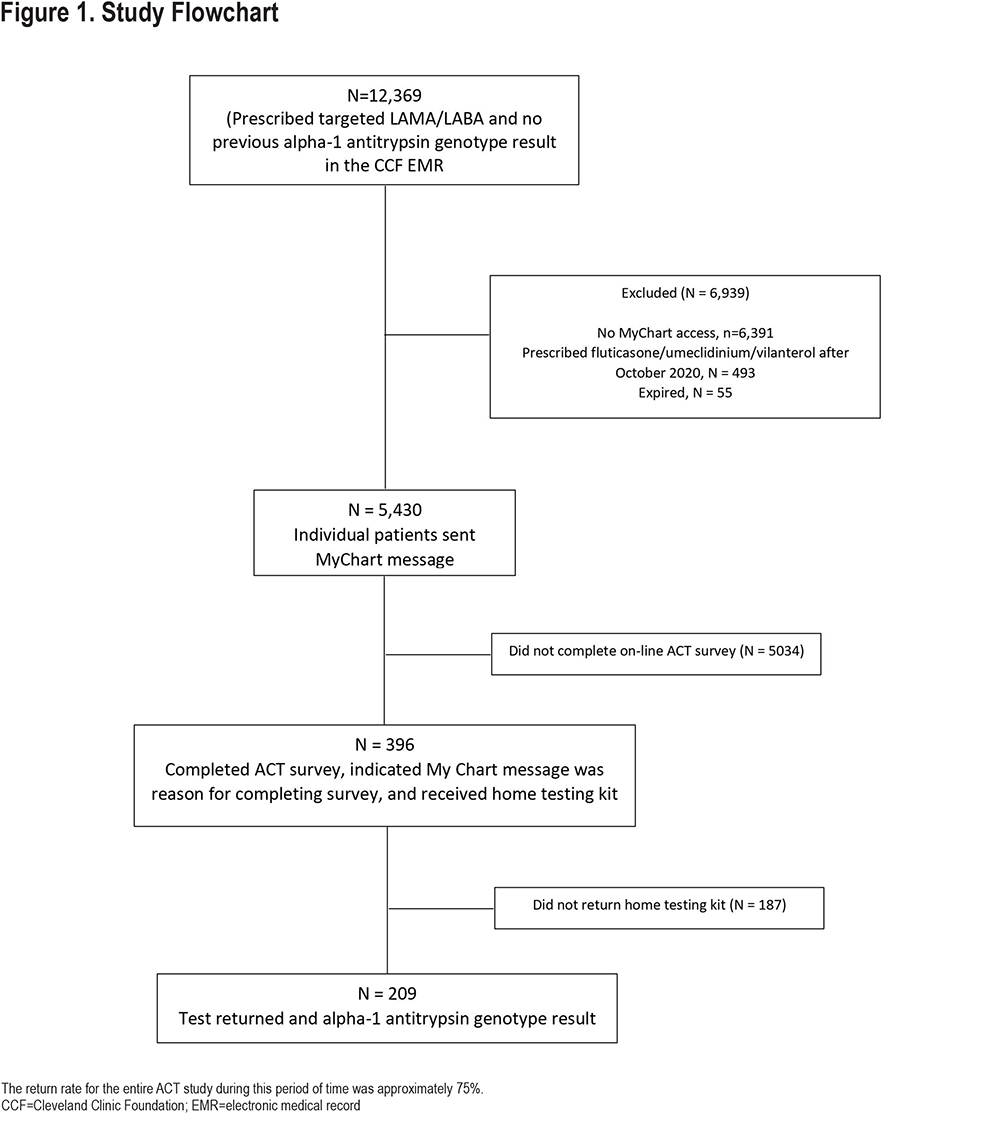
A report of patients was generated automatically from the EMR of patients who were prescribed one of the targeted inhaled long-acting muscarinic antagonist / long-acting beta2-agonist (LAMA/LABA) or triple therapy inhalers in 2018 and who met the other inclusion criteria. Participants were invited to participate between January 2019 and May 2021. Eligible participants were sent an electronic text message through MyChart. The message indicated that they were being contacted because they were prescribed a medication indicated only for COPD, emphasized guideline recommendations for AATD testing in patients with COPD, provided basic information about AATD, and informed them of the availability of free, home-based AATD testing. In addition, the message provided electronic links to obtain more information about AATD and instructions on how to obtain the free, home-based testing kit, called the Alpha-1 Coded test (ACT) kit provided by the Alpha-1 Foundation.16 Patients who followed the link to obtain the free ACT kit were directed to complete a survey and consent form. The survey to receive a free ACT test kit asked the patient to indicate what prompted them to order the ACT kit, i.e., whether the prompt was receiving a message in their EMR. Respondents who affirmed that were eligible could then be linked to the Alpha-1 Coded Testing results (performed initially at the Medical University of South Carolina and later at the University of Florida).
