Running Head: Patient/Primary Care Factors in COPD Outpatient Care
Funding Support: This study was supported by Dr. Laura Feemster’s National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) K23 award (HL 111116) and an NIH institutional training grant (T32 HL 007287, PI Robb Glenny, MD). Dr. Donovan is supported by VA Health Services Research and Development Career Development Award 18-187 and Dr. Spece is supported by an NIH NHLBI award (5K12 HL137940-04). The funding sources were not involved in the data analysis and interpretation and had no role in the writing of the manuscript.
Date of Acceptance: November 29, 2021 │ Published Online: December 16, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; primary care provider, PCP, standard deviation, SD; emergency department, ED;Global initiative for chronic Obstructive Lung Disease, GOLD; University of Washington, UW; attending and resident physicians, MDs; advanced registered nurse practitioners, ARNPs; International Classification of Diseases, 9th Revision, ICD-9; electronic medical record, EMR; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; long-acting bronchodilator, LABD; body mass index, BMI; hypertension, HTN; bronchodilator, BD; oxygen saturation via pulse oximetry, SpO2; smoking cessation therapy, SCT; modified Medical Research Council, mMRC; pulmonary function tests, PFTs; interquartile range, IQR; confidence interval, CI
Citation: Keller TL, Wright J, Donovan LM, et al. Association of patient and primary care provider factors with outpatient COPD care quality. Chronic Obstr Pulm Dis. 2022; 9(1): 55-67. doi: http://doi.org/10.15326/jcopdf.2021.0232
Online Supplemental Material: Read Online Supplemental Material (366KB)
Introduction
Chronic obstructive pulmonary disease (COPD) remains the 4th leading cause of death in the United States, contributing to 1.5 million emergency department (ED) visits and 725,000 hospitalizations each year, with an estimated $49 billion in annual costs.1-4 Seeking to address this burden, multiple organizations including the Global initiative for the chronic Obstructive Lung Disease (GOLD), the American Thoracic Society, and the American College of Physicians have created evidence-based guidelines for the diagnosis and management of COPD.5-8
Despite prior implementation initiatives,9-13 large gaps exist between guideline-recommended outpatient COPD care and actual clinical practice.14-22 While some recommended care processes (e.g., annual assessment of dyspnea and resting oxygen saturation) occur frequently in clinical practice,14,16,17 others do not. Although professional organizations consistently define COPD as the presence of non-reversible airflow obstruction during spirometry, fewer than 50% of patients diagnosed with COPD ever complete this confirmatory test.23,24 In addition, many patients with COPD use inhaled treatment regimens that depart from guideline-recommended indications.18,25,26 Notably, fewer than 5% of patients attend pulmonary rehabilitation despite strong evidence supporting its use.27-29
Identifying factors associated with the achievement of high-quality outpatient COPD care represents an important first step toward the design of effective interventions to improve care quality. Most studies to date have evaluated only 1 aspect of recommended outpatient care (e.g., pharmacotherapy or spirometry) without considering care quality as a whole.17,24,27,28,30-32 The relative importance of primary care provider (PCP) characteristics to outpatient COPD care quality is unclear. Moreover, the impact of pulmonary specialty care on outpatient care quality remains unknown. Striving to address these limitations, we examined the association of patient and PCP characteristics with the receipt of evidence-based outpatient care quality measures among a cohort of patients clinically diagnosed with COPD.
Methods
Data and Study Population
We conducted an observational study of adults aged ≥40 years with clinically diagnosed COPD who received care at 2 University of Washington (UW)-affiliated primary care clinics between June 1, 2011, and June 1, 2013. Staffed by attending and resident physicians (MDs) and nurse practitioners (ARNPs), these clinics provide comprehensive medical care to nearly 11,000 patients annually. We included patients who had at least 1 outpatient or primary hospital discharge diagnosis of COPD (International Classification of Diseases- 9th revision [ICD-9] codes: 491.X, 492.X, 493.2, or 496.X) and 2 or more outpatient PCP visits (any diagnosis) over 1 year. We required more than 1 outpatient PCP visit to ensure patients routinely received primary care within our system. We set the index date as the first date during the study period that patients met the inclusion criteria. We strove to identify a real-world population of patients receiving outpatient management for COPD. As PCPs often continue to manage patients for COPD despite the lack of airflow obstruction on spirometry,33 we believed that it was important to include this subset of patients.
