Running Head: Review: Home Non-Invasive Ventilation in COPD
Funding Support: This study was funded by a non-commercial grant from ResMed/Phillips. The funders were not involved in design or direct conduct of the study and have not been involved in any aspect of the analyses, interpretation of findings nor drafting, editing, or submission of this manuscript.
Date of Acceptance: March 3, 2022 │ Published Online: March 8, 2022
Abbreviations: non-invasive ventilation, NIV; chronic obstructive pulmonary disease, COPD; randomized controlled trials, RCTs; long-term oxygen therapy, LTOT; adverse events, AEs; relative risk, RR; hazard ratio, HR; inspiratory positive airway pressure, IPAP; arterials partial pressure of carbon dioxide, PaCO2; World Health Organization International Clinical Trials Registry, WHO ICTRP; confidence interval, CI; weighted mean difference, WMD; intensive care unit, ICU; Severe Respiratory Insufficiency questionnaire, SRI
Citation: Dretzke J, Wang J, Yao M, et al. Home non-invasive ventilation in COPD: a global systematic review. Chronic Obstr Pulm Dis. 2022; 9(2): 237-251. doi: http://doi.org/10.15326/jcopdf.2021.0242
Online Supplemental Material: Read Online Supplemental Material (1731KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease characterized by respiratory symptoms, airflow limitation, and recurrent exacerbations. It is associated with significant morbidity, mortality, and poor quality of life, and places a substantial and increasing burden on health care systems.1 Non-invasive ventilation (NIV) has been shown to be beneficial in treating patients hospitalized with acute hypercapnic respiratory failure due to COPD exacerbations.2 The evidence on long-term use of NIV at home for management of COPD is much more uncertain, as reflected by conditional recommendations made by the European Respiratory Society.3
There are several systematic reviews4-7 on home NIV, the most recent one by Wilson et al,8 which included 31 studies (RCTs and non-randomized studies). It is likely that these reviews have underestimated the available evidence at the time they were undertaken, as most have not considered, or fully considered, studies undertaken in China, which are only published in the Chinese language. Despite Chinese bibliographic databases being included in a past review, 7 only 23 RCTs were found, which we show in this paper is less than half of the RCTs undertaken. The difference is likely related to the way databases have been searched and/or eligibility criteria (e.g., patient characteristics) of studies for review.
The systematic review reported in this article is the most comprehensive to date. It includes both RCTs and non-randomized studies, and is based on comprehensive search strategies, including Chinese language databases. The aim was to: (1) present an up-to-date and comprehensive evidence base on the effectiveness of home NIV for COPD by including studies published in any language, and (2) explore the impact of the inclusion of more recent and/or Chinese language studies on the current evidence base.
Methods
This review updates and extends a previous systematic review by the authors 6,9 A summary of the methods is presented here.
Bibliographic databases (MEDLINE, MEDLINE In Process, EMBASE, Cochrane CENTRAL, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Science Citation Index Expanded, China National Knowledge Infrastructure (CNKI) and Wanfang databases) and clinical trial registries were searched to February 2020 (see the online supplement). There were no restrictions on study design or language of publication. Citation lists of relevant studies were checked. Randomized controlled trials or non-randomized studies comparing any form of domiciliary NIV with any form of usual care, in adult patients with COPD, with or without hypercapnia or long-term oxygen therapy (LTOT) were included. Relevant outcomes (at any time points) were mortality, hospitalizations, exacerbations, quality of life, adverse events, (AEs) and adherence to NIV.
Study selection was performed by 2 reviewers independently (JD and either JH, RM, AA, or DMu), with disagreements resolved by AT. Chinese titles and abstracts were screened by 1 reviewer (JW). Full texts were checked where necessary. Data extraction and risk of bias assessment was performed by 1 reviewer (JD, MY, NG, ML, or EZ) using a standardized, piloted data extraction form. All extracted data was checked for consistency by JD. Risk of bias assessment was based on the Cochrane Collaboration risk of bias tool10 for RCTs and adapted for non-randomized studies.
