Running Head: Increased Endurance Time as a Patient-Focused Outcome
Funding Support: Funding for the CBQC Constant Work Rate Exercise project was provided by Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, and Chiesi.
Date of Acceptance: January 5, 2022 │ Published Online: January 10, 2022
Abbreviations: Chronic Lung Disease Biomarker and Clinical Outcome Assessment Qualification Consortium, CBQC; chronic obstructive pulmonary disease, COPD; constant work cycle ergometry, CWRCE; U.S. Food and Drug Administration, FDA; drug development tool, DDT; clinical outcome assessment, COA; St George’s Respiratory Questionnaire, SGRQ; concept of interest, COI; American Thoracic Society, ATS; European Respiratory Society, ERS; forced expiratory volume in 1 second, FEV1; 6-minute walking test, 6MWT; incremental shuttle walk test, ISWT; endurance shuttle walk test, ESWT; peak oxygen uptake, V̇O2 peak; pulmonary carbon dioxide output, V̇CO2; pulmonary ventilation, V̇E
Citation: Casaburi R, Merrill DD, Harding G, et al. A conceptual framework for use of increased endurance time during constant work rate cycle ergometry as a patient-focused meaningful outcome in COPD clinical trials. Chronic Obstr Pulm Dis. 2022; 9(2): 252-265. doi: http://doi.org/10.15326/jcopdf.2021.0258
Introduction and Overview
In 2006, the U. S. Food and Drug Administration (FDA) issued the “Critical Path Opportunities Report,”1 which described 6 key areas along the path to improved therapies. The report noted that attention to a needed drug development tool (DDT) often only occurs when the clinical study protocols to facilitate the registration of a drug are being developed, at which time it is recognized that the available DDTs are inadequate. The report further noted that the efficiency of drug development could be improved by developing a new product development toolkit containing innovative scientific and technical methods. New and improved DDTs are among the methods that can help streamline the drug development process, improve the chances for clinical trial success, and yield more information about the treatment and/or disease.
In 2010, the FDA issued a draft guidance intended to represent the agency's thinking on the qualification process for DDTs; the final guidance2 was formally adopted in January 2014 and was recently updated3 in November 2020. According to the final DDT guidance, as well as other relevant sources,4-7 establishing a well-understood relationship of a clinical outcome assessment (COA) to a meaningful aspect of how a patient feels or functions in his or her usual life is central to the conclusion that the observed effect is actually a treatment benefit, i.e., an aspect of health that the patient cares about and has a preference that this aspect either does not become worse, improves, or is prevented. As an illustrative example of this important initial phase of the COA development process, we describe here the necessary first steps in establishing endurance time during constant work rate cycle ergometry (CWRCE) as a COA for use in interventional studies by describing its link to physical function. We discuss:
- How the disease process that characterizes COPD has meaningful effects on patient symptoms and physical functioning during everyday life.
- How limitations in physical functioning are directly linked to exercise endurance among people with COPD.
- How endurance time during CWRCE can be objectively measured, yielding consistent interpretable results.
Combining these elements, we conclude that endurance time during CWRCE is an appropriate candidate COA for the measurement of exercise endurance in interventional studies. In future publications, we will describe the validation requirements to support the COA as a qualified DDT.
Background: The COPD Biomarker Qualification Consortium Initiative
Stimulated by the publication of the FDA’s DDT guidance, the COPD Biomarker Qualification Consortium (CBQC) was formed under the management of the COPD Foundation to focus efforts on the qualification of DDTs in COPD, following processes consistent with the newly released draft DDT guidance. The organizational structure of the CBQC was set-up to foster contributions of clinical and scientific expertise from both academia and the pharmaceutical industry.8,9 To date, the work of the CBQC has resulted in qualification of plasma fibrinogen as a stratification tool10 and qualification of the St George’s Respiratory Questionnaire (SGRQ) as a COA in interventional studies.8 Based on the recognition that many COAs are relevant across chronic lung diseases such as bronchiectasis, pulmonary fibrosis, alpha-1 antitrypsin deficiency, and asthma, the CBQC was refocused as the Chronic Lung Disease Biomarker and Clinical Outcome Assessment Qualification Consortium (CBQC) in 2020.
A working group within the CBQC was formed in 2013 to evaluate opportunities for qualification of a COA based on exercise endurance. Within the working group (see list in Acknowledgements), a broad request for input from both academia and the pharmaceutical industry has resulted in a significant contribution from clinical/scientific experts in clinical exercise testing; National Heart, Lung, and Blood Institute staff members have served in an advisory capacity, while independent contractors have assisted with data collection and analysis. Feedback from the FDA has helped to shape the project’s progress.