We obtained all patient data from the electronic medical record (EMR). The UW Institute for Translational Health Sciences identified patient demographics nearest the index date and used ICD-9 codes to determine comorbid conditions documented in the year prior to cohort entry. Additional patient data was ascertained via chart abstraction using standardized forms. We reviewed all primary care and pulmonary clinic notes within 1 year prior to the index date to determine baseline health behaviors, health care utilization, symptom assessments, and supplemental oxygen and medication use. We abstracted spirometry records (including those documented in clinic notes) within 5 years prior to the index date. We reviewed all discharge summaries, ED notes, and outpatient documentation within 2 years prior to the index date to identify COPD exacerbations, defined as a clinician-diagnosed exacerbation accompanied by prescription of systemic antibiotics and/or glucocorticoids. We performed duplicate chart abstractions for ~20% of the cohort to ensure consistency and adjudicate discrepancies, with fewer than 5% noted between reviewers. We identified primary care and pulmonology providers from outpatient notes. We determined PCP characteristics from data available to UW faculty, including residency rosters and curricula vitae.
Outcomes
Our primary outcome was the proportion of guideline-recommended outpatient COPD care measures received through primary or pulmonary specialty care. We used the guidelines contemporaneous with the study to determine recommended care measures.5,6,34 Patients could receive a maximum of 9 measures, if eligible (e-Table 1 in the online supplement). Among all patients, measures included completion of spirometry (within 5 years prior to index), assessment of respiratory symptoms, smoking status, and oxygen saturation (at least once in year prior to index), and receipt of guideline-concordant inhaled therapy (nearest to index). Among those eligible, additional measures included confirmation of airflow obstruction (within 5 years prior to index, defined as a post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] ratio < 0.7), prescription for oxygen therapy, any smoking cessation intervention, and pulmonary rehabilitation referral (at least once in year prior to index).
Obtaining an accurate diagnosis is an important aspect of COPD care quality. We thus included completion of spirometry and the accuracy of COPD diagnosis among patients completing spirometry (post-bronchodilator FEV1/FVC <0.7) as secondary outcomes. In addition, given strong evidence supporting its use, we also evaluated the receipt of indicated inhaled long-acting bronchodilator (LABD) therapy (e-Table 1 in the online supplement).5,6,34
Predictors of Outpatient Care Quality
We a priori identified patient and PCP characteristics hypothesized to influence the receipt of guideline-recommended outpatient COPD care. Patient characteristics included demographics (age, sex, insurance status, marital status, and race), presence of obesity (body mass index [BMI] ≥ 30), comorbidities (heart disease, non-COPD lung disease, obstructive sleep apnea, and depression), substance use, outpatient health care utilization (total number of PCP and pulmonary clinic visits, completed pulmonology referral), continuity of primary care (number of unique PCPs seen, proportion of primary care clinic visits with most frequent PCP), and the number of hospitalizations for COPD within 1 year of the index date. PCP characteristics included gender, highest degree attained (MD versus ARNP), years since highest degree attainment, and the proportion of cohort patients co-managed with a pulmonologist of each patient’s most frequent PCP (e-Methods in the online supplement)
Statistical Analyses
We used multivariable mixed effects linear regression to estimate the association of patient and PCP characteristics with the proportion of eligible guideline-recommended outpatient COPD care measures received. For secondary outcomes, we generated multivariable mixed effects logistic regression models to estimate the association of patient and PCP characteristics with each outcome. For all models, we specified a random intercept by most frequent PCP and chose an independent covariance matrix with robust variance estimators. We tested for multicollinearity using variance inflation factors and excluded covariates with values > 5 (e-Methods in the online supplement).35
To account for differences in follow-up time, we prespecified a sensitivity analysis in which we restricted the cohort to patients who had 1 full year of data available prior to the index date. We performed an additional sensitivity analysis that assessed the proportion of guideline-recommended outpatient care measures received through primary care alone. We performed all analyses using Stata version 16.1 (StataCorp, College Station, Texas). The UW institutional review board approved this study (UW 41666 EA).
