Running Head: Disparities in Hospitalized COPD Exacerbations
Funding Support: Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM104938. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Date of Acceptance: January 12, 2022 │Published Online: January 27, 2022
Abbreviations: chronic obstructive pulmonary disease, COPD; American Indian, AI; non-Hispanic White, NHW; intensive care unit, ICU; odds ratio, OR; confidence interval, CI; diabetes mellitius, DM; University of Oklahoma Medical Center, OUMC; International Classification of Diseases, 9th revision, ICD-9; electronic health record, EHR; computed tomography, CT; length of stay, LOS; body mass index, BMI; area deprivation index, ADI; interquartile range, IQR; standard deviation, SD; reference, ref; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC
Citation: Wu H, Rhoades DA, Chen S, et al. Disparities in hospitalized chronic obstructive pulmonary disease exacerbations between American Indians and non-Hispanic Whites. Chronic Obstr Pulm Dis. 2022; 9(2): 122-134. doi: http://doi.org/10.15326/jcopdf.2021.0246
Online Supplemental Material: Read Online Supplemental Material (157KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and one of the top 3 causes of death worldwide.1 Cigarette smoking is the most common risk factor for COPD, and type 2 diabetes mellitus (DM) and obesity are common comorbidities.1 COPD has only rarely been studied among American Indian (AI) populations, despite their having the highest prevalence of smoking, 2-5 diabetes,6,7 and obesity.8,9 Many AI communities are in medically underserved areas,10 with reduced access to specialty care. AIs are, therefore, likely to have a high risk of poor COPD outcomes. Although COPD heath disparities in the United States have been reported for non-Hispanic White (NHW) and African American patients,11-15 no published research on COPD health disparities has included AI persons.
COPD research of any kind among AI populations is also scarce. The most recent report of COPD prevalence among AIs in the United States was in 2011, when the prevalence (11%) was higher among AIs than among any other racial group in the United States.16 We recently provided one of the few descriptive studies of COPD exacerbations among AI persons who had emergency department visits or were hospitalized with COPD,17 finding high prevalence of comorbidity and need for intensive care.
Oklahoma has the highest percentage by population of AIs affiliated with federally recognized tribes in the country.18 Furthermore, Oklahoma has a very high COPD prevalence and mortality.19 We conducted a single-center retrospective pilot study to investigate COPD health disparities between AIs and NHWs at the University of Oklahoma Medical Center (OUMC).
Methods
Study Population and Setting
The University of Oklahoma Health Sciences Center Institutional Review Board approved this study (number: 10716). A waiver of informed consent was granted due to the retrospective chart review nature of the study.
OUMC is the largest academic medical center and major transfer center in Oklahoma and has the state’s largest intensive care unit (ICU). The study population included AI and NHW men and women ages 18 and older who were hospitalized for COPD exacerbations between July 2001 and June 2020 at OUMC. Patients were included if they were discharged from the medical center or died during the hospitalization with a diagnosis of COPD, defined by International Classification of Diseases, 9th revision (ICD-9) and 10th revision (ICD-10) codes (490, 491, 494, 496, ICD-10: J41-44), and were treated for COPD-related acute events, such as worsening shortness of breath and acute respiratory failure. Encounters for patients who had stable COPD but were admitted for management of another condition or for other chronic respiratory disease management, such as interstitial lung disease, bronchiectasis, and lung transplantation, were excluded.
Patients were also included if they self-identified as AI or NHW during the hospital intake process, as noted in the electronic health record (EHR). AI patients were matched initially 1:20 with NHW patients in the same month of first COPD hospitalization. During data collection, the power was recalculated, as investigators noticed that higher numbers of matching chart reviews did not increase the study power. AI patients were matched 1:4 with NHW patients for final data analysis.
Data Collection
The data collected from the EHR included race, age at last encounter, gender, smoking history as defined at the last visit (current, former, never, unknown), home address, insurance information, date of visit, height, weight, admission locations, comorbidities (described below), use of supplemental oxygen on discharge, history of prior OUMC hospitalization for COPD exacerbation during the study time frame, and any recorded pulmonary function test and ever recorded chest computed tomography (CT) result during the study time frame. The COPD health outcome variables include hospital length of stay (LOS) (from the day of admission to the day of discharge or death), ICU days, invasive mechanical ventilator use, supplemental oxygen use on discharge, discharge disposition (home with self-care, home with home health, rehabilitation facility, long-term acute care, skilled nursing, and others), 30-day readmission, and death.
Comorbidities included cardiovascular diseases (coronary artery disease, heart failure, hypertension, peripheral vascular disease, arrhythmias, and stroke), metabolic diseases (DM and dyslipidemia), pulmonary and sleep disorders (asthma, lung cancer, and obstructive sleep apnea), and others (obesity, osteoporosis, anxiety/depression, gastroesophageal reflux, chronic kidney disease, and anemia). Comorbidity index is the total number of the above comorbid diseases. Obesity was defined by standard categories of the body mass index (BMI) (weight in kilograms divided by the square of the height in meters). BMI was classified into 6 categories: underweight (BMI <18.5), normal (BMI 18.5–24.99), overweight (BMI 25.0–29.99), obesity class I (BMI 30.0–34.99), obesity class II (BMI 35.0–39.99), and obesity class III (BMI of 40.0 or higher).
