Running Head: Sedentary Time, Activity and COPD Quality of Life
Funding Support: This work was funded by grants K24HL138150 and R01HL140486, from the National Heart, Lung, and Blood Institute at the National Institutes of Health (PI: R Benzo). This publication was also made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Date of Acceptance: November 9, 2021 │ Published Online: November 15, 2021
Abbreviations: chronic obstructive pulmonary diseases, COPD; Global initiative for chronic Obstructive Lung Disease, GOLD; Chronic Respiratory Questionnaire, CRQ; percentage of forced expiratory volume in 1 second, FEV1%; minimal clinically important difference, MCID; metabolic equivalents, METs; least absolute shrinkage and selection operator, LASSO; standard deviation, SD, hospitalization, Hx; modified Medical Research Council, mMRC; adjusted, adj; confidence interval, CI; difference, diff
Citation: Driver CN, Novotny PJ, Benzo RP. Differences in sedentary time, light physical activity, and steps associated with better COPD quality of life. Chronic Obstr Pulm Dis. 2022; 9(1): 34-44. doi: http://doi.org/10.15326/jcopdf.2021.0230
Online Supplemental Material: Read Online Supplemental Material (198KB)
Introduction
Reducing time spent in sedentary behaviors is increasingly being recognized as an important target for patients with chronic obstructive pulmonary disease (COPD). Physical activity is the strongest predictor of mortality and sedentarism is an independent predictor of mortality among COPD patients.1-3 Many studies have explored interventions to optimize physical activity in this population, but with little success.4 While changing physical activity patterns is difficult in the general population, patients with COPD face additional disease-specific barriers including dyspnea and oxygen therapy. Because this population is highly sedentary, recently there has been a shift of focus from promoting participation in exercise to the more realistic goal of replacing sedentarism with light physical activity in the form of activities of daily living.5,6
The Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines recommend physical activity for all stages for COPD; they state, “Patients should be encouraged to increase the level of physical activity although we still don’t know the likelihood of success.”7 While pulmonary rehabilitation has been shown to increase exercise capacity and health status in COPD, there is limited evidence that pulmonary rehabilitation leads to lasting improvement in physical activity, and conversely, sedentary time, likely due to a lack of lasting behavioral modification.8 Thus, we know that patients with COPD do not get enough physical activity and increasing physical activity in this population is important to curb decay in functional status. The best interventions to increase physical activity are currently unknown, and even targets for activity behavior change are currently not established.
This analysis investigates the association between the primary aspects of patient-perceived quality of life and direct, gold-standard measures of physical activity, sedentary time, and steps using a validated activity monitor. We provide initial estimates for the magnitude of differences in sedentary time, light physical activity, and number of steps per day that associate with an unquestionable improvement in perceived quality-of-life in multiple domains. Our results inform clinicians and researchers on differences in daily activity related to better quality-of-life in COPD.
Methods
Data Source
We merged baseline de-identified data from 11 clinical trials of patients with moderate and severe COPD and experiencing respiratory symptoms. All trials were conducted by the Mindful Breathing Laboratory at the Mayo Clinic in Rochester, Minnesota. Trials were included if they measured physical activity or sedentary time with an accelerometer and measured quality-of-life with the Chronic Respiratory Disease Questionnaire (CRQ) at baseline. All of the studies measured the effects of non-pharmacologic interventions, such as pulmonary rehabilitation, motivational interviewing-based health coaching, breathing awareness practices, and coordinated family-care team communication, on outcomes such as hospital readmissions9 and lifestyle (self-management), emotional, and cognitive function.10 While the studies measured the effects of different interventions on different outcomes, we used only pre-interventional baseline data to ensure internal validity of the sample. All patients were in a stable condition, free of recent exacerbations, during baseline measurements. All trials used the same definition of disease severity (moderate to severe) using the GOLD guidelines.7 No institutional review board approval was required as the use of de-identified data was deemed “Not Human Subjects” research.