Physical Functioning in Daily Life: A Meaningful Aspect of Health Impacted by COPD
Qualitative research among people with COPD consistently shows that limitations in the ability to perform activities of daily life are a significant burden to patients. Table 1 provides a summary of relevant qualitative research conducted among people with COPD, highlighting the specific ways in which people with COPD adapt their physical functioning.11-14
The qualitative research described in this table offers important insight into the experience of living with COPD. There is a consistent theme; people with COPD describe the impact of the disease in terms of the adverse effects and necessary adaptations they must make in physical functioning:
- Sustained symptom burden (breathlessness, fatigue) resulting in loss of physical functioning; breathlessness impacts every aspect of life, including everyday activities.
- On good days there is an increase in physical activity and on bad days a reduction in physical activity.
- When expecting a high level of discomfort from an activity, people impacted by COPD consider alternative methods of performance, reducing standards of performance, or relinquishing the activity altogether.
- People with COPD frequently describe the course of their COPD in terms of symptom-induced changes in activity that are discouraging and frustrating and express a desire to return to previous levels of performance.
Of particular relevance, people with COPD express the need to modify activity patterns with progressing disease:
- Utilize pacing (self-regulated performance to minimize symptoms), planning (devising strategies to accomplish the activity within known constraints on capacity), assistive devices, and personal assistance to accomplish activities they want to perform.
- Report unpredictable symptoms that gradually worsen over time, and this results in pacing or slowing down, interrupting activities to recover, or allowing for a longer recuperation period.
Exercise Endurance: A Specific, Relevant Concept of Interest Related to Physical Functioning
An explanatory conceptual model of the progressive limitations in physical functioning over time in COPD patients (the “downward spiral of disability”)15-18 postulates that:
- In the face of expiratory flow limitation, the respiratory response required to support the increased metabolic demands of a given intensity of muscular work results in disproportionate breathlessness.
- In an effort to avoid breathlessness, patients reduce the intensity and/or amount of activity performed during daily life.
- The reduced activity leads to muscular de-conditioning, especially of the leg muscles.
- Other extra-pulmonary intrinsic factors related to COPD (e.g., systemic inflammation) also contribute to muscle dysfunction.
- The consequences of muscle dysfunction (e.g., early onset of lactic acidosis during exercise) further stimulate breathing, increase breathing work, and increase breathlessness, amplifying the downward spiral of disability.
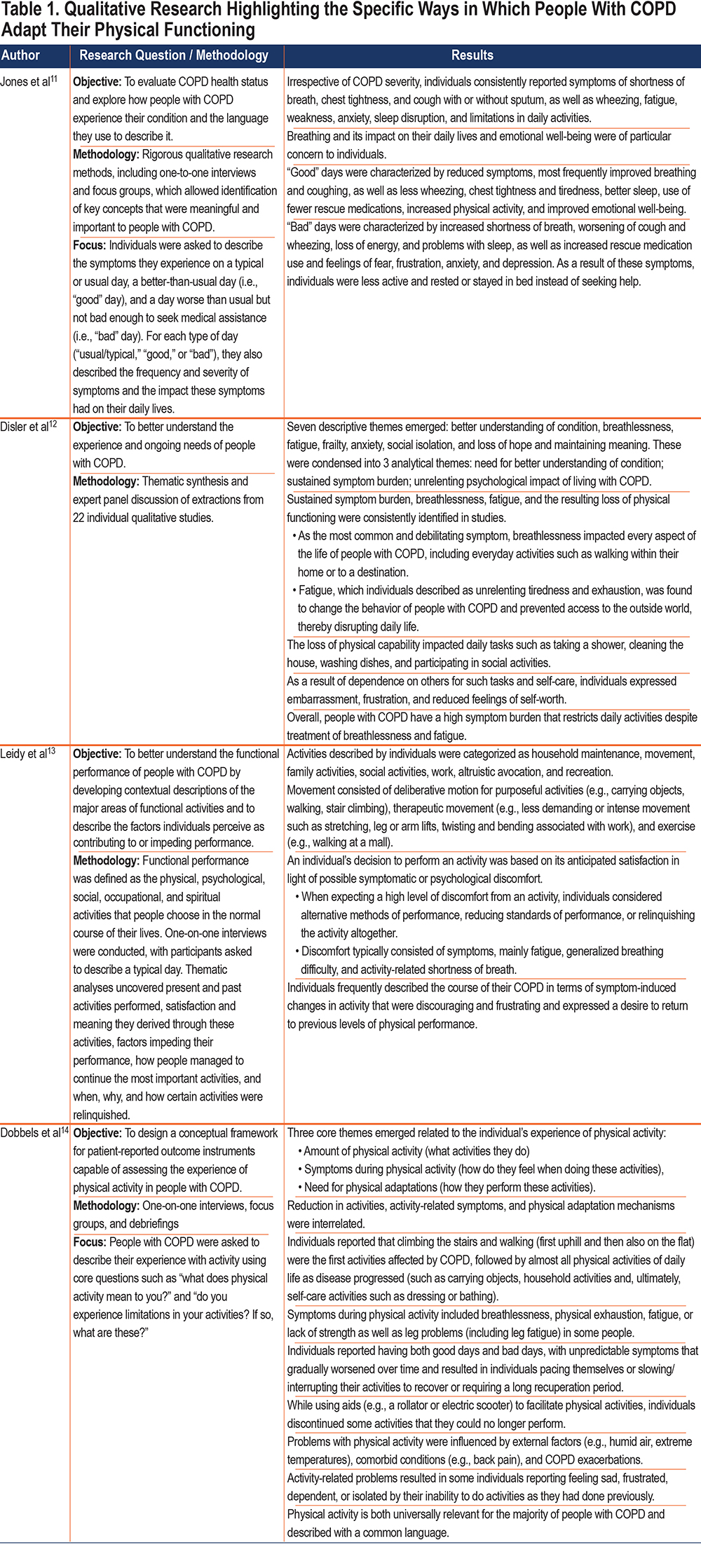