Patients’ addresses were used to identify their respective U.S. Census block group. An area deprivation index (ADI) system was used to rank patients’ neighborhood socioeconomic disadvantage according to the respective U.S. Census block group.20 The ADI includes factors of income, education, employment, and housing quality. The ADIs are provided in national percentile rankings at the block group level from 1 to 100. Group 1 is the lowest ADI (the lowest level of “disadvantage”) and group 100 is the highest ADI (the highest level of “disadvantage”).
The payment sources were classified as Medicare, Medicaid, commercial insurance, Indian health coverage (including Indian Health Service or a tribal health program), other insurance, and uninsured. A patient may have more than 1 payment source.
Trained research staff reviewed every chart manually to confirm eligibility for inclusion, treatment for COPD exacerbation, smoking history, and comorbidities. The project principal investigator, a board-certified practicing pulmonologist, reviewed more than 20% of the charts abstracted by research staff to ensure quality control.
Analysis Plan
Continuous variables are expressed as mean±SD, or median (interquartile range [IQR]), while categorical variables are shown as percentages. For between group comparisons, if the normality assumption held, we used independent t-test; if normality assumption was not correct, we used nonparametric tests, including the Wilcoxon rank sum test (for 2 groups). Chi-square test was used to evaluate the relationship between 2 categorical variables if we did not have sparse cells. Fisher’s exact test was used for sparse cells. A 2-tailed p value <0.05 was considered statistically significant.