The data were merged between the trials because the variables of interest (e.g., CRQ and activity) were measured before any interventions were enacted. Trials included patients with a diagnosis of moderate to severe COPD or interstitial lung disease. Inclusion criteria about age differed between the trials, with some recruiting patients above age 18 and others recruiting patients above age 40, but the average ages of the populations did not differ between the trials. Overall, the populations from which the samples were drawn for each study were homogeneous with each other.
Sample Selection and Demographics
Individuals in the databases with no CRQ data and with no activity data (demographics only) were removed from the analysis. The demographic variables from all the studies were simplified to enable a merge between multiple datasets. Race was recoded as White, Black, or other. Ethnicity indicated if the patient was Hispanic or Latino or not. Education was coded as a binary variable between high school graduate or more education, or some high school or less. Marital status was simplified to married or not married.
Six of the 11 trials included questions about exacerbations and hospitalizations within the past year. These data, along with percentage of forced expiratory volume in 1 second (FEV1%), were used to classify the corresponding patients into GOLD groups A through D.11
Chronic Respiratory Disease Questionnaire
The CRQ is the instrument most commonly used to assess quality of life in clinical trials for chronic lung disease. All studies used the Self-Administered Standardized Format of the questionnaire. The questionnaire items are meaningful to the day-to-day life of patients with COPD and include patient perceptions of both physical and emotional health through evaluation of dyspnea, fatigue, emotional function, and mastery (a feeling of control over the disease).
Each domain includes 4 to 7 items each graded on a 7-point Likert scale (range 1 to 7). Higher scores indicate higher health-related quality of life.12 The total score of the CRQ ranges from 1 to 28 and is the sum of the 4 subdomain scores. The method of development of the CRQ allows the subdomains to be interpretable on their own. More, the developer discourages use of the total score and recommends using the individual subdomains.13 The instrument has been validated13 and is used internationally in the care and study of patients with COPD. The minimal clinically important difference (MCID) in the CRQ is a difference in score of 0.5 points on a 7-point scale.14
Accelerometers and Physical Activity Definitions
Physical activity and sedentary time were measured by a multisensor armband (SenseWear Pro Armband; BodyMedia, Inc., Pittsburgh, Pennsylvania). The SenseWear Pro armband is worn on the upper right arm over the biceps and triceps muscles. The device is a triaxial accelerometer capable of recording number of steps per day, and metabolic equivalents (METs), an indicator of energy expenditure. The SenseWear Pro has been validated as one of the most accurate devices to measure physical activity in patients with chronic diseases such as COPD (correlation 0.65 compared to indirect calorimetry in GOLD 3/4).15 Days were counted if the monitor was worn for at least 20 hours per day. Average physical activity was calculated if at least 3 days of activity monitor data were available in the 7 days of baseline monitoring.
Sedentary time was defined as activity <1.5 METs, light physical activity was defined as activity ≥1.5 and <3 METs, moderate physical activity was defined as activity ≥3 and <6 METs, and vigorous physical activity was defined as ≥6 METs, according to the 2018 edition of the Physical Activity Guidelines for Americans.16 Average physical activity for some of the trials were originally calculated using the 2008 definitions of physical activity behavior (<2 METs for sedentary time, ≥2 and <4 METs for light physical activity, ≥4 and <6 METs for moderate physical activity, and ≥6 METs for vigorous physical activity).17 For these patients, we recalculated the average time spent in each of the activity levels from the accelerometer outputs using the 2018 guidelines. We omitted physical activity averages for patients for whom the raw data were not available to do the recalculations. Physical activity and sedentary time were only measured during waking hours.
Statistical Analyses
Patients were described by demographic and disease characteristics at baseline. Spearman rank correlations between the CRQ subdomain scores and physical activity measures assessed bivariate relationships. Normality was assessed visually; steps, sedentary time, and light physical activity data were transformed using square root transformations before inclusion in the multivariable analysis.