In people with COPD, adaptations to their activity patterns such as pacing, slowing down, interrupting activities to recover, and allowing for a longer recuperation, reflect a reduced exercise endurance. Exercise endurance may be defined as the ability to sustain intense aerobic exercise or activity (intense relative to the individual’s peak capabilities). Thus, as a concept of interest (COI) of meaningful treatment benefit, exercise endurance has a direct relationship to the experience of physical functioning in daily life. Figure 1 presents a conceptual framework of this relationship. In the figure, the concept of physical function refers to relevant physical tasks in everyday life that are impacted among people with COPD. Physical function is identified as a distal concept in that many contextual factors, including environmental (e.g., air quality, products or substances for personal consumption, most especially cigarette smoking) and demographic (e.g., age, psychosocial status, ethnicity) factors, influence physical function in addition to the effects of the impairment in bodily function caused by the disease and its associated comorbidities (including skeletal muscle dysfunction, cardiovascular abnormalities, and orthopedic limitations). Within the domain of physical function, the separation into upper limb and lower limb activities has value in general.19 This separation has particular relevance when considering physical function among people with COPD due to the significantly larger muscle mass involved in lower limb activities compared with upper limb activities, and the consequently greater ventilatory response required to support lower limb activities. COPD imposes difficulty performing upper extremity activities (especially those requiring lifting arms above the head), in part related to recruitment and use of accessory muscles of respiration. However, the central role of the muscles of ambulation in supporting activities most important to people with COPD (see Table 1) justifies a primary focus on the lower limbs.
Therefore, our work in the CBQC has focused on lower limb activities since limitations in these types of activities are ubiquitous among people with COPD. In developing our conceptual framework, the next step was to identify a proximal concept of treatment benefit that was more directly associated with the disease-defining concepts of impaired pulmonary function and muscle dysfunction. An important concept relates to the reduced physiological capacity (pulmonary dysfunction, muscle dysfunction) and its impact on the ability to sustain aerobic muscular work (i.e., muscular work requiring a significant cardiorespiratory response to support bioenergetic requirements), which defines exercise endurance.
In summary, exercise endurance (the ability to sustain intense aerobic exercise or activity) provides an appropriate link between pulmonary and muscular dysfunction and limitations in lower limb activities experienced by people with COPD, observed in daily life as limitations in the ability to complete a given physical task.