Because the ACT protocol required preserving the confidentiality of ACT study participants, results were reported in aggregate as de-identified data.
Statistical Analysis
As this is a proof-of-concept study, no formal power analysis was completed. The study was designed to conclude when 250 patients who indicated the electronic message was the reason for obtaining a test kit were actually tested, though terminated early due to personnel changes. The impact of the electronic message detection strategy was assessed with 3 metrics: (1) the proportion of patients among those contacted who actually obtained a test kit, (2) the proportion of patients, among those who actually ordered the ACT kit, who returned the test kit and received test results (AAT genotype and serum level), and (3) the proportion of abnormal genotypes among those who received ACT kit results.
Results
During the course of the study (January 2019 to May 2021 [Figure 1]), 12,369 unique patients were prescribed one of the targeted inhaler combinations; 5430 (43.9%) patients met all inclusion criteria and were sent an electronic message. Of these, 396 (7.3%) responded to the electronic message and requested an ACT kit. Of the 5430 eligible patients, 209 patients (3.8%) ultimately returned the ACT test kit for testing and have genotype information available. Table 1 presents the age and medications prescribed for the 5430 patients sent an electronic message.
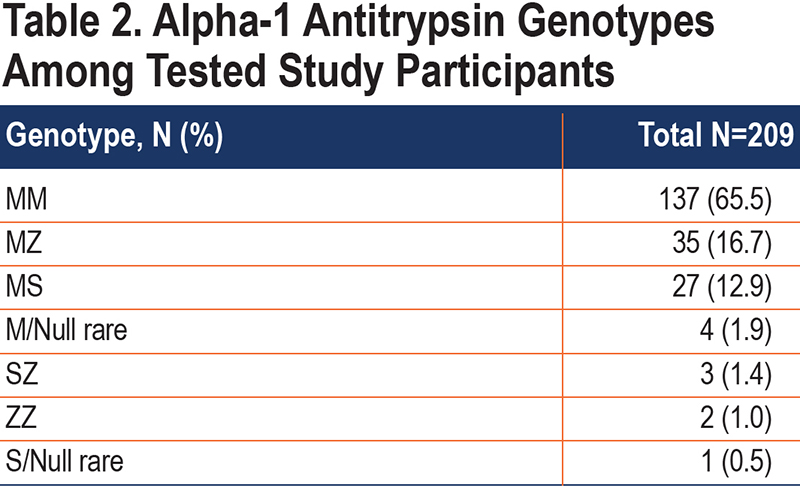
For the 209 participants with genotype results, most (65.5%) had a normal genotype (PI*MM), while 31.6% were heterozygous for an abnormal AAT allele, and (2.9%) had a genotype associated with severe deficiency of AAT (Table 2). Among those heterozygous for AATD, the most common genotype was PI*MZ (53%). Among the patients with severe deficiency, the most prevalent genotype was PI*SZ (3 patients), with 2 patients and 1 patient with PI*ZZ and PI*S/Null Rare, respectively.