Study populations were classified as stable if they had no recent exacerbations, hospital admissions, or other major change in clinical parameters over a defined period (4 or more weeks). Study populations were classified as post-hospital if they started domiciliary NIV immediately after an admission to the hospital or an exacerbation; while not always reported, we would expect these patients to have required acute NIV while in the hospital, with domiciliary NIV being initiated on return to the patient’s home. Random effects meta-analysis was performed in STATA (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, Texas: StataCorp, LLC.) where there was clinical and methodological homogeneity for studies reporting the same outcome. This was the case for mortality (reported as relative risk [RR], hazard ratio [HR]), and number of hospitalizations and days in the hospital (reported as mean difference). Separate analyses were undertaken for randomized and non-randomized studies, stable or post-hospital populations, and follow-up times up to, and beyond, 2 years. Sensitivity analyses were performed to explore the effect of excluding non-Western or Western studies. All non-Western studies contributing to meta-analyses were conducted in China. Statistical heterogeneity was estimated with the I2 statistic. Results for other outcomes were tabulated and described. Funnel plots were generated where meta-analysis included 10 or more studies.
NIV pressure may influence effectiveness. Formal sub-group analysis according to mean inspiratory positive airway pressure (IPAP)/pressure support was not possible due to poor reporting of these aspects of ventilation. Sub-group analysis according to level of hypercapnia was also not possible, due to inconsistent reporting of mean arterial partial pressure of carbon dioxide (PaCO2) or variable cut-offs for inclusion based on PaCO2. Sensitivity analysis was performed to explore the effect of including only studies in Western/high income countries (World Bank/ Organisation for Economic Co-Operation and Development definition).
Results
A total of 103 studies were included (49 RCTs and 54 non-randomized studies) (Figure 1). A full list of included studies can be found in the online supplement.
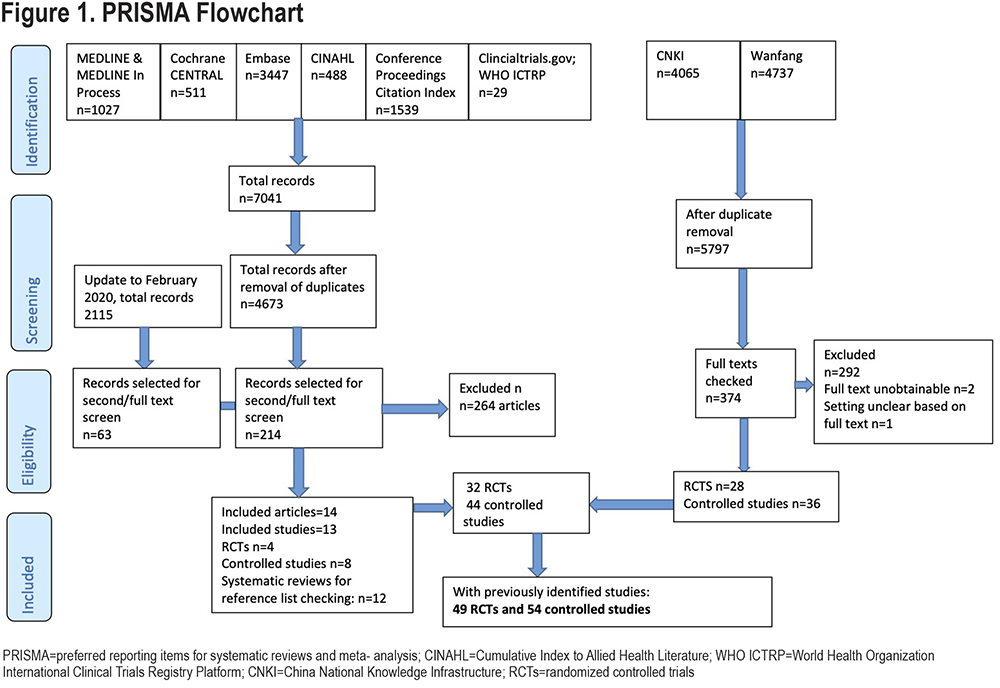
Most non-randomized studies (80%) were prospective. Follow-up times varied between 3 months and 10 years (retrospective analysis) but were most commonly 12 months. Studies included between 13 and 201 participants with the exception of 3 retrospective analyses11-13 which included substantially higher numbers (between n=1435 and n=37,014). Not including these latter 3 studies, the total number of patients across all studies was 6617, with varying proportions contributing to the respective outcomes.