Exercise endurance as a relevant COI in COPD is also supported by guidance developed by clinical and scientific bodies and regulatory authorities. The American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force on Outcomes for COPD Pharmacological Trials,20 assembled with the aim of informing the COPD research community about current outcomes and markers for evaluating the impact of a pharmacological therapy, identified exercise tolerance as a necessary supplement to the measurement of lung function among people with COPD. From the task force:
“Changes in forced expiratory volume in 1 second (FEV1) with therapy should not be regarded as a surrogate for changes in dyspnea, exercise performance, or health-related quality of life. These variables should be measured separately to complement other markers of physiological impairment when assessing a therapy for COPD.”20
The FDA issued a draft guidance for developing drugs in COPD8 in 2016 which described exercise tolerance (which is synonymous with exercise endurance) as a potential objective physiological assessment. The draft guidance was withdrawn for undisclosed reasons in 2018 and a final guidance has not been released. In this document, efficacy assessments were grouped into the following broad categories: (1) objective physiological assessments, (2) patient- or evaluator-reported outcome measures, and (3) biomarkers and surrogate endpoints. Within this framework, reduced capacity for exercise was described as a potential objective physiological assessment:
“…reduced capacity for exercise is a typical consequence of airflow obstruction in COPD patients, particularly because of dynamic hyperinflation occurring during exercise. Assessments of exercise capacity by treadmill or cycle ergometry combined with lung volume assessment potentially can be a tool to assess efficacy of a drug.”8
The European Medicines Agency considers exercise testing among people with COPD to be useful in the clinical setting to assess the degree of impairment, prognosis, and the effects of treatment interventions ,21 and recommends its use as a co-primary endpoint in confirmatory trials for therapies intended for the symptomatic treatment of COPD:
“…measurement of lung function parameters alone is considered to be insufficient in the assessment of therapeutic effect. If lung function is selected as a primary endpoint (FEV1 would be the parameter of choice), additional evidence of efficacy must be demonstrated through the use of a co-primary endpoint, which should either be a symptom-based endpoint or a patient-related endpoint. In moderate/severe COPD this might be the number of exacerbations and/or symptoms such as dyspnea on exertion, or health status assessed through the use of a disease-specific questionnaire such as the SGRQ and/or assessment of exercise capacity.”21
Thus, there is general consensus that exercise endurance represents a clinically meaningful aspect of patient function in COPD. Currently, no products approved for treatment of COPD in the United States have claims related to exercise endurance and, as such, the regulatory pathway in the United States is not established.22 Therefore, there is a clear need to develop and qualify COAs associated with the concept of exercise endurance within the framework of the FDA DDT qualification process.
Identification of an Appropriate Clinical Outcome Assessment for the Measurement of Exercise Endurance
There are several exercise tests that have been used in the evaluation of patients with COPD. An ERS Task Force conducted a comprehensive review of the value and limitations of different exercise tests for the assessment of therapeutic interventions.23 The task force summarized the available evidence for:
- laboratory-based incremental work-rate tests (cycle ergometer; motorized treadmill),
- high-intensity constant work rate tests (cycle ergometer; motorized treadmill),
- field tests (6-minute walk test [6MWT], incremental shuttle walk test [ISWT], endurance shuttle walk test [ESWT]).
Laboratory-based incremental work rate tests (using a cycle ergometer or a motorized treadmill) permit evaluation of both submaximal and peak exercise responses and have an important role in establishing exercise prescriptions for exercise training. Peak oxygen uptake (V̇O2peak) is a key measurement during these tests and is closely reflective of the individual’s maximum oxygen uptake (V̇O2), the gold-standard index of aerobic capacity. However, in terms of a measure of exercise performance, the necessary incremental nature of the work rate control during the test means that the exercise test is not representative of the type of activity pattern performed in everyday life; it is analogous to climbing up a hill that becomes steeper and steeper as the individual climbs. Furthermore, individuals do not habitually choose to perform exercise at peak work rate or peak V̇O2. As such, the measurement of peak work rate (i.e., the work rate associated with V̇O2peak) is not a relevant measure of exercise endurance in the context of activity limitation in COPD. Similarly, the ISWT, in which walking speed increases progressively, was developed as a field test for the estimation of peak aerobic capacity24 and, as such, is also not a relevant measure of exercise endurance within the present context.
The task required of the individual performing the 6MWT is to “walk as far as possible during a 6-minute period.”25 The individual, therefore, has the task to self-select a walking speed that he/she assesses to be appropriate for maximizing the distance walked in the 6-minute testing period. As the test continues, the individual has the option to adjust the walking speed based on a continuous re-assessment of the time to test-end within the requirement to maximize distance walked. If necessary, the individual is allowed to rest during the test. Therefore, the 6MWT is a suitable test for the measurement of “walking performance,” but is not a measure of exercise endurance, defined as “the ability to sustain intense aerobic exercise or activity.” Importantly, it has been demonstrated operationally26 that the 6MWT does not consistently elicit limitation in physiologic variables. Most individuals self-select walking speeds that are comfortable, rather than maximal. As a result, pharmacological interventions that ameliorate physiologic limitations to exercise have generally not been found to increase 6MW distance.26
The ESWT is a field test that was developed based on the same construct as laboratory-based CWR tests27; the individual is tasked with walking for as long as possible (i.e., to the point of exercise intolerance) at an externally regulated walking speed that is pre-determined to be at a high relative intensity compared with peak walking speed. The ESWT was developed to reflect a specific functional activity performed in daily life (walking along level ground); the ESWT is the focus of a separate COA qualification initiative that focuses on the concept of interest of “walking endurance.”