Discussion
The main finding of this proof-of-concept study of a novel EMR-based strategy for detecting individuals with AATD is that, although the response and return rates for test kits were low, the prevalence of abnormal genotypes among those tested was high compared with other testing strategies and highest of all reported results for detecting heterozygotes. Furthermore, for heterozygotes, the prevalence in the tested population was 5-6-fold higher than the population prevalence estimates. For example, PI*MZ individuals comprised 16.7% of the tested population but only 3% of the unselected U.S population. The prevalence of individuals with severe deficiency of AAT generally resembles the prevalence estimates in COPD populations.17
As reviewed by Stoller et al, prior yields of other testing strategies (including targeted AATD detection among patients with COPD) are mostly lower than those described here, i.e., ranging from 1% to 62% for PI*MZ heterozygotes and 0% to12% for PI*ZZ genotype individuals.2 The rate of detecting PI*MZ genotypes in this study – 16.7% – is exceeded by only 1 study by Corda et al in which targeted detection among COPD patients was undertaken.18 The rate of detecting individuals with severe deficiency of AAT in the current study is on par with prior studies and consistent with the prevalence of AATD in the COPD population.17 In addition to the high detection yield of the current EMR-based strategy, other favorable attributes of the current approach include its simplicity, the automated generation of lists of eligible individuals (using EMR filters), and its adaptability to scale; specifically, filtering using the EMR and linking notifications to the electronic patient-facing portal (MyChart) allows the current strategy to be readily, easily expanded to larger populations.2 Different EMR-based strategies to enhance detection of AATD have been previously examined, with lower yields. For example, Jain et al reported rates of testing and AATD detection before and after implementing an electronic prompt in the EMR-triggered by pulmonary function test results that showed fixed airflow obstruction, a guideline-based criterion for AATD testing.10,13 That experience showed that 13 although no significant difference was observed in rates of detecting AATD after versus before deploying the prompt, the prompt was associated with an increased rate of testing from 5.3% of eligible patients (i.e., those with newly diagnosed COPD) to 15.1% (p<0.001) in the post-implementation period. Despite these modest gains in testing, the long-term efficacy of an EMR physician reminder alert approach is uncertain, since clinicians may experience “alert fatigue” as the number of EMR alerts proliferate. 19
The current study also attempts to activate patients in service of their own health, as demonstrated by their requesting the free, home-based ACT test. In another study that prompted patients to seek ACT testing, Stoller et al empowered respiratory therapists (RTs) who were conducting pulmonary function tests to invite patients with fixed airflow obstruction on testing to undergo AATD testing through the ACT study.20 Over the course of 1 year, 62 patients were referred by an RT and returned a test kit, with 3.2% identified as severely deficient and 24% as heterozygotes. While promising, this PFT lab-based strategy requires the continued education and training for RTs to identify at-risk individuals and so is less scalable than the novel approach reported here.
This current study adds to experience showing that the EMR is a viable and efficient way to reach out to patients to enhance communication and patient-engagement. In other disease contexts, several studies have illustrated the importance of patient engagement in improving satisfaction, outcomes, and cost-efficiency.21,22 Specifically, patient-facing portals have demonstrated benefit in patient engagement by improving medication adherence, discovery of medical errors, and patient-physician communication.23
Also, as in the current study, the EMR can be easily used to generate lists of eligible patients based on clinical criteria. The current study identified patients based on the use of inhaled LAMA/LABA or triple therapy, which are specifically indicated for COPD patients. Future studies may explore whether the use of additional medication criteria and/or diagnosis codes may further identify patients who are suitable for AATD EMR messaging. The interface with a patient-facing portal facilitates contacting patients rapidly and with minimal effort. While the current study required manually sending an electronic message to targeted patients, automating the process for future deployment of this strategy is easily imaginable. Furthermore, this experience with using the EMR and the patient-facing portal to enhance disease detection could easily be extended to other health conditions, including management. For example, patients receiving prescriptions for diabetes medications could be offered instructional materials about ancillary therapies or health care notifications, e.g., seeking routine eye examinations, etc. Of course, replication of these results in a larger study or other patient populations will be important.