Forty-one studies were conducted in a stable population, and 53 in a post-hospital population (9 studies could not be categorized regarding the stable/post-hospital status). There was little information on the length of time before initiation of NIV. Classification into stable or post-hospital was in some cases stated by authors, but where it was not obviously stated, or if the statement of stability was questionable based on other data shown, reported reasons for admission (exacerbation or worsening of symptoms), type of treatment prescribed (oral steroids, antibiotics), pH (arterial blood gases), length of hospital stay, and time after discharge were all considered to ensure that patients were truly clinically stable.
Where reported, patients had Global Initiative for Chronic Obstructive Lung Disease stage 3 and/or stage 4 COPD,1 i.e., severe disease (Table 1 in online supplement). Mean age was usually above 60 years (range 52 to 74), and most studies included more men than women (40%–98% men). A total of 75% of studies reported details on hypercapnia, mean PaCO2 or had a (variable) cut-off for inclusion based on PaCO2. Most studies included hypercapnic patients; only 4/103 studies14-17 (4%) stated that patients were (mostly) normocapnic. There was little information on exacerbation history. Reporting of NIV characteristics was variable. Most studies reported ranges for IPAP and expiratory positive airway pressure, but not averages. NIV was mainly pressure-targeted, and a variety of NIV machines were used. Masks were mainly nasal or oro-nasal (Table 2 in online supplement).
Risk of Bias
For RCTs, there was a lack of reporting of details related to blinding of outcome assessment (94% of studies), allocation concealment (86%), and generation of randomization sequence (55%) (Table 3 in online supplement). Only 3 RCTs17-19 included a “sham NIV” arm, and a further 3stated that LTOT or continuous positive airway pressure was used as a “placebo NIV” in the control arm.20-22 This lack of blinding to the intervention may have led to bias in patient-reported quality of life but may be less important for mortality and hospitalization outcomes. Risk of bias related to missing data was unclear or high for at least 1 study arm and/or outcome in 57% of RCTs.
Non-randomized studies are, by definition, more likely to be biased. Most were prospective and indicated that NIV and control groups were similar for selected baseline characteristics, but the effect of unknown confounders cannot be ruled out. Some retrospective studies had more evidence of baseline imbalances. Reporting on blinding of outcome assessment, incomplete data, and similarity of follow-up for NIV and control arms was poor (Table 4 in online supplement).