CWR tests (cycle or treadmill) require the patient to perform an activity that reflects the symptom-limited exercise intolerance experienced while performing activities of daily living: the duration for which a given task can be sustained. Importantly, it standardizes the intensity of the activity performed by the individual, thus avoiding the confounding influence of the behavioral adaptations seen in everyday life, consistent with the recommendations in the International Classification of Functioning, Disability, and Health published by the World Health Organization.19 Constant work rate tests have the following important characteristics:
- A physical task that is continued until the point of symptom limitation (“symptom-limited”).
- A high intensity activity (relative to the individual’s exercise capacity) involving large muscle groups, which most commonly brings the person with COPD to 1 of 2 physiologic limitations:
- a limitation in pulmonary ventilation and/or gas exchange, which elicits a limiting intensity of breathlessness;
- a limitation in leg muscle oxidative metabolism and/or accumulation of fatigue-associated metabolites, which elicits a limiting intensity of neuromuscular fatigue.
Both CWRCE28 and CWR treadmill walking are recognized as appropriate measurement tools for exercise endurance.23 The CBQC CWR Exercise Working Group decided to focus its initial qualification efforts on CWRCE due to the more extensive evidence available for CWRCE compared with CWR treadmill walking.28 Specifically, the Working Group agreed to move forward with an evaluation of endurance time during CWRCE as a potentially important COA for drugs developed for COPD that reflects the concept of interest “exercise endurance.” As a basis for the evaluation, the context of use was stipulated as a key efficacy endpoint in clinical trials that incorporate standard features, e.g., randomization and double-blind study treatment(s). In such a trial setting, the endpoint assessed with this COA is anticipated to be defined as an increase in exercise endurance measured as change from pre-treatment baseline endurance time during CWRCE.
Description of the Clinical Outcome Assessment: Endurance Time During Constant Work Rate Cycle Ergometry
CWRCE is performed on an electronically braked stationary cycle ergometer (specifically, an ergometer in which the work rate is controlled and is independent of the pedaling cadence). At baseline, an individualized work rate is established for each individual, based on a preceding incremental cycle ergometer exercise test in which work rate is incremented in a pre-specified protocol.
During the CWRCE, the individual begins to pedal at a self-selected pedaling cadence (usually 60 rpm) and is encouraged to maintain this frequency throughout the exercise test. A stopwatch (or other time recording device) is started when the work rate is increased to the pre-determined level. The individual is encouraged to continue exercising for as long as possible (i.e., to intolerance or maximal exertion).
Although not an absolute requirement (and engendering additional complexity), measurement of physiological and sensory responses are typically collected during this laboratory-based exercise test to allow changes in endurance time to be interpreted in relation to changes in physiological and sensory responses: e.g., V̇O2, pulmonary carbon dioxide output (V̇CO2) pulmonary ventilation (V̇E) inspiratory capacity, breathing frequency, heart rate, or patient-reported ratings of dyspnea and leg effort.
The individual supervising the exercise test provides standardized and continuous encouragement to the individual. If an individual selects a pedaling rate of 60 rpm, the encouragement during the test would focus on ensuring that the patient is motivated to maintain the pedaling rate of 60 rpm. Should the pedaling cadence drop below the selected rpm, the individual is encouraged immediately to increase the pedaling cadence back to the selected rpm, and to maintain it for as long as possible.
The limit of exercise tolerance is defined as the point at which the individual is: (1)limited by symptoms (i.e., is unwilling to continue exercising because of the discomfort associated with the exercise), or (2) unable to maintain the self-selected pedaling cadence (e.g., the cadence drops more than 10 rpm below the self-selected cadence and is not increased even with continued encouragement), or (3) unable to continue safely (in the opinion of supervising personnel).
It should be noted that the latter reason for termination is uncommon, as CWRCE is preceded by an incremental cycle ergometer test in which a safety evaluation is conducted.
At the end of exercise, the duration of exercise is recorded (in minutes and seconds).
Comparing Work Rate During Constant Work Rate Cycle Ergometry and During Lower Limb Activities in Daily Life
A critical issue in CWRCE is the selection of a work rate that is a relevant indicator of work rates required during daily activities. There is a critical relationship between the intensity of the muscular work performed, degree of muscular conditioning, and the associated ventilatory response, which interacts with the magnitude of the individual’s expiratory flow limitation resulting in the degree of breathlessness experienced. While the work rate imposed during CWRCE can be measured in a straightforward manner, this is generally not the case for activities of daily living. However, the well-established relationship between work rate and V̇O2 allows for a close approximation of the work rate during daily life activities by using V̇O2 as the critical linkage parameter.