Results
Patient Characteristics
We included 641 patients with a clinical diagnosis of COPD and at least 2 outpatient primary care visits within a 1-year period. Of these, 382 (59.6%) were male with mean age 63.6 (standard deviation [SD] 10.6) years. Most patients were of White race (N=400, 62.4%) with insurance through Medicare (N=377, 58.8%). Patients frequently had comorbid asthma (N=159, 24.8%) and prior pneumonia (N=125, 19.5%). Over 60% (N=386) of patients had current smoking documented at least once during the study period. Among those completing spirometry, most had moderate airflow obstruction with a mean percent predicted FEV1 of 60% (SD 21%) (Table 1).
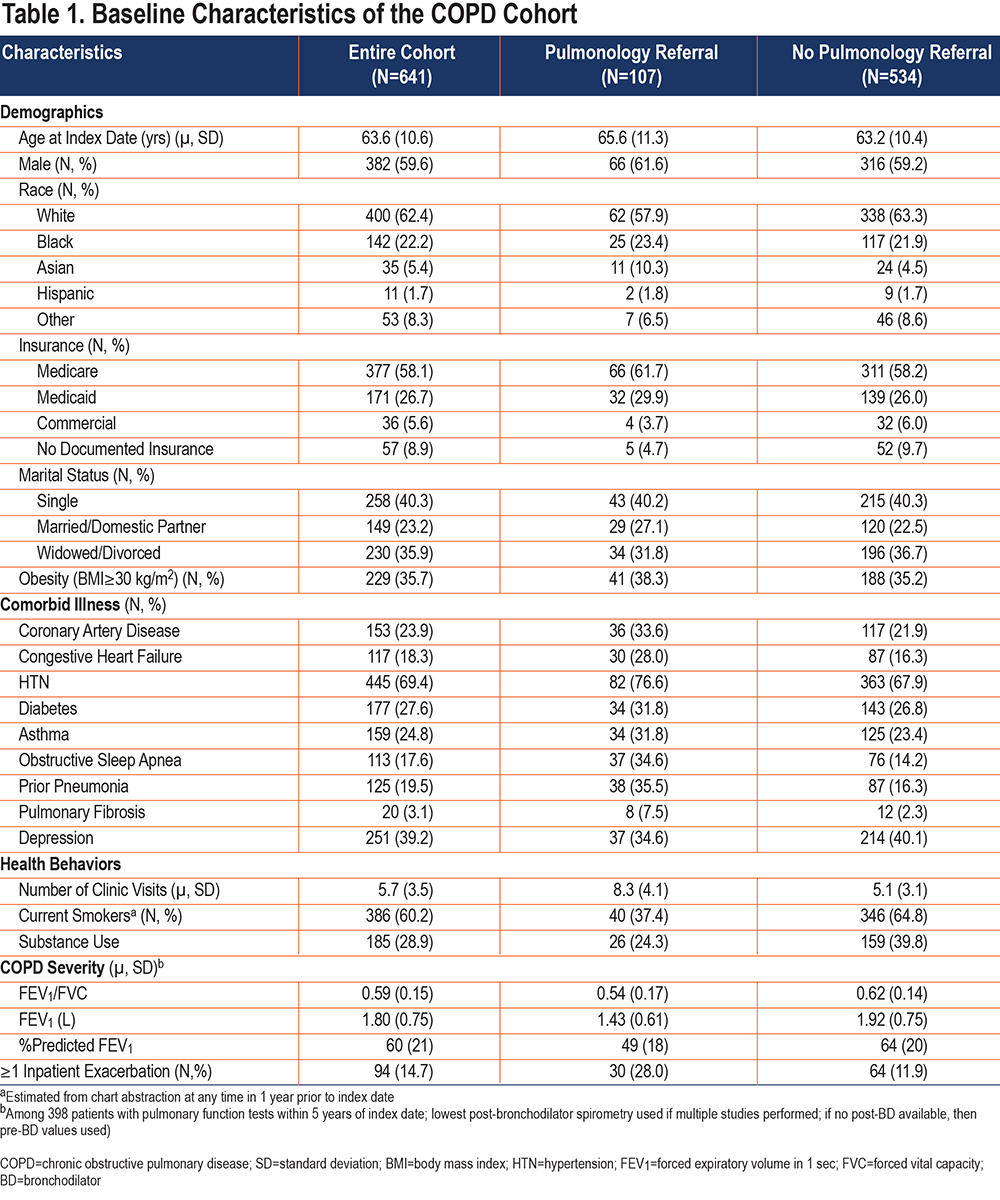
Patients saw 150 different PCPs during a mean of 5.3 (SD 3.2) primary care visits. Most patients received care from allopathic physicians (N=593, 91.0%) and women providers (N=359, 56.0%) with median time since completion of training among PCPs of 12.2 (interquartile range [IQR] 2.5–22.6) years. Only 107 patients (16.7%) completed outpatient pulmonary consultation.