Multivariable linear regression was used to quantify the association between CRQ subdomain scores (dyspnea, fatigue, mastery, and emotions) and the transformed physical activity measures (steps, sedentary time, and light physical activity) according to the following models:
√(Physical Activity) ~ β0 + β1 CRQ + β2 Age + β3 FEV1% + … + Bx OtherCovariates
The dependent variable was the square root of physical activity. The independent variable was the CRQ subdomain. All models were adjusted for age and FEV1%. Covariates with prevalence <0.5% or >90% were excluded from selection. Other covariates were selected using shrinkage methodology (least absolute shrinkage and selection operator, [LASSO], with the R package glmnet18) choosing a value for lambda that was within 1 standard error of the minimum value to balance accuracy and simplicity.
Multivariable linear regression models were used to predict the average difference in activity between 2 individuals with a difference of 1.0 point (double the MCID of 0.5 points) in the corresponding CRQ subdomain. The robustness of the estimates was tested using bootstrapping of the models. All analyses were conducted19 using R version 3.5.3.
Results
Sample Selection and Summary Data
A total of 467 patients from trials using the SenseWear Pro accelerometer were included in the original sample. Of these patients, 13 patients were missing all CRQ data, and 66 patients were missing physical activity data due to incomplete wear time. Overall, 85.9% of patients wore the monitor for at least 20 hours per day for at least 3 of 7 days for the baseline period across the trials. Accelerometer data from 137 patients was not in a format in which the data could be recalculated for physical activity level cut-offs according to the 2018 guidelines. With additional missingness of the FEV1%, marital status, and education covariates, this gave a final total sample of 241 patients for models including sedentary time, 243 for models including light physical activity, and 292 patients for models including steps. Paired t-tests were used to compare the original and final samples and found no significant differences in the demographic characteristics between the removed and retained participants, except FEV1%. Patients removed from the sample had, on average, higher FEV1% at 47.8% compared to 41.2% (p=0.04), but this difference is not clinically meaningful (MCID for FEV1% is 12%).