Main Findings
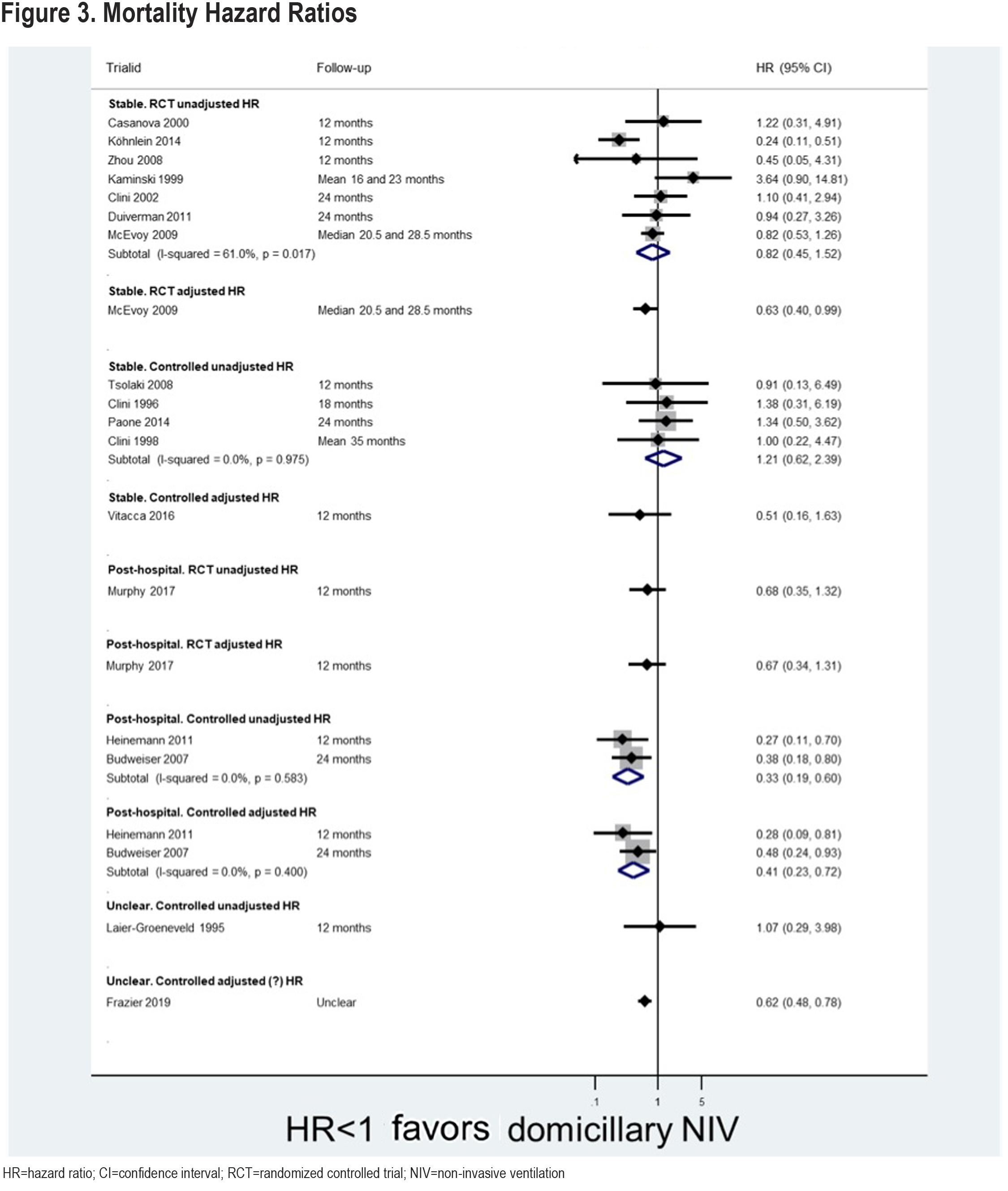
Mortality: Forty-six studies (45%) are represented in 1 or more forest plots. Figure 2 shows the RR for mortality (up to 24 months) across the different sub-groups. There was no statistically significant difference in mortality for the stable population based on 9 RCTs (RR=0.83 [0.54, 1.28], I2=51%) or 10 non-randomized studies (RR=0.85 [0.56, 1.28], I2=0%). This is consistent with hazard ratios (HRs) reported in some studies (Figure 3). Limiting to Western studies, or to only studies conducted in China (all non-Western studies) does not substantially change the findings (figures not shown).