Receipt of COPD Care Quality Measures
Patients received a mean of 67.5% (SD 18.4%) of eligible (median 7 [IQR 6–7]) guideline-recommended outpatient COPD care quality measures. Only 46 patients (7.2%) received “ideal” (100% of eligible) outpatient COPD care. Most patients had their respiratory symptoms (N= 584, 91.1%) and smoking status (N= 599, 93.5%) assessed at least once in the year prior to index, with these assessments occurring at 58.5% (SD 32.5%) and 77.3% (SD 32.6%) of visits, respectively (Table 2). Fewer patients completed spirometry (N=398, 62.1%) or received guideline-recommended inhaled therapy (N=382, 59.6%). Among those with high symptom burden or hospitalization for COPD, 37 (10.7%) completed or received a referral to pulmonary rehabilitation. Notably, 283 active smokers (73.3%) received smoking cessation counseling at least once, while 92 (23.8%) received a prescription for smoking cessation pharmacotherapy.
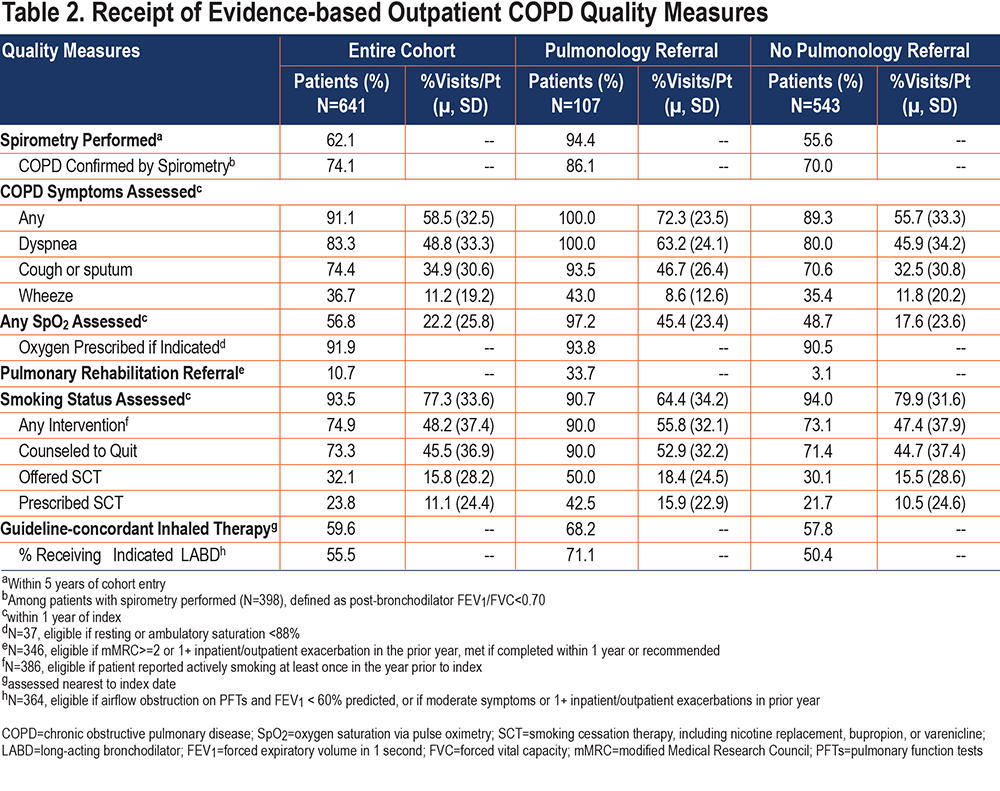
Patients who saw a pulmonologist received a mean of 83.3% (SD 13.3%) of eligible guideline-recommended outpatient COPD care, while those who did not received 64.3% (SD 17.6%) (Figure 1). Those who saw a pulmonologist were more likely to complete spirometry (94.4% versus 55.6%), have oxygen saturations assessed at least once (97.2% versus 48.7%), receive any smoking intervention if currently smoking (90.0% versus 73.1%), and receive an indicated referral to pulmonary rehabilitation (33.7% versus 3.1%) than those who did not.