Linear regression model analysis was performed to determine the association between continuous health outcome variables (hospital LOS, ICU days, and mechanical ventilator support days) and predictors (e.g., race, age, gender, smoking history, ADI, DM, obesity, number of comorbidities, number of COPD rehospitalizations). Log transformations were performed for non-normally distributed variables: hospital LOS, ICU days, and ventilator support days. Logistic regression model analysis was performed to determine the association between categorical health outcome variables (admission locations, history of mechanical ventilator use, supplemental oxygen use, discharge disposition, and 30-days readmission), and predictors. Exact logistic regression was used for low numbers of cases.
Single variable analysis (α level=0.05) and collinearity were used in both linear and logistic models. For linear models, assumptions including independence, normality, and equal variance were examined by using residual plots and qq plots.
Data analysis was performed using SAS software version 9.4 and R.21 Before analysis was conducted missing values were removed.
Results
A total of 630 encounters with the diagnosis of COPD in AI patients were initially identified in the OUMC EHR. After excluding emergency department visits, ambulatory encounters, and no confirmed treatment for COPD exacerbation, 76 AI patients with 128 encounters met the inclusion criteria (Figure 1). The index COPD hospitalization of each of these 76 AI patients was matched 1:4 to 304 NHW patients (448 encounters) by the same month of the admission.
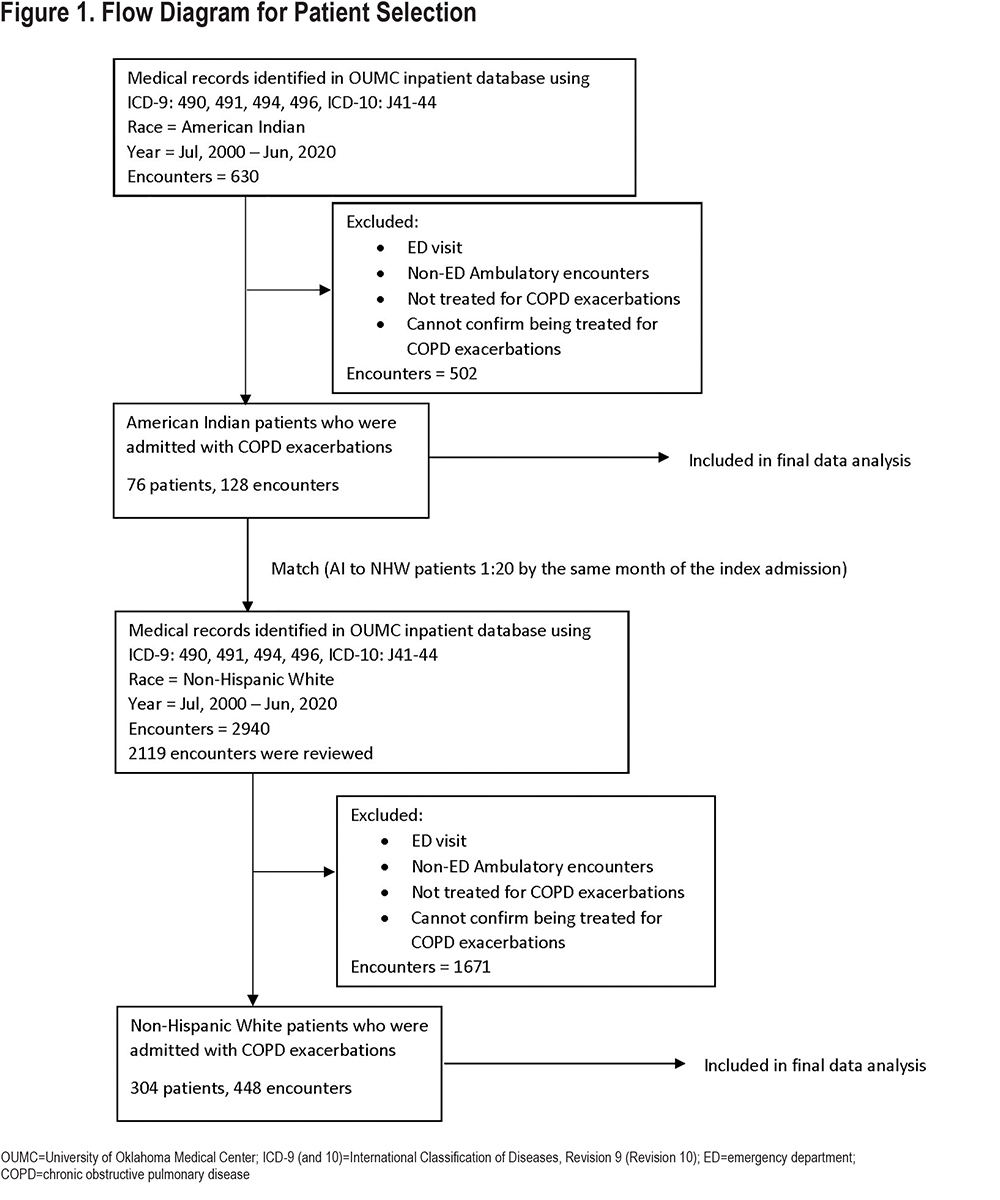
Table 1 shows single variable comparisons between AI and NHW patients. Although not statistically significant, we found that AI patients were on average 3 years younger than NHW patients. A higher proportion of females were present in the AI group (61%) than in the NHW group (48%). In both groups, most patients were current or ex-smokers. Most participants in both groups lived in urban areas and