Running Head: COPD Assessment Test and Exacerbation Risk: IMPACT
Funding support: This study was funded by GlaxoSmithKline (GSK study number CTT116855; NCT02164513). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report.
Date of Acceptance: December 7, 2021 │ Published Online: December 22, 2021
Abbreviations: InforMing the PAthway of COPD Treatment, IMPACT; fluticasone furoate, FF; umeclidinium, UMEC; vilanteral, VI; chronic obstructive pulmonary disease, COPD; COPD Assessment Test, CAT; Global initiative for chronic Obstructive Lung Disease, GOLD; St George’s Respiratory Questionnaire, SGRQ; forced expiratory volume in 1 second, FEV1; intent-to-treat, ITT; adverse events of special interest, AESI; standard deviation, SD; body mass index, BMI; inhaled corticosteroid, ICS; long-acting muscarinic antagonist, LAMA; fractional polynomial, FP; long-acting beta2-agonist, LABA; akaike information criterion, AIC
Citation: Thomashow B, Stiegler M, Criner GJ, et al. Higher COPD Assessment Test score associated with greater exacerbations risk: a post hoc analysis of the IMPACT trial. Chronic Obstr Pulm Dis. 2022; 9(1): 68-79. doi: http://doi.org/10.15326/jcopdf.2021.0259
Online Supplemental Material: Read Online Supplemental Material (223KB)
Note: Data in this paper were presented in part at the American Thoracic Society 2019 conference as a poster presentation. The poster abstract was published in: Am J Resp Crit Care Med. 2019;199:A1591. doi: https://doi.org/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A1591.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease characterized by airflow limitation and persistent respiratory symptoms. It is a major cause of morbidity and mortality worldwide and as such is associated with substantial clinical and economic burden. To address these burdens, the 2021 Global initiative for chronic Obstructive Lung Disease (GOLD) strategy document recommends COPD management to include reducing symptoms, improving health status and exercise tolerance, and reducing the risk of exacerbations to ultimately prevent disease progression and reduce mortality.1-3
The COPD Assessment Test (CAT) is a validated, disease-specific, patient-completed questionnaire that is simple and quick to perform.4 It comprises 8 items which assess: cough, phlegm, chest tightness, breathlessness when going uphill or upstairs, any activity limitation at home, confidence leaving home, sleep, and energy. Each item is scored 0–5 to provide an overall total score of 0–40. Higher CAT scores indicate increased disease burden and worse health status.4 The CAT score has also been incorporated into the GOLD strategy document to help guide initial pharmacological treatment of COPD.1
A history of frequent exacerbations has been shown to be predictive of future exacerbations in patients with COPD.5-7 The CAT and other tools such as the St George’s Respiratory Questionnaire (SGRQ) quantify the impact of COPD symptoms on the health status of patients. Elevated baseline CAT scores have been observed in patients with stable COPD with a history of frequent exacerbations,8 and the CAT and SGRQ have demonstrated their ability to predict future exacerbations.6,9,10 High baseline CAT scores have also been associated with shorter time-to-first exacerbation in patients with a history of exacerbations in the preceding year.11 However, there is a paucity of data evaluating the relationship between CAT score and treatment on the risk of future exacerbations.
In this post hoc analysis of the large-scale InforMing the PAthway of COPD Treatment (IMPACT) trial,12 we examined the relationship between baseline CAT score as a continuous variable and the efficacy and safety of fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) versus FF/VI and UMEC/VI.
Methods
Study Design
The IMPACT study (GSK study CTT116855; NCT02164513) was a 52-week, randomized, double-blind, parallel-group, multicenter Phase 3 study comparing single-inhaler triple therapy with FF/UMEC/VI with FF/VI or UMEC/VI dual therapy. Patients were randomized (2:2:1) to receive FF/UMEC/VI 100/62.5/25mcg, FF/VI 100/25mcg or UMEC/VI 62.5/25mcg, all administered once daily via the ELLIPTA dry-powder inhaler. The study design and primary results have been previously published.12,13
Study Population
Inclusion and exclusion criteria have been described previously.12,13 Briefly, eligible patients were ≥40 years of age with symptomatic COPD (CAT score ≥10 at screening), and either a forced expiratory volume in 1 second (FEV1) <50% of predicted normal values and ≥1 moderate or severe exacerbation in the previous year, or FEV1 50% to <80% of predicted normal values and ≥2 moderate or ≥1 severe exacerbation in the previous year.13 The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines and received approval from local institutional review boards or independent ethics committees. All patients provided written informed consent.