Several limitations of the study warrant comment. First, the described experience is based on a relatively small number of patients, truncated a bit by early termination of the study as the principal investigator relocated. Still, it seems unlikely that the expansion of the study from 209 patients reported to the initial goal of 250 tested participants would materially affect the results. While the study does not permit reasons for non-response, possibilities include general reluctance to be tested for AATD, prior testing for AATD outside the Cleveland Clinic, an aversion to finger sticks required by the ACT test,24 or the perceived cumbersomeness of the ACT testing protocol. In this context, future availability of a buccal swab test for AAT testing or testing through a local laboratory might encourage higher participation in future studies. Another limitation is that the study is based on a single institutional experience, so the generalizability of these findings is uncertain. The number of individuals who failed to request an ACT Study test because of previous AATD testing outside of the Cleveland Clinic is unknown. Whether the expertise of the Cleveland Clinic in AATD drew more COPD-affected individuals who have AATD is also unclear. Furthermore, while the yield in detecting AATD is notably high in this study, the study offers little insight into why the yield is high. Specifically, because the study population was de-identified (as a condition of being able to use ACT testing), characterizing the study population, including comparing responders and non-responders, to optimize demographic and clinical correlates of high yield was not possible.
A third study feature with potentially important interpretive implications is its timing; the study was conducted during high surge periods of the COVID-19 pandemic. Consequent offsetting effects are possible. On the one hand, the pandemic eclipsed people’s attention to their own well-being (as in deferring routine health screening), potentially prompting individuals to ignore other important health issues like diagnosing AATD. This factor could contribute to the low observed rate of uptake of invitations to seek ACT testing. On the other hand, social distancing and the mandate to self-quarantine caused many people to revert to virtual strategies of self-care. Accessing the ACT test required only completing an online request and posting the completed test in the mail. The net effect of these potentially offsetting effects is unknown, of course, and will require replicating the study in a post-pandemic era. Finally, as a feasibility study, the current detection strategy exploited only a single, simple criterion for establishing patient eligibility, i.e., receiving a prescription for an inhaler reserved for a COPD indication. More detailed mining of the EMR for features predictive of COPD associated with AATD (e.g., obstructive pulmonary function tests perhaps with a positive family history and minimal smoking history, etc.) could potentially enrich the pool of eligible patients for those likely to have AATD and, therefore, conceivably enhance the detection yield yet more. Future studies could explore such alternate selection strategies, as well as multiple automated reminders to seek testing, etc.
In conclusion, this study has demonstrated that an electronic messaging program that targets patients receiving medications for COPD can enhance AATD testing and did lead to a high rate of detecting individuals with abnormal genotypes for AATD. These results invite further exploration of EMR-based strategies for detection of AATD and other conditions.
Acknowledgements
The authors acknowledge and thank Michelle Owens from the University of Florida and Laura Schwarz from the Medical University of South Carolina for their help in querying the necessary data from the ACT databases.
Author contributions: SWL takes responsibility for the content of the manuscript, including the data and analysis. SWL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SWL, CS, MLB, and JKS contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Data sharing statement: Selected de-identified data will be available through the Alpha-1 Foundation biomaterial exchange. Please refer to this website for more information (https://www.alpha1.org/investigators/resources/biomaterials-exchange/).
Declaration of Interest
SWL and MLB have nothing to disclose. JKS serves on the Board of Directors of the Alpha-1 Foundation. JKS also serves as a consultant to: Grifols, Vertex, CSL Behring, Takeda, 23andMe, InhibRx, Insmed, 4DMT, Korro, and Bridgebio. CS had AATD research monies paid to his university during the tenure of this study from the Alpha-1 Foundation, Adverum, AstraZeneca, CSL Behring, Grifols, MatRx, Takeda, and Vertex. He consulted for AstraZeneca, Dicerna, GlaxoSmithKline, and Vertex. CS is an employee of AlphaNet.