areas above the national median of ADI national rank (most disadvantaged) (Figure 2). Although the difference was not statistically significant, the proportion of AI patients living in the least disadvantaged areas was lower than the proportion of NHW patients living in the least disadvantaged areas. Most patients had an identified health care payment source. The proportion of patients without health care coverage did not differ by race. AI patients had more comorbid diseases than did NHW patients. The prevalence of DM among AI patients was nearly twice that among NHW patients. Lung cancer, peripheral vascular disease, and anemia were nearly 3 times as prevalent among AI patients. Although the difference was not statistically significant, more AI patients were overweight or obese than were NHW patients (69.7% versus 58.5%, respectively). The prevalence of class III obesity among AI patients was more than twice that among NHW patients.
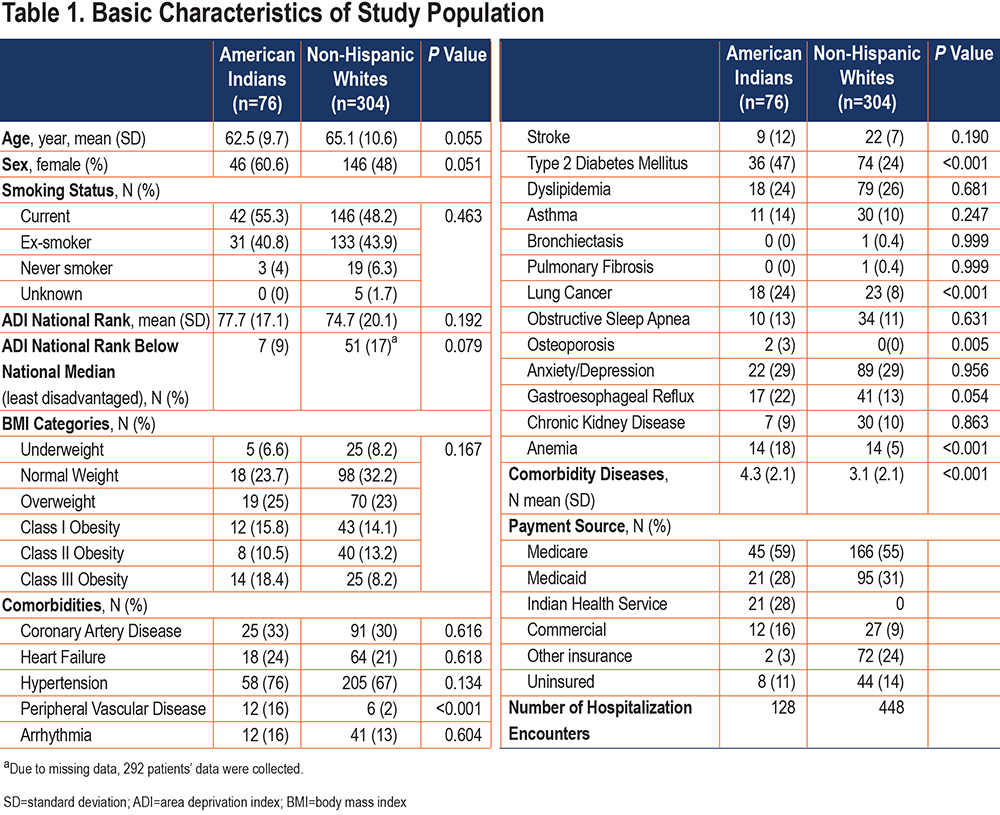
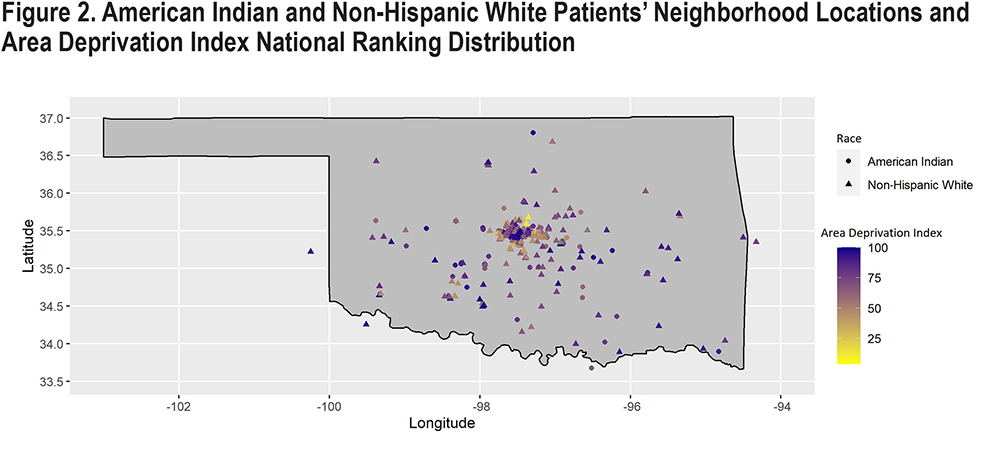
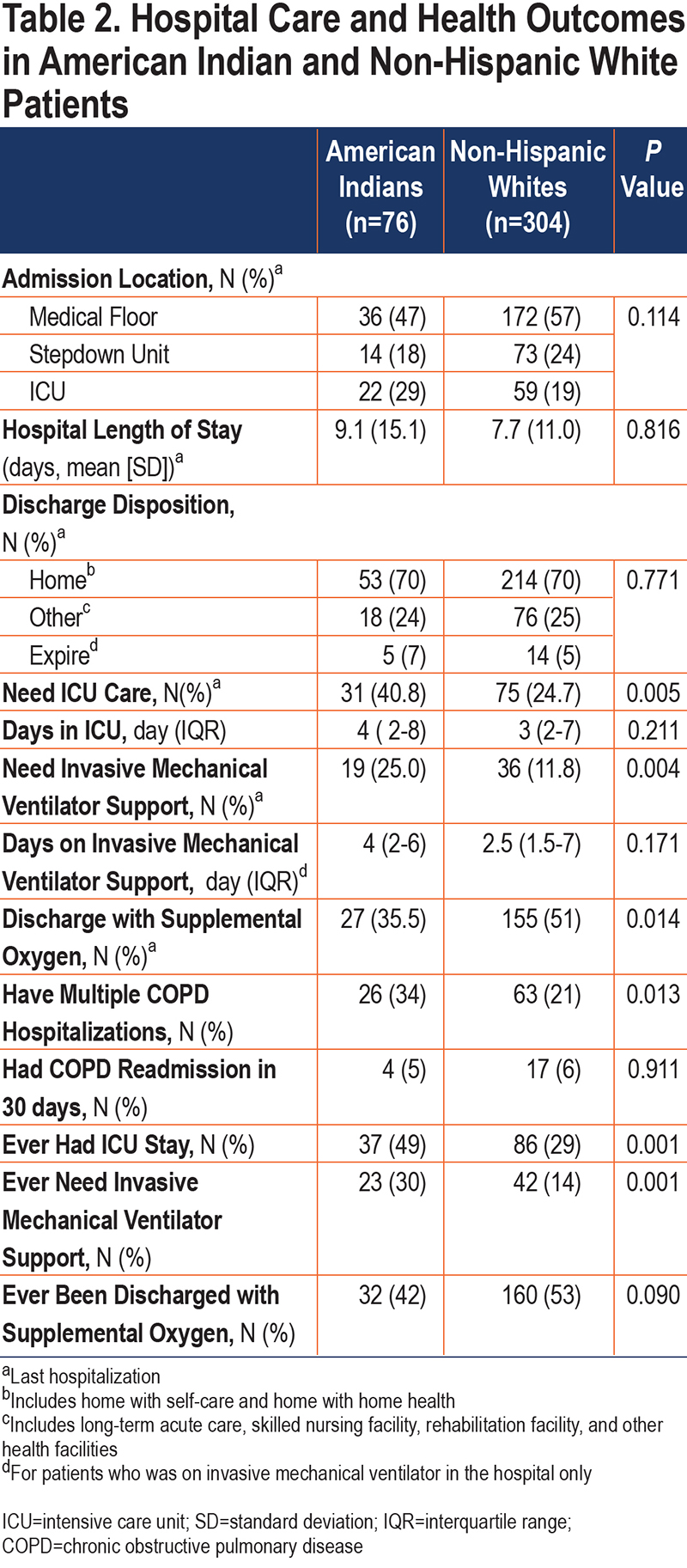
Table 2 shows single variable hospital care and outcomes by AI and NHW race. Initial admission locations, hospital LOS, and discharge disposition (including death) did not differ by race. However, the percentage of AI patients ultimately needing ICU care and invasive mechanical support was significantly higher than that for NHW patients. Similarly, the number of days of ICU and invasive mechanical ventilator support was greater for AI patients than for NHW patients. On the other hand, the proportion of AI patients discharged with supplemental oxygen was lower than that in NHW patients. Although the percentage of COPD readmission in 30 days was comparable and low in both groups, AI patients more often had a history of prior COPD hospitalization, prior ICU stay, and prior invasive mechanical ventilator support.