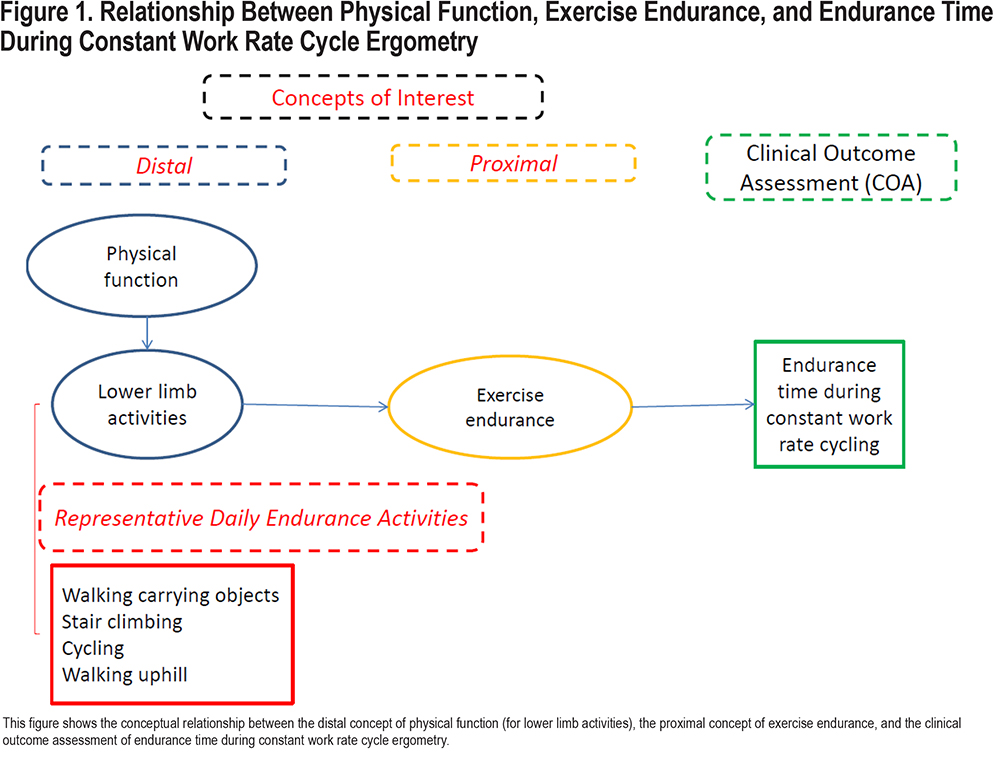
The Compendium of Physical Activities29 documents the metabolic requirements for a multitude of daily activities. In the document, metabolic requirements are described as metabolic equivalents (METs), defined as a multiple of the metabolic requirement at rest. One MET is defined as the oxygen uptake required in the resting state, which by convention is approximated as 3.5 milliliters of oxygen uptake per minute per kilogram of body weight (mL/kg/min). For example, “stair climbing, slow pace” requires 4.0 METs, which means a metabolic requirement that is 4 times the resting metabolic requirement. While the concept of METs has value in describing activity intensity to the lay public, the V̇O2 requirement has greater value for relating the work rate during CWRCE to the work rate required during everyday activities. So, the example of “stair climbing, slow pace” equates to a V̇O2 of 14.0ml/kg/min. The V̇O2 requirement may then be transformed into an estimate of the associated work rate by using the well-established relationship between work rate and V̇O2; for a 70kg individual, based on a V̇O2 requirement of 14.0ml/kg/min, “stair climbing, slow pace” has an associated work rate of 42 watts (W). Table 2 and Figure 2 illustrate a representative list of activities in daily life involving the lower limbs, accompanied by the MET equivalent, the associated V̇O2 requirement and the estimated work rate (for a 70kg individual30,31; the equations in the footnote of Table 2 allow calculations for individuals of other body weights). Thus, the work rates performed (and the associated cardiorespiratory response) during CWRCE are representative of the work rates (and the associated cardiorespiratory response) of many lower limb activities that people with COPD perform during daily life.