Endpoints
This post hoc analysis evaluated endpoints by baseline CAT score from the IMPACT intent-to-treat (ITT) population.12 Baseline CAT scores were assessed on the randomization visit (Day 1) of the study, approximately 2 weeks following the screening visit.13 CAT scores of 10–20 indicate medium impact of COPD on health status, scores of 20–30 and >30 indicate high and very high impact, respectively.14,15
Baseline characteristics were described by baseline CAT score subgroup (<20 and ≥20). Analysis of the efficacy of FF/UMEC/VI versus FF/VI and UMEC/VI using continuous CAT score at baseline was carried out for the following outcomes: annual rate of on-treatment moderate/severe exacerbations, change from baseline in trough FEV1 and percentage FEV1 responders (patient achieving a ≥100 mL increase from baseline in trough FEV1) at Week 52. Moderate exacerbations were defined as requiring treatment with antibiotics and/or oral/systemic corticosteroids, and severe exacerbations were defined as events resulting in hospitalization or death.
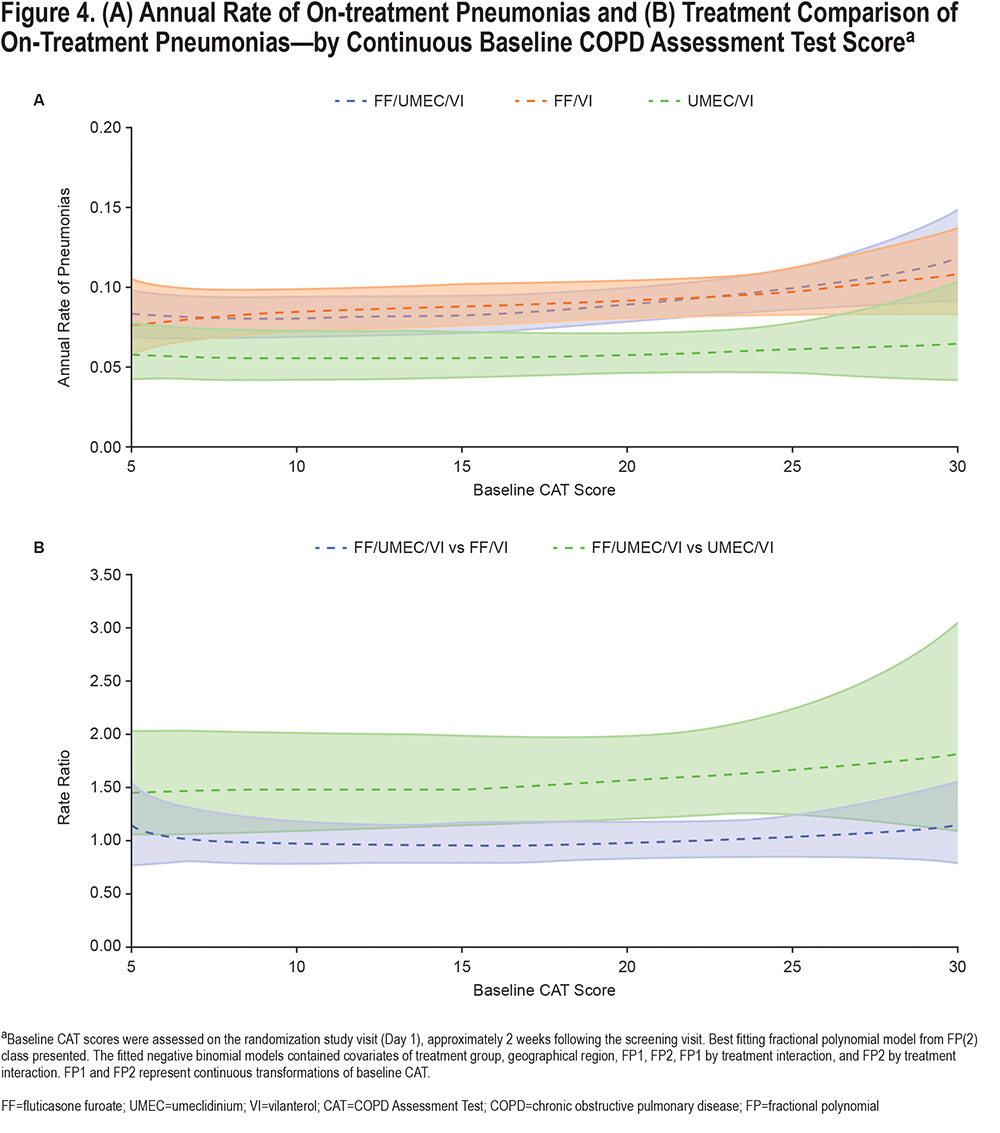
Safety endpoints included the post hoc assessment of annual rate of on-treatment pneumonias by continuous CAT score at baseline. Adverse events of special interest (AESI) by baseline CAT score subgroup (<20 and ≥20) were also evaluated. AESIs were defined using Standardized Medical Dictionary for Regulatory Activities Queries and allowed for a comprehensive review of safety data that is not limited to a specific preferred term.