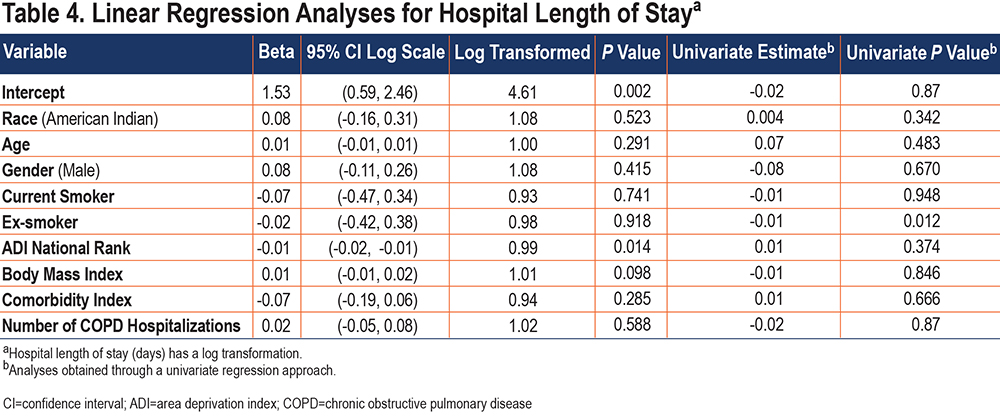
Analyses assessing whether AI race was independently associated with hospital outcomes are presented in Tables 3-7. Although not statistically significant, the odds of requiring admission to the ICU for AI patients were twice as great as that for NHW patients after adjustment for other significant cofactors (Table 3). Admission to stepdown did not differ by race. Being male was significantly associated with both ICU and stepdown admission. In the linear regression model with LOS as the outcome, each unit increase in the ADI national rank resulted in a 0.59% decrease in average of LOS (Table 4).
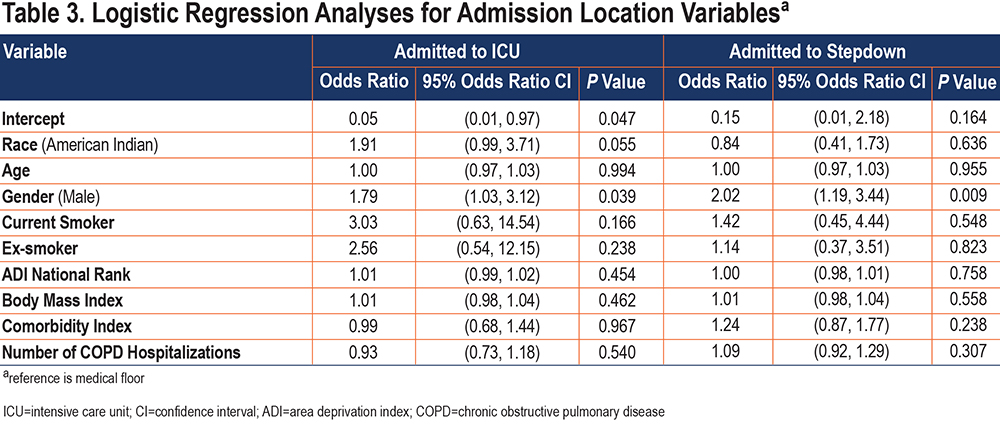
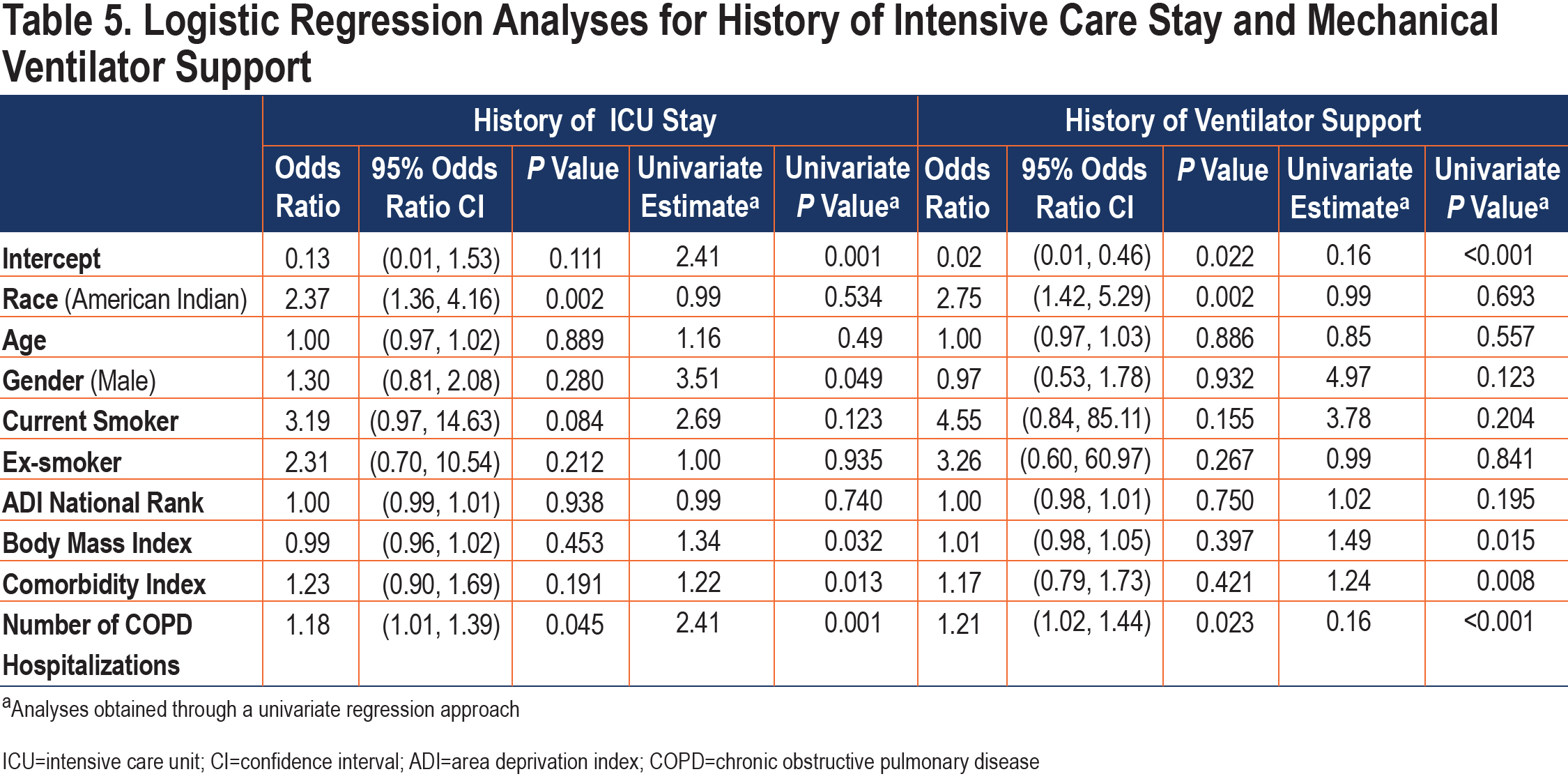