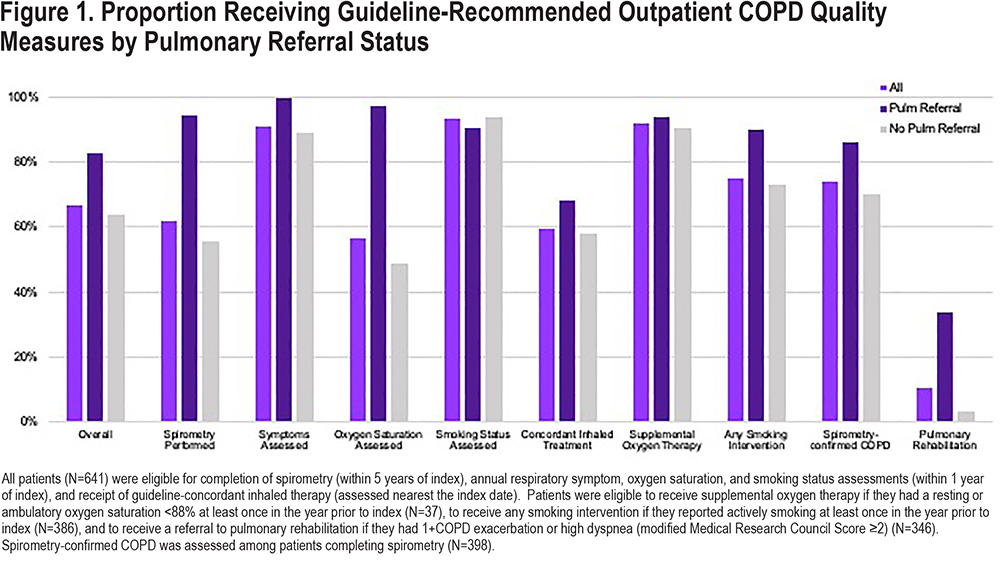
Predictors of COPD Care Quality
In the multivariate analysis, patients completing pulmonary referral achieved 17.7% higher outpatient COPD care quality than those who did not (ß117.7%, 95% confidence interval [CI]: 12.6%, 22.7%). We found no significant difference in the proportion of care quality measures received among patients of non-White race (ß1-1.1%, 95% CI: -3.5%, 1.2%), insured via Medicaid (ß1 1.0%, 95% CI: -2.5%, 4.5%), without documented insurance (ß1 3.4%, 95% CI: -1.2%, 8.0%), or reporting substance use (ß1 -0.3%, 95% CI: -3.6%, 2.9%). We similarly found no strong evidence of an association between PCP characteristics and the receipt of guideline-recommended outpatient COPD care (Table 3). The patient’s most-frequent PCP contributed minimally to the observed variance in receipt of care quality measures (intraclass correlation coefficient < 0.001%).