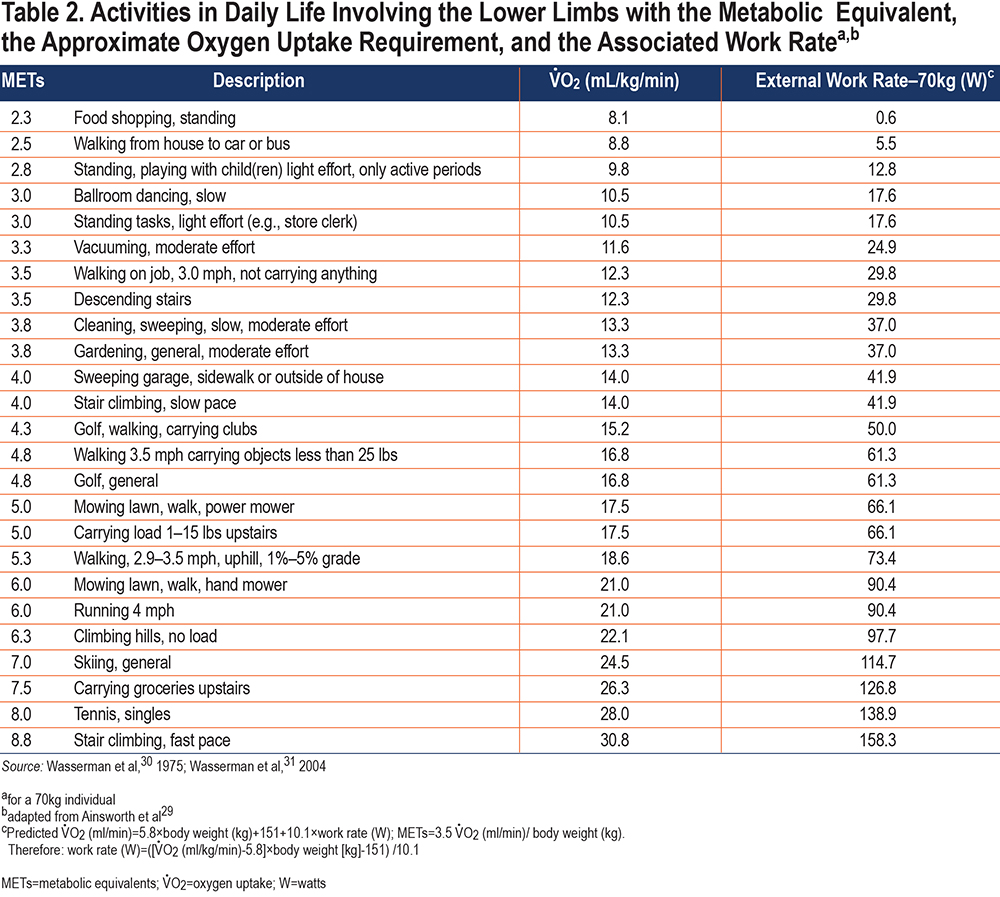
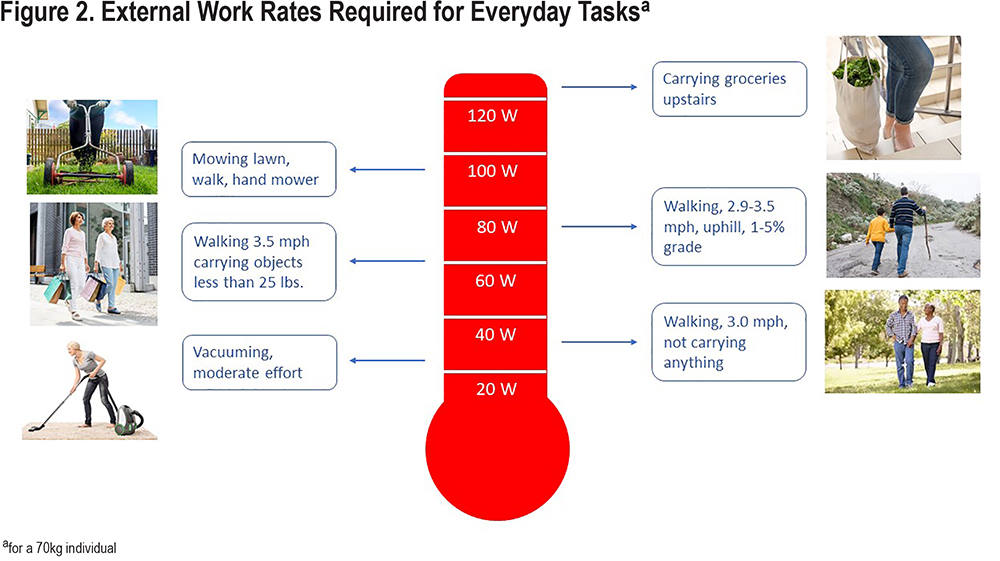
Work rates for CWRCE testing are selected to be ones that the given individual with COPD can sustain for only a limited period of time (e.g., 6 minutes). Using Table 2, that work rate can be related to a relevant activity the individual may wish to perform. An improvement in CWR exercise time as a result of an intervention implies that the individual will be capable in everyday life of performing that activity for a longer duration without stopping.