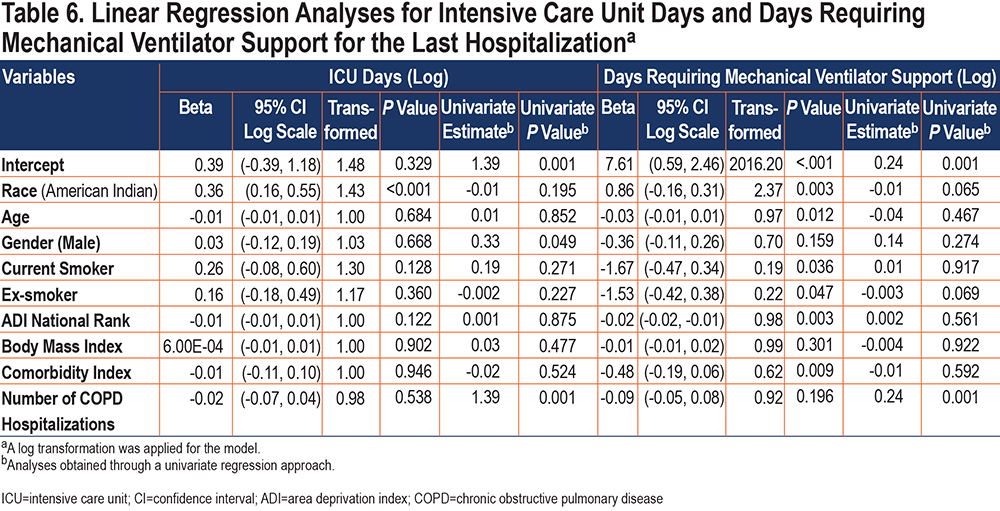
AI patients’ odds of needing ICU and mechanical ventilator support during the study time frame were 2 or more times as large as those for NHW patients (Table 5). AI race was independently associated with longer ICU stay (Table 6). When on mechanical ventilator support, our records indicated the median duration of ventilator support was 4 days for AI patients and 2.5 days for NWH patients. The association between AI race and days requiring mechanical support was also significant in multiple variable analysis (Table 6). From the log transformed linear regression model, the average number of days on mechanical ventilator support increased by 137.3% for an AI patient compared to an NHW patient.