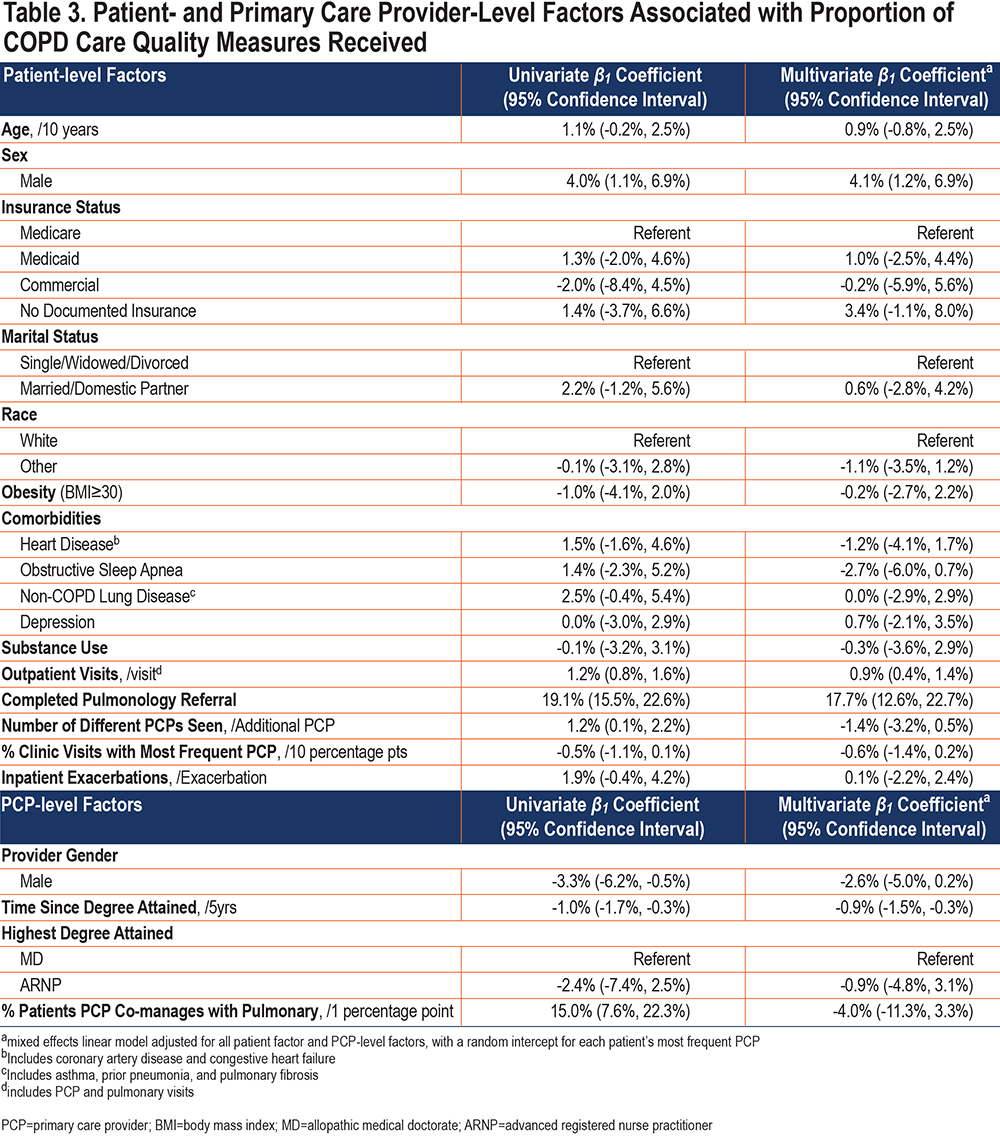
Secondary Analyses
A total of 398 patients (62.1%) completed spirometry at least once in the 5 years prior to cohort entry. In the multivariate model, a completed pulmonary referral strongly predicted the completion of spirometry (odds ratio [OR] 12.48, 95% CI: 4.20, 37.13) (e-Table 2 in the online supplement). We found no evidence of an association between patient race, insurance status, or marital status and the completion of spirometry. In contrast, a patient whose PCP completed their highest degree 5 years prior had a 10% lower odds of completing spirometry than a patient whose PCP just graduated (for each 5-year period since degree attainment OR 0.90, 95% CI: 0.83, 0.98).
Among those completing spirometry, 295 (74.1%) had confirmed COPD (post-bronchodilator FEV1/FVC < 0.70). In the multivariate model, the strongest predictors of a spirometry-confirmed COPD diagnosis were a completed pulmonary referral (OR 4.88, 95% CI: 1.92, 12.41) and each hospitalization for COPD (OR 4.10, 95% CI: 1.70, 9.95) (e-Table 3 in the online supplement). Non-White race was associated with 48% lower odds of spirometry-confirmed COPD (OR 0.52, 95% CI: 0.34, 0.82). Similarly, individuals with comorbid heart disease (OR 0.38, 95% CI: 0.20, 0.72) or obstructive sleep apnea (OR 0.48, 95% CI: 0.24, 0.96) had lower odds of spirometry-confirmed COPD than individuals without these diagnoses.