In conclusion, endurance time during CWRCE is proposed as an efficacy endpoint for use in clinical trials evaluating drugs or other interventions such as pulmonary rehabilitation. We have demonstrated here that improved exercise endurance time has a direct relationship to an individual with COPD’s experience of physical functioning in daily life, which is a meaningful patient-centered benefit. Future publications will focus on the process of assembling a database of endurance time responses of individuals with COPD to endurance-enhancing interventions and subsequent characterization of the factors contributing to endurance time improvement.
Acknowledgements
Author contributions: All authors have significantly contributed to the intellectual content of this article and have given final approval of the version that has been submitted for publication.
Medical writing assistance, provided by Nena O’Keefe, was funded by the CBQC Constant Work Rate Exercise project funders.
COPD Biomarkers Qualification Consortium Constant Work Rate Exercise Working Group Members: Richard Casaburi, PhD, MD, (academic co-chair), the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, California; Alan Hamilton, PhD, (industry co-chair), Boehringer Ingelheim, Canada, Ltd; Christopher B. Cooper, MD, University of California, Los Angeles; Gale Harding, MA, Evidera, Bethesda, Maryland; Nick Hopkinson, FRCP, Brompton Hospital, London, United Kingdom; Nancy Kline Leidy, PhD, Evidera, Bethesda, Maryland; Nicholas Locantore, PhD, GlaxoSmithKline, Collegeville, Pennsylvania, (at the time of the study); Debora Merrill, MBA, COPD Foundation, Washington, DC; Francois Maltais, MD, Université Laval, Quebec, Canada; Divya Mohan, MRCP, PhD, GlaxoSmithKline, Collegeville, Pennsylvania (at the time of this study); Alberto Neder, MD, Queens University, Kingston, Canada; Andrea Noronha, Syneos Research, Morrisville, North Carolina; Dennis O’Donnell, MD, Queens University, Kingston, Canada; Michael Polkey, PhD, FRCP, Brompton Hospital, London, United Kingdom; Janos Porszasz, MD, PhD, the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, California; Stephen Rennard, MD, University of Nebraska Medical Center, Omaha, Nebraska; Harry B. Rossiter, PhD, the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, California; Frank Sciurba, MD, University of Pittsburgh, Pittsburgh, Pennsylvania; Sally J. Singh, PhD, University of Leicester, United Kingdom; Martijn A. Spruit, PhD, CIRO, Expertise Centre for Chronic Organ Failure, Horn, Netherlands; Ruth Tal-Singer, PhD, COPD Foundation, Washington, DC, (formerly at GlaxoSmithKline); Martyn Walker, Syneos Research, Ebchester, England, United Kingdom; Susan Ward, PhD, Crickhowell, Wales, United Kingdom; Ren Yu, Evidera, Bethesda, Maryland.
All Working Group members were provided this manuscript prior to submission for publication.
Declaration of Interest
Richard Casaburi reports consulting fees/honoraria from Boehringer Ingelheim, GlaxoSmithKline, Regeneron, Abbott, and Respinova. He is involved in contracted clinical research with Regeneron, United Therapeutics, and Boehringer Ingelheim. Debora Merrill has no conflicts to disclose. Gale Harding is employed by Evidera, a health care research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In this salaried position, she works with a variety of companies and organizations and receives no payment or honoraria directly from these organizations for services rendered. Nancy Kline Leidy is employed by Evidera, a health care research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In this salaried position, Dr. Leidy works with a variety of companies and organizations. She receives no payment or honoraria directly from these organizations for services rendered, with the exception of honoraria received for her advisor role on several National Institutes of Health and FDA-funded programs: PATIENTS, PCAR, and NUCOAT. Harry Rossiter is supported by grants from the National Institutes of Health (R01HL151452, R01HL153460, P50HD098593, R01DK122767, P2CHD086851), the Tobacco-Related Disease Research Program (T31IP1666), and the University of California, Office of the President. He reports consulting fees from Omniox Inc., and is involved in contracted clinical research with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Astellas, United Therapeutics, Genentech, and Regeneron. Ruth Tal-Singer is a former employee (retiree) and current shareholder of GlaxoSmithKline. She reports personal fees from Immunomet, Vocalis Health, Teva and ENA Respiratory. She is a member of ENA Respiratory Board of Directors. Alan Hamilton was an employee of Boehringer Ingelheim during the writing of this manuscript.