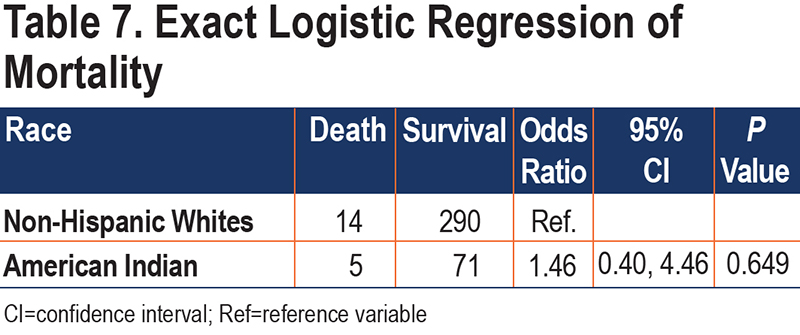
Although AI race was associated with a higher risk of all-cause mortality, the number of events was low, and the association was not significant in exact logistic regression (Table 7). AI race was not significantly associated with discharge to other health facilities, such as long-term acute care, skilled nursing facilities, or rehabilitation facilities (OR 0.98, 95% CI 0.52–1.83, p=0.94). However, AI patients with DM were more likely to be discharged to other health facilities than were AI patients without DM (OR 2.05, 95% CI 1.22–3.51, p<0.01).
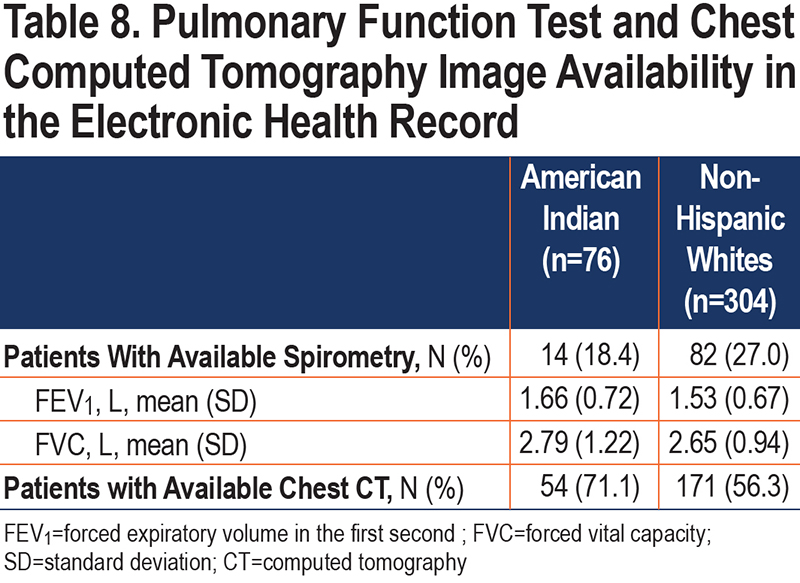
Only 18% of AI patients and 27% of NHW patients had pulmonary function data ever recorded in our health system (Table 8). While more than half the patients in both groups had ever received a chest CT, AI patients had chest CT images more often than did NHW patients.
Uninsured NHW patients had less supplemental oxygen use (p<0.01) and were less likely to be discharged to other health facilities (p<0.01) than were insured NHW patients (supplemental Table 1 in the online supplement). There was no difference by payment source in admission locations, ICU stay, ventilator use, or 30-day readmissions for insured versus uninsured in both races.
Discussion
Increasing attention is being paid to differences in health outcomes in critical illness between racial and ethnic groups. Most available literature regarding COPD reflects differences between African Americans and NHWs.22 For example, a prior study revealed that African Americans with COPD exacerbations who were admitted to Veterans Affairs hospitals were more likely to be admitted to the ICU and to receive mechanical ventilation than were NHWs with COPD exacerbations.23 However, little data are available to investigate COPD among AI populations.17 Our study shows that AI race was associated with worse health outcomes, including more ICU admissions, longer ICU days, and longer invasive mechanical ventilator use, than was NHW race. Due to the limited sample size and retrospective chart review, we could not further characterize the underlying reasons for disparate outcomes.
In our study, hospitalized AI patients were slightly younger than NHW patients, although the difference did not reach statistical significance. Nonetheless, COPD pathology likely develops insidiously before the spirometric threshold for diagnosis is met.24 Identifying early COPD among AI populations is, therefore, critical to begin effective interventions.