Among patients with guideline-recommended indications for inhaled LABDs (N=364), 202 (55.5%) received this therapy. In the multivariate model, each hospitalization for COPD was associated with 89% higher odds of receiving LABD therapy (OR 1.89, 95% CI: 1.32, 2.72) (e-Table 4 in the online supplement). We found little evidence of an association between the completion of a pulmonary referral and the receipt of LABD therapy (OR 1.59, 95% CI: 0.69, 3.64). In contrast, a patient of non-White race (OR 0.60, 95% CI: 0.35, 1.04) or without documented insurance (OR 0.43, 95% CI: 0.20, 0.96) had lower odds of receiving LABD therapy than a White patient or a patient insured via Medicare, respectively.
We obtained similar results when we: (1) restricted the cohort to patients with at least 1 year of data available (e-Table 5 in the online supplement), or (2) evaluated the association of patient and PCP factors with the proportion of guideline-recommended outpatient care measures received only through primary care (e-Table 6 in the online supplement).
Discussion
In a cohort of adults aged ≥ 40 years with clinically diagnosed COPD, we found that overall outpatient COPD care quality was poor. Pulmonology referral was strongly associated with the receipt of evidence-based outpatient COPD care, with patients completing this referral estimated to achieve nearly 18% higher mean care quality compared with those who did not. This corresponds to the achievement of an additional 1–2 quality measures per patient. In contrast, we observed little association between markers of socioeconomic status, COPD severity, or comorbidities and overall care quality. Similarly, PCP characteristics were not strongly associated with the receipt of outpatient COPD quality measures.
The quality of care among outpatients with COPD was suboptimal with patients in our study achieving only 67% of eligible quality measures over a 1-year period. Notably, fewer than 10% of patients in our study received “ideal” outpatient COPD care. Our findings add to the literature that, despite prior improvement efforts,9-12 the quality of outpatient COPD care is poor. Studies consistently show that spirometry is performed in only 35%–50% of patients with clinically diagnosed COPD.23,24,30 Similarly, only 50%–60% of patients receive inhaled therapy per guideline recommendations.15,17,18,25,26,36 Moreover, few patients receive smoking cessation pharmacotherapy or pulmonary rehabilitation despite strong evidence supporting their use.20,21,27,28,37
Consistent with prior studies, patients in our cohort classified as racial or ethnic minorities, who were uninsured, or with documented substance use did not receive lower overall care quality than those not classified within these traditionally marginalized groups.17,20 Although racial minorities in our study had a similar likelihood of completing spirometry, they had lower odds of spirometry-confirmed COPD. This suggests that racial minorities are more likely than White patients to be misdiagnosed with COPD. The etiology of this disparity is unclear and warrants further investigation. Similarly, patients without documented insurance and, although not statistically significant, those of non-White race, had lower odds of receiving an indicated LABD. The high out-of-pocket cost of inhaled therapy may contribute to these observed disparities. Further studies are needed to clarify the importance of addressing socioeconomic status or racial bias in future initiatives to improve outpatient COPD care quality.
While the observational nature of our study limits causal interpretation, our results suggest that engaging pulmonary specialty providers in the outpatient management of patients with COPD may improve care quality. Patients in our cohort who completed a pulmonology referral were more likely to complete spirometry, have oxygen saturations assessed, receive pharmacotherapy for smoking cessation, and receive a referral to pulmonary rehabilitation than those who did not. Prior literature has consistently demonstrated the benefit of involving subspeciality providers in the outpatient management of patients with other chronic conditions, such as heart failure and diabetes.38-43 Multiple aspects of the pulmonary specialty visit could produce higher quality outpatient COPD care. Pulmonologists likely have up-to-date knowledge of COPD-specific guidelines while the longer duration of subspecialty visits enables them to devote more time toward the evaluation and management of COPD and patient education. In addition, embedding respiratory therapists in pulmonary clinics may result in higher completion of spirometry and more frequent oxygen status assessments.44,45 The optimal proportion of patients with COPD who should be co-managed with a pulmonologist, and which subset would benefit the most from pulmonary consultation, remain unknown. Future studies should investigate these topics.