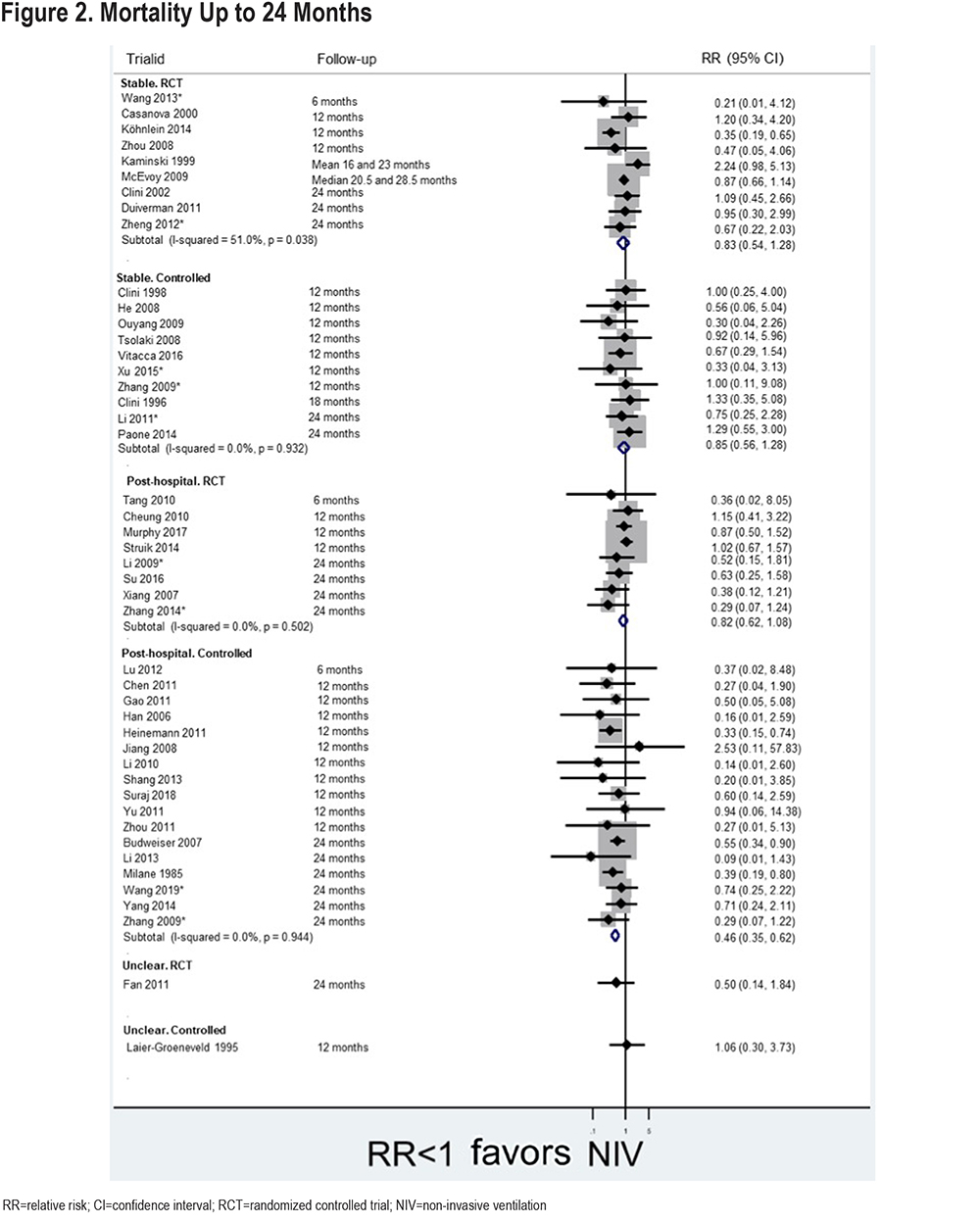
For the post-hospital population, pooled evidence from 9 RCTs also showed no significant difference in mortality (RR=0.78 [0.60, 1.03], I2=0%), but there was a statistically significant difference based on 17 non-randomized studies (RR=0.46 [0.35, 0.62], I2=0%). This is consistent with the limited number of HRs reported for this population. Limiting the analysis to Western studies does not substantially alter the findings; limiting to all non-Western studies, results in a statistically significant difference (in favor of NIV) based on 6 randomized studies (RR=0.58 [0.35, 0.95], I2=0%).