Statistical Analyses
Fractional polynomials were used to model the relationship between CAT score as a continuous variable and the treatment outcomes. The selected best fitting model was plotted as CAT score versus moderate/severe exacerbation rate, trough FEV1, probability of FEV1 response, and annual rate of pneumonias in each treatment group. The non-fractional polynomial covariates included in each of the models mirror those covariates that were included in the analysis of those endpoints in the primary study and were defined a priori (please see figure footnotes for covariate details). Pointwise confidence bands for fractional polynomials are included to provide an indication of whether the difference in the estimates occurred by chance, they are not formal statistical tests with p values. Safety data were also summarized descriptively.
Results
Patients
Of the 10,355 patients randomized in the ITT population, 10,157 had baseline CAT score data available and were included within this analysis. Of these, 5952 (59%) had a baseline CAT score <20 and 4205 (41%) had a score ≥20. Demographics and baseline characteristics are shown in Table 1 and were generally similar across treatments within each CAT score subgroup, with some notable differences. Patients with a baseline score ≥20 were slightly younger and had lower post-bronchodilator FEV1 % predicted than patients in the <20 subgroup; a greater proportion of patients with a score ≥20 were current smokers.
Efficacy Endpoints
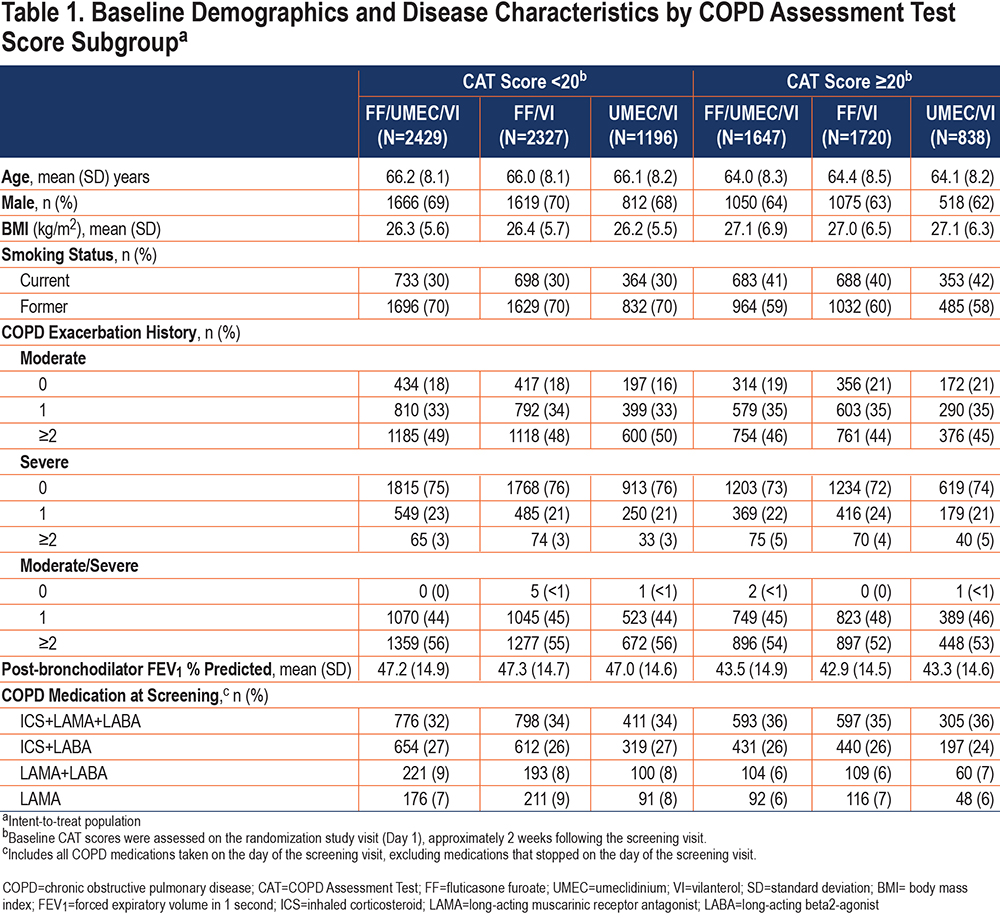
Annual Rate of On-treatment Exacerbations:
The annual rate of on-treatment moderate/severe exacerbations was higher in patients with higher baseline CAT scores (Figure 1).