Summary data from baseline are shown in Table 1. This population had advanced COPD; average FEV1% was 41.2% (standard deviation [SD] 16.8%). The majority of patients (62.7%) were levels 3 or 4 on the modified Medical Research Council (mMRC) dyspnea score. Of the 163 patients for whom we had data, 57 (35.0%) had at least 2 exacerbations or a hospitalization within the past year, meeting the criteria for GOLD Group C (11 patients, 6.7%), or Group D (46 patients, 28.2%). Most patients were GOLD Group B (83 patients, 50.9%).
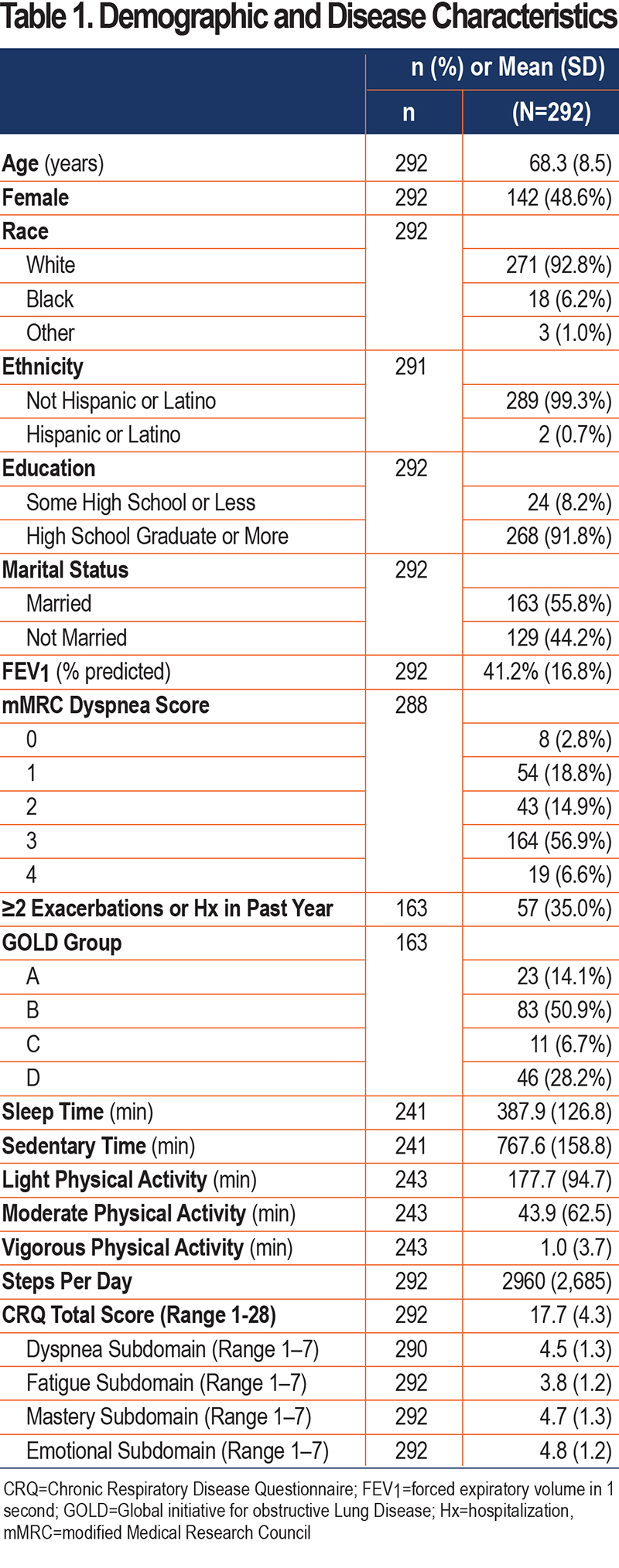
Patients on average achieved 2960 steps per day, but there was a wide range (SD 2685) of stepping activity. The majority of time was spent in sedentary behaviors (767.6±158.8 minutes per day), and patients achieved on average 177.7±94.7 minutes of light physical activity per day. Because average moderate (43.9±62.5 minutes per day) and vigorous physical activity (1.0±3.7 minutes per day) were very low with many patients obtaining no minutes in moderate or vigorous physical activity per day, we chose to focus the multivariable analyses on sedentary time, light physical activity, and steps.
Multivariable Analysis
The results of the multivariable analyses are shown in Table 2. All models adjusted for age and FEV1%. LASSO regression did not select sex for inclusion in any of the models. Marital status was included in all models using steps per day and the model regressing light physical activity on CRQ mastery. Education was included in the models including steps per day regressed on CRQ fatigue, mastery, and emotions. These decisions to include adjustment by sex, marital status, and education were determined by LASSO. Individuals with 1-point better dyspnea scores averaged 24.5 (8.4–40.5) minutes less sedentary time per day. Individuals with 1-point better dyspnea and fatigue scores averaged 21.5 (10.9–32.3) minutes or 12.5 (2.0–23.2) minutes more light physical activity per day, respectively. Individuals with 1-point better dyspnea, fatigue, mastery, and emotions scores averaged 762 (546–984), 579 (351–814), 418 (207–636), and 392 (157–634) more steps per day, respectively. The full model results can be found in the online supplement e-Table 1.