Nonetheless, involving pulmonologists in the outpatient management of patients with COPD is unlikely to produce “ideal” care quality without additional interventions. Our findings agree with a prior study demonstrating that patients co-managed with pulmonologists are more likely to receive guideline-concordant inhaled therapy.17 However, our study also demonstrates that for these patients, large gaps remain. Among patients who saw a pulmonologist in our cohort, only 68% received guideline-concordant inhaled therapy. In addition, fewer than 34% received an indicated referral to pulmonary rehabilitation. EMR-based clinical decision support tools may help optimize these aspects of outpatient COPD care.46,47
Moreover, the prevalence of COPD, the limited availability of pulmonologists across the United States, and the current fee-for-service model make widespread in-person pulmonary consultation impractical.2,3,48 Given the recent liberalization of Centers for Medicare and Medicaid Services reimbursement for telemedicine services,49 future implementation initiatives could focus on engaging pulmonary specialty providers in the population management of COPD via e-consultations or shared telemedicine visits. Prior literature suggests these modalities may improve care quality and outcomes among patients with HIV and diabetes.50,51 Expanding access to pulmonary specialty care by incorporating alternate care providers (e.g., pharmacists or registered nurses) into the workforce may also improve care quality.52,53 Alternatively, collaborative approaches between specialty care providers and PCPs, such as those evaluated in project ECHO, could be leveraged to improve COPD care quality.54 Finally, aside from these pulmonary-based approaches, the behavioral health economics literature also suggests a role for initiatives (e.g., nudge strategies) that empower PCPs to independently deliver high quality outpatient COPD care.55
Our study has a number of limitations. First, although we included many patient- and PCP-level characteristics hypothesized to affect outpatient COPD care quality, unmeasured confounders may have influenced our results. We were unable to ascertain if our measures of care quality were indicative of care provided by the PCP, the pulmonologist, or both. We also were unable to determine which pulmonary visits resulted directly from PCP referrals. As a result, it is possible that pulmonary referral could be a marker of higher care quality provided by primary care clinicians. Our findings should thus be considered hypothesis generating. Second, we a priori chose our primary composite outcome with the goal of assessing overall outpatient COPD care quality. We recognize that some aspects of outpatient COPD care may be more important than others toward the achievement of optimal outcomes. Third, although patients in our study have demographic characteristics similar to the U.S. population of patients with COPD, our study occurred within a single, academically affiliated health care system in an urban center and, thus, our results may not be generalizable. Fourth, we relied upon chart abstraction to determine most patient-level exposures and outcomes. Providers may not have documented every care process that patients received (e.g., counseling). Patients may not have taken documented medications as prescribed. In addition, we were unable to capture important aspects of care such as patient preference. Fifth, we were unable to directly ascertain any care that occurred outside our health care system (e.g., exacerbations or spirometry), which could have resulted in misclassification. In addition, we performed multiple comparisons, which increases the chance of type I error.
Finally, we acknowledge that our study evaluates care processes that occurred more than 8 years ago. Despite this limitation, current COPD guidelines continue to recommend most of the care quality measures assessed in our study.56 Although specific inhaled treatment recommendations have changed as new evidence has emerged, the indications for inhaled long-acting bronchodilators are similar. Moreover, recent publications suggest that the quality of COPD care has not changed substantially over the past 10 years,25,26,28,45,57 and we believe that our findings remain relevant to the outpatient management of patients with COPD.
Conclusions
In a single center study of adults aged ≥ 40 years with clinically diagnosed COPD, we found that the overall quality of outpatient COPD care was suboptimal. The completion of a pulmonary referral was associated with higher receipt of evidence-based outpatient COPD quality measures. Future studies should investigate if the engagement of pulmonary specialty providers in the population management of patients with COPD can improve the quality of outpatient COPD care.
Acknowledgements
Author contributions: TLK had full access to the data and assumes responsibility for the integrity and accuracy of data analysis. TLK, JW, LJS, LMD, KD, JRC, DHA, and LCF contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. NS and AD provided the source data and contributed substantially to data interpretation and writing the manuscript.
Declaration of Interest
Dr. Keller, Dr. Wright, Dr. Donovan, Dr. Spece, Dr. Duan, Ms. Sulayman, Ms. Dominitz, and Dr. Curtis have nothing to disclose. Dr. Feemster reports receiving consulting fees from the National Center for Quality Assurance. Dr. Au reports serving on the data monitoring committee for Novartis. No additional conflicts of interest relevant to this article were reported.