COPD historically affected men with a history of smoking. Our study showed a higher proportion of women hospitalized for COPD in the AI group than in the NHW group. The high percentage of women in the AI group also exceeded that reported for women of other races hospitalized for COPD.25,26 This may reflect a higher proportion of smoking among AI women.3 Although being male was associated with higher risk of ICU and stepdown admission for both groups in our study, the high percentage of women in the AI group may be a unique feature that warrants additional study. Whether this demonstrates a greater willingness among AI women than men to seek care before becoming critically ill is unknown and merits further investigation.
Medical comorbidities are prevalent among patients with COPD.1 It is well known that COPD comorbidities may have a significant impact on disease course and prognosis.27 Most patients in our study had comorbidities. But AI patients had more comorbidities, especially DM, lung cancer, and peripheral vascular disease, than did NHW patients. They also had a non-significantly higher prevalence of class III obesity. A greater number of comorbid diseases may contribute to the poor COPD health outcome in AI patients, but our sample size may have limited our ability to detect meaningful associations. Early diagnosis and treatment of COPD comorbidities may be an important management strategy for AI patients with COPD.
Neighborhood disadvantage has been linked with health disparities in a large COPD cohort study in non-AI patients.28 COPD patients who resided in more-disadvantaged neighborhoods had worse COPD outcomes than did those residing in less-disadvantaged neighborhoods. While our study also showed this association, the average ADI national ranking was 75.34±19.58, much worse than patients in the aforementioned study at 41.0± 29.4. However, ADI rankings did not differ significantly between AI and NHW groups in our study even though the proportion of least disadvantaged was lower for the AI group. Again, larger studies are needed to further explore the role of socioeconomic status. Strategies at the state level also have the potential to improve neighborhood socioeconomic status as a whole.
Although supplemental oxygen was prescribed less often to AI patients on discharge, AI patients had more use of ICU and invasive mechanical ventilation, as well as a history of repeated COPD hospitalizations. Our medical record review could not assess outpatient severity of COPD. Reasons for these seemingly disparate findings are unclear. Potential explanations include lack of access to pulmonary specialty care or post-hospitalization primary care, shortage of medications, lack of COPD education in self-management, and patient preferences. Systematic studies are needed to characterize COPD within AI communities and to identify the risk factors for repeated hospitalizations.
Both AI and NHW patients had very low documentation of any spirometry testing in the system. Some patients may have had spirometry in other health facilities. Others may not have had spirometry due to transportation or no spirometry capability in their primary care providers’ office. The lack of spirometric data limits the accuracy of COPD diagnosis and disease progression monitoring. On the other hand, a high percentage of AI patients had at least 1 chest CT result in the system. Although documenting the reason for obtaining the chest CT was beyond the scope of this study, chest CT may be another tool to diagnose COPD.29
In assessing the limitations of our study, of first note is the small sample size from a single center. After applying exclusion criteria, the number of eligible cases was insufficient to reach the desired power to confidently identify factors associated with 30-day COPD readmission, the study’s initial primary outcome. Second, the medical center is a tertiary care facility and transfer center; some patients at this facility may have more advanced disease than do patients in community health facilities, limiting generalizability. Similarly, the majority of our AI patients resided in urban areas and may not reflect the experience of AI persons in rural areas, who may have fewer medical resources.25 This also hampers our ability to generalize our results to AI populations statewide or nationwide. Third, as a retrospective medical record review, this study is subject to limitations including misdiagnosis and racial misclassification. For instance, the diagnosis of COPD exacerbation was based on physician documentation and coding and may not have been recorded in a uniform manner in the EHR. If encounters with COPD exacerbation were not coded correctly, these medical records would not have been reviewed by the research team. Similarly, race was determined by EHR demographic documentation, which is prone to misclassification of AI persons to another race,30 as are disease registries and clinical databases.31-33 Our study may not have identified all AI patients with COPD. Finally, the nature of the study precluded exploration of other effects that may be important among AI people, such as intergenerational or historical trauma and medical distrust.34,35 Despite these limitations, data regarding COPD among AI people are lacking and this study is the first to attempt an in-depth exploration of COPD health disparities in this population. These findings may support efforts to design large, systematic studies to assess and address COPD in AI communities.
In conclusion, AI patients hospitalized with COPD exacerbations had unique characteristics and worse health outcomes than did NHW patients. This study provides preliminary data for future larger multicenter studies to address COPD heath disparities in AI communities.
Acknowledgements
Author contributions: HW, DAR, SC, and BB conceptualized the study. HW and DAR produced the original manuscript. HW, MS, and CG participated in the acquisition of the data and take responsibility for the integrity of the data. SC, AW, and HW performed data analysis. All coauthors critically assessed methodological and statistical approach, critically reviewed the manuscript, and revised the manuscript.
We thank Ms. Kathy Kyler, a University of Oklahoma employee, for medical writing assistance.
Data Sharing statement: Data sharing is not available.
Declaration of Interest
The authors report no conflict of interest related to the publication of this paper.