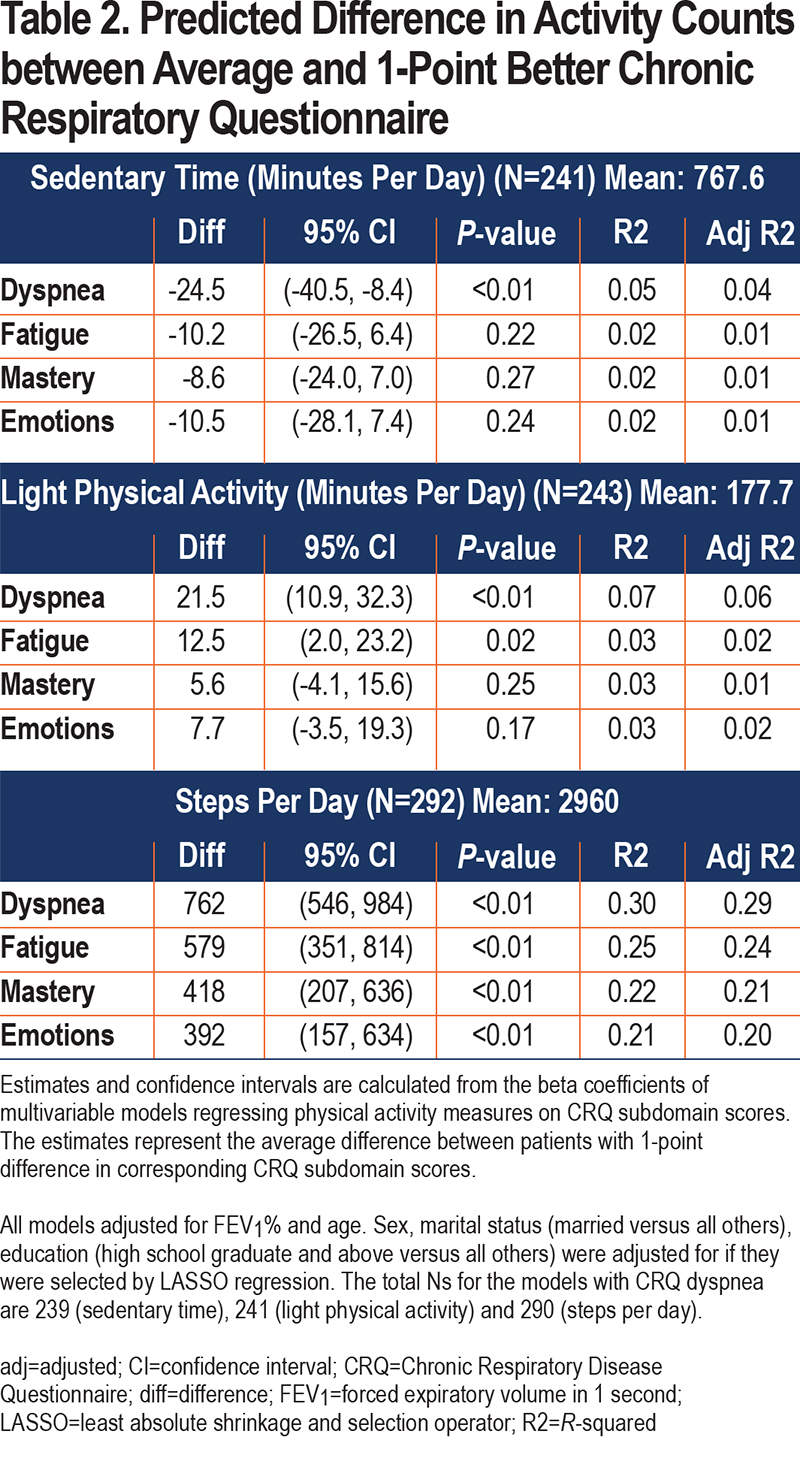
Estimates from the bootstrapped models were very similar to the results from the main analyses. They are provided in online supplement e-Table 2 along with the results of the bivariate analysis in online supplement e-Table 3.
Discussion
Although the importance of reducing sedentary behavior and increasing physical activity in the management of COPD is well-established, there are currently no guidelines on recommendations for physical activity behaviors, particularly sedentary time. Understanding sedentarism in severe COPD is essential to improving patient care and all health outcomes, and it has been shown that physical activity and sedentary time are more related to disease prognosis than lung function.20
In an initial attempt to close this gap, we used data from one of the most widely validated accelerometers and a common, well-validated quality-of-life questionnaire in patients with COPD. We provide quantitative estimates of the association between physical activity and quality-of-life measures, namely that decreasing sedentary time about 25 minutes per day and /or increasing light physical activity for about 22 minutes per day, may associate with improved symptoms of dyspnea and fatigue, or increasing steps by about 750 per day may associate with better physical and emotional quality of life. Other studies report 2000–6000 average steps per day among patients with COPD across disease severities with most studies reporting 3000–4000 on average2,21-30; our average of 2960 steps per day is on the lower end, but consistent with this range and reflects a population with more severe disease (our average FEV1% of 41% is lower than many studies).
While general population trends in sedentary behavior show that older adults have become increasingly more sedentary in the past 2 decades,31 patients with COPD have different patterns of activity than the general older adult population. Patients with COPD spend the majority of time in sedentary behaviors,32 and achieve none to very little moderate to high intensity activity.33-35 There is an emotional response to dyspnea that is important to recognize in these patients that distinguishes them from those with other chronic diseases.36
However, recommendations for sedentary behaviors are included in no COPD-specific clinical practice guidelines.37 Guidance for physical activity in COPD generally mimics the guidelines in the general adult population (30 minutes per day or 150 minutes per week of moderate to vigorous physical activity and strength training), but these guidelines are unachievable to many patients with COPD, especially those with more advanced disease. In our sample, the median daily average of moderate physical activity was 43.9 minutes, with 28.7% of patients achieving fewer than 10 minutes of moderate physical activity per day and 68.3% of patients achieving 0 minutes of vigorous physical activity per day. The guidelines do not account for limitations imposed by symptoms of dyspnea, fatigue, negative emotions, and comorbidities. There is evidence that with more advanced disease, people do fewer chores and increase screen time.34 Sports and exercise are low across all disease states, even in mild COPD.34 These observations make recommendations around increasing chores and other light physical activities potentially more actionable for patients as they progress in their disease. Establishing guidelines that include COPD-specific goals is of utmost importance to the future care of these patients.
To that end, the MCIDs in sedentary time and physical activity in COPD have yet to be established. Having MCIDs in sedentary time and physical activity would provide a link between the health-related quality-of-life outcomes, which arguably are the most important outcomes from the patient’s perspective, and mortality and prognosis, which are generally important outcomes from the clinician’s perspective.38 Because we had less than 50% correlation between the CRQ and physical activity variables (eTable 3), however, we could not use the CRQ as an anchor for establishing an MCID in physical activity in our analysis.
One previous study attempted to define an MCID for steps in COPD24 and our data highly coincide with their findings. The correlation between CRQ dyspnea and steps was 0.38 in our data, consistent with a 0.29 correlation found by Demeyer et al24 which precluded CRQ dyspnea from serving as an anchor for the MCID in their calculations. These authors found the one-half population standard deviation to be 1121 steps, which is consistent with our data’s distribution-based estimate of baseline steps of one-half the standard deviation of 1364 steps. Finally, our finding of a 762 steps per day difference based on a 1-point difference in CRQ dyspnea is near to that study’s MCID using the standard error of measurement of 599 steps.24 Overall, our data are very consistent with the estimates for the MCID in steps defined by the previous study as between 600 and 1100 steps per day.
Our multivariable results for associations between sedentary time and light physical activity were meaningfully associated with the physical subdomain scores for dyspnea and fatigue but were not associated with emotions and mastery. Number of steps per day, however, was significantly associated with all 4 subdomains, and has higher strengths of association with the CRQ subdomains than physical activity (eTable 3). It may be that activity that is directed and goal-oriented, such as steps, is more related to emotional subdomains than general physical activity movements (or lack thereof, i.e., sedentary time). It is important to recognize the fact that average daily steps were significantly associated with emotions and mastery (self-management), with approximately 400 steps associated with 1-point positive difference in these scores. As emotions and mastery have the ability to affect all outcomes in COPD,11 the more modest associated differences propose a hopeful prospect. In the absence of targetable interventions to improve self-management and emotions in COPD, our results provide a starting point for setting goals of behavioral change in patients particularly deficient in these aspects.
Lastly, although the focus of our analysis was on quality of life, the minimum difference in activity we found may have an impact on hospitalization and mortality in this population as well. Twenty-five minutes per day is 2.9 hours per week. The Copenhagen Heart Study showed that for patients with COPD, a level of physical activity equivalent to walking or cycling (light physical activity) for 2 hours per week or more was associated with a 30%–40% reduction in hospitalizations and mortality.39
Overall, the strengths of this study are that we used data from a large sample of well-characterized patients with moderate and severe COPD using objective measurement of physical activity and a well-validated quality-of-life questionnaire. Previous studies have looked at strengths of associations between these variables, but the results were not quantified, and activity was measured using self-report.40 A systematic review of sedentary time and disease found sedentary time was quantified using self-report in all but 1 study.41 Objectively measured physical activity is important to quality study design. All patients were in a stable condition, free of recent exacerbations at the time their baseline data were collected. There was also a high degree (85.9%) of compliance for wearing the accelerometers.
Limitations include generalizability. All studies were performed among a predominantly White, non-Latino population at a single tertiary care center in the upper Midwest; results may not be generalizable to other populations. Due to a lack of pre-and post-measurements and <0.5 correlation between CRQ scores and physical activity, we could not use our data to put forth estimates for the MCID. Instead, we chose to calculate the difference in physical activity between patients in our population who differ by 1 point, or twice the MCID for the CRQ, to quantify an unquestionable difference. Although there were different inclusion criteria for some of the studies with regard to age, the average age of the studies was the same (mean 68 years). This is not surprising because COPD is a disease that usually develops in the geriatric population. Another limitation is the low R-squared and adjusted R-squared values for the models. While these may be seen as non-representative models for predicting physical activity, it is not uncommon for models predicting behavior to have low R-squared values.42 Activity behavior has many biopsychosocial and environmental factors that are difficult to measure and model, and our primary aim was not to generate a highly predictive model, but to quantify the associations between physical activity and common COPD symptoms. The SenseWear Pro armband is no longer available; while this may represent a limitation to reproduce our results, the monitor has been validated against other monitors currently in the market. This was a secondary analysis of previously collected data, with limitations due to missingness. For example, one variable we could not account for is the time of year when activity was measured. This has been shown to influence physical activity levels in COPD.43 Missingness in variables from different studies led to a smaller final sample size and has the potential to introduce selection bias due to differences between individuals who were retained or removed. However, paired t-tests between removed and retained individuals were not significant except for the variable FEV1%. On average, patients who were retained appeared to have slightly more severe disease (lower FEV1%), but these differences are not clinically meaningful. Thus, it is unlikely to have impacted the results. We furthermore acknowledge that our lower limit of wearing the activity monitor to be included in the models was 3 days, which is less than the 4-5 days currently recommended.25
Of course, because our data are cross-sectional, we are reporting associations and cannot conclude any causal relationships between these variables (we cannot imply, based on our results, that by decreasing sedentary time by 25 minutes "will" meaningfully improve dyspnea) and results should be tested in future prospective studies. One study of patients with advanced COPD found improvement in CRQ scores with increased physical activity, but physical activity was measured using self-report 44; prospective studies with objective measurements would greatly add to the evidence base.
In summary, we provide initial estimates for differences in sedentary time, light physical activity, and steps that correspond to unquestionable differences in corresponding CRQ scores. We found that decreasing sedentary time by about 25 minutes and increasing light physical activity by about 22 minutes per day associates with better symptoms of dyspnea and fatigue or increasing by about 750 steps per day associates with both physical and emotional quality of life, in patients with advanced COPD.
Acknowledgements
Author contributions: CND had full access to the de-identified data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CND, PN, and RB contributed substantially to the study design, data interpretation, and writing of the manuscript.
Data Sharing Statement: Unpublished data is available upon request by contacting the corresponding author.
Declaration of Interest
C. Driver, P. Novotny, and R. Benzo report no conflicts of interest.