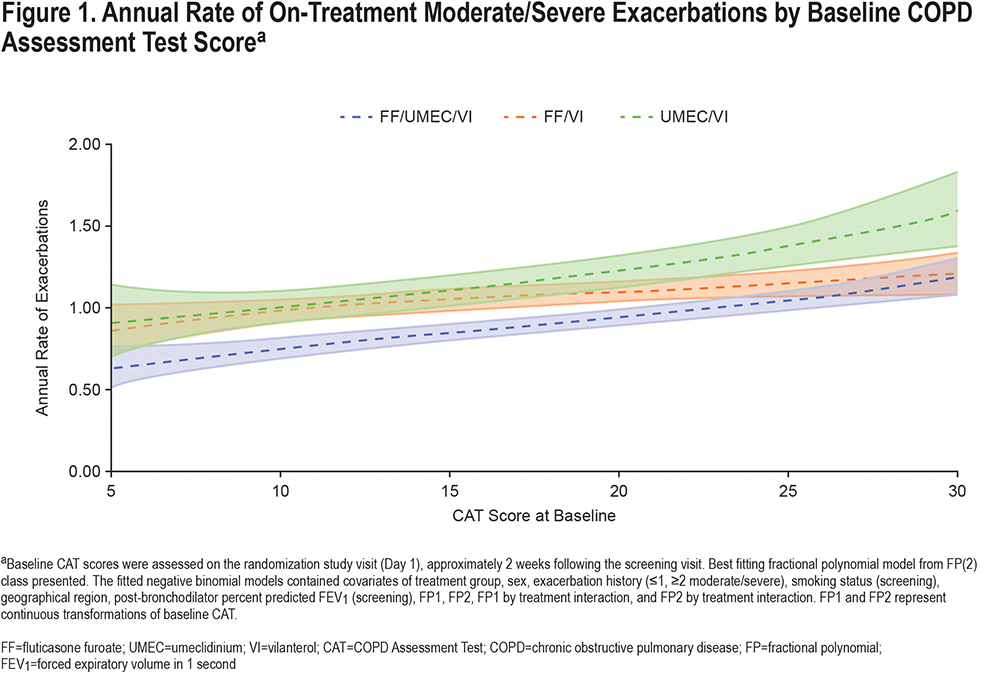
A consistent reduction in the annual rate of on-treatment moderate/severe exacerbations of similar magnitude was observed with FF/UMEC/VI compared with UMEC/VI (i.e., the effect of the inhaled corticosteroid [ICS] component), regardless of baseline CAT score. FF/UMEC/VI reduced on-treatment moderate/severe exacerbation rates versus FF/VI (i.e., the effect of the long-acting muscarinic antagonist [LAMA] component) at lower CAT scores, but at higher CAT scores (approximately above 25) the 95% confidence interval crossed 1 (Figure 2).
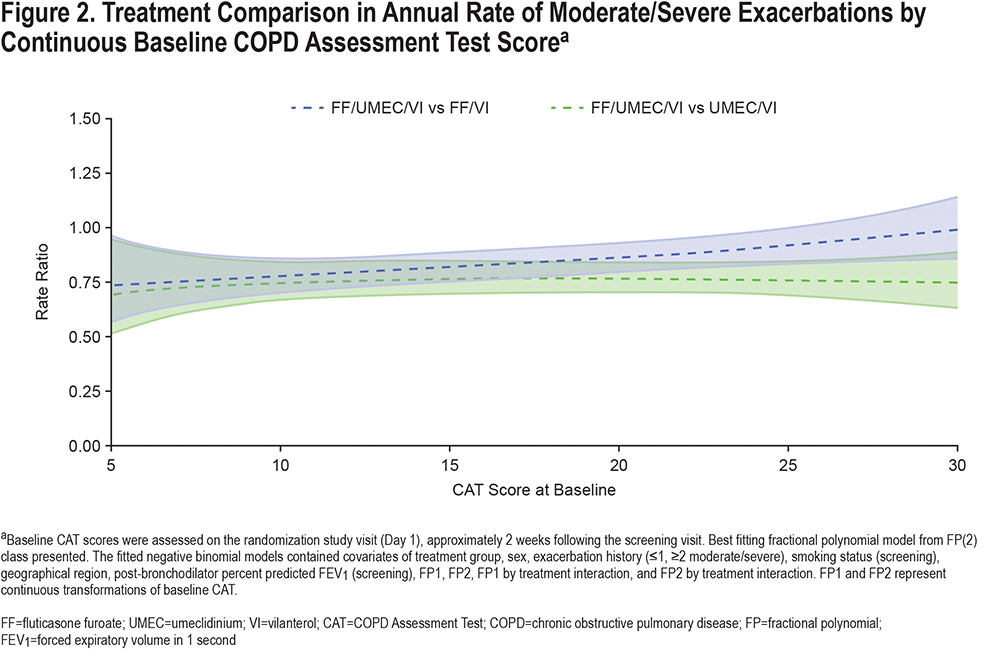
Lung Function:
Trough FEV1 at Week 52 remained consistent across all treatment groups, regardless of CAT score at baseline. Irrespective of baseline CAT score, improvements in trough FEV1 Week 52 were observed with FF/UMEC/VI compared with either FF/VI or UMEC/VI therapy (Figure 3).16
Safety
The annual rate of on-treatment pneumonias was marginally higher in patients with higher CAT scores across all treatment groups (Figure 4). The AESI profile of FF/UMEC/VI was similar to FF/VI and UMEC/VI in both CAT subgroups and no new safety signals were identified (Table S1in the online supplement). These results are consistent with the overall ITT population.12
Discussion
In this post hoc analysis of patients with COPD and a prior history of exacerbations enrolled in the IMPACT trial, patients with greater CAT scores (worse health status) at baseline experienced a higher rate of moderate/severe exacerbations during the 1-year treatment period. The benefit of the ICS component (i.e., FF/UMEC/VI versus UMEC/VI) was the same across the whole range of baseline CAT scores, but the benefit of the LAMA component (i.e., FF/UMEC/VI versus FF/VI) was less apparent at higher baseline CAT scores (above ~25), as shown in Figure 2. The risk of pneumonia appeared to be slightly higher at very high CAT scores in the ICS-containing treatment groups; however, the sparseness of the data, resulting in wider confidence intervals in this region, limit interpretation (Figure 4). Overall, the benefit-risk profile of FF/UMEC/VI versus UMEC/VI appears to be very similar in patients with low and high CAT scores.
The benefit of the LAMA component on reducing exacerbation rates diminished at CAT scores >20, but it is noteworthy that the benefit of the LAMA on trough FEV1 was also slightly lower with CAT scores in this range. This suggests that the lung function benefit and the exacerbation benefit are linked, as has been shown before.17 These observations are consistent with a similar analysis using baseline CAT to examine the benefit of UMEC/VI versus UMEC in which the symptomatic benefit of adding the long-acting beta2-agonist (LABA) was a little lower at higher CAT scores.18 A small study has also shown that higher baseline CAT score was a predictor of short-term ineffectiveness (defined as COPD exacerbations, need for additional treatment, and no improvement in functional parameters) for the LAMA tiotropium.19 Regardless of mechanisms, the clinical importance of these findings is that, in terms of exacerbation reduction, the benefit of triple therapy over ICS/LABA or LAMA/LABA is at least as great in patients with better preserved health status as in those in whom the disease impact is severe. Furthermore, in terms of the ICS component, this benefit in the less symptomatic patients does not come at a greater risk of pneumonia. Patients with worse health status (CAT score ≥20) at baseline experienced a higher rate of moderate/severe exacerbations, corroborating findings from other studies that have identified an association between higher CAT score and a greater exacerbation risk.8,11 Furthermore, higher CAT scores in the period after an exacerbation have also been shown to predict risk of recurrence, hospitalization, and death.10,20,21 In a study that followed 45 patients admitted to the hospital for an exacerbation of COPD, those who re-exacerbated within 3 months had higher CAT scores during their first admission compared with patients who did not.22 Our results greatly strengthen the evidence for a relationship between CAT score and rate of COPD exacerbations, since the previously noted studies, each of which recruited less than 600 patients, whereas this analysis of the IMPACT trial included 10,157 of the 10,355 patients in the ITT population.
Patients with a baseline CAT score ≥20 were slightly younger, had worse lung function, and were more likely to be current smokers than those with a baseline score <20. This association between current smoking status and higher CAT and SGRQ scores in patients with COPD is consistent with previous reports.23 There was only a weak correlation between CAT score and lung function,24 indeed in this analysis there was a slight trend for better on-treatment FEV1 in patients with worse baseline CAT score (Figure 3A). The benefit of FF/UMEC/VI over the other 2 therapies, however, was generally consistent over the range of baseline CAT scores (Figure 3B).
Some limitations of this investigation should be considered. For instance, these analyses were conducted post hoc. The study also enrolled patients with a prior history of exacerbations (and, therefore, were at risk of further exacerbations), which limits the generalizability of the findings to patients of this type. Nevertheless, the IMPACT study was a large, prospective COPD clinical trial in which patients were well characterized at baseline, providing an extensive and robust dataset for these analyses.
In conclusion, in this population of patients at risk of COPD exacerbations, patients with worse health status at baseline experienced a higher rate of exacerbations, confirming that CAT is predictive of exacerbation risk. Regardless of CAT score, treatment with FF/UMEC/VI reduced exacerbation rates versus FF/VI and UMEC/VI. While pneumonia rates increased slightly at the highest CAT scores in patients receiving ICS-containing therapy, the overall safety profile was similar across the range of CAT scores studied in this analysis. Overall, these results indicate that single-inhaler triple therapy provides treatment benefit over dual therapy in patients with COPD and at risk of exacerbations regardless of symptom burden severity.
Acknowledgements
Author contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. P Jones takes responsibility for the integrity of the work as a whole. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All authors contributed to data analysis and interpretation. GJ Criner, MT Dransfield, and DMG Halpin also contributed to the acquisition of data and data analysis and interpretation. DA Lipson and M Tabberer also contributed to study conception and design, and data analysis and interpretation. All authors contributed to the writing and reviewing of the manuscript and have given final approval for the version to be published.
Data availability: Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com
Declaration of Interest
All authors report other and non-financial support from GlaxoSmithKline (GSK) (funding the study and funding medical writing support by Katie Baker and Eloise Morecroft at Fishawack Indicia Ltd, United Kingdom). Marjorie Stiegler, Dawn Midwinter, and David A. Lipson are GSK employees and hold stock/shares in GSK. Paul Jones and Maggie Tabberer were employed by GSK at the time of the study and hold stocks/shares in GSK. Gerard J. Criner has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, CSA Medical, Eolo, GSK, HGE Technologies, Novartis, Nuvaira, Olympus, Pulmonx, and Verona. Mark T. Dransfield is the editor-in-chief for Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation and has received personal fees and contracted clinical trial support from AstraZeneca and GSK, and personal fees from Teva and Pulmonx. MeiLan K. Han reports personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, United Therapeutics, Medscape, and Integrity. She has received either in-kind research support or funds paid to the institution from the National Institutes of Health, Novartis, Sunovion, Nuvaira, Sanofi, Astrazeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association. She has participated in data safety monitoring boards for Novartis and Medtronic with funds paid to the institution. She has received stock options from Meissa Vaccines. David M.G Halpin reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, and Pfizer, and non-financial support from Boehringer Ingelheim and Novartis. Peter Lange has received personal fees from GSK, AstraZeneca, and Boehringer Ingelheim, and grant support from Boehringer Ingelheim and GSK. Fernando J. Martinez reports personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech/Roche, GSK, Sunovion, and Teva, non-financial support from ProterrixBio, and other support from AstraZeneca, Boehringer Ingelheim, ProterrixBio. Dave Singh reports personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Theravance, and Verona, and grant support from AstraZeneca, Boehringer Ingelheim, Chiesi, Glenmark, Menarini, Mundipharma, Novartis, Pfizer, Pulmatrix, Therevance, and Verona. Dave Singh is supported by the National Institute for Health Research Manchester Biomedical Research Centre. Robert A. Wise has received personal fees from Boehringer Ingelheim, Contrafect, AstraZeneca, GSK, Merck, Verona, Mylan/Theravance, Propeller Health, Novartis, Bristol Myers Squibb, Galderma, Kiniksa, ChimiRx, and PureTech and grant support from Boehringer Ingelheim, AstraZeneca, Sanofi, Verona, and GSK. Byron Thomashow has taken part in advisory boards for AstraZeneca, Boehringer Ingelheim, and GSK, and has received personal fees for consultancy from GSK. ELLIPTA is owned by or licensed to the GSK Group of Companies.
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Katie Baker, at Fishawack Indicia Ltd., United Kingdom, part of Fishawack Health, and was funded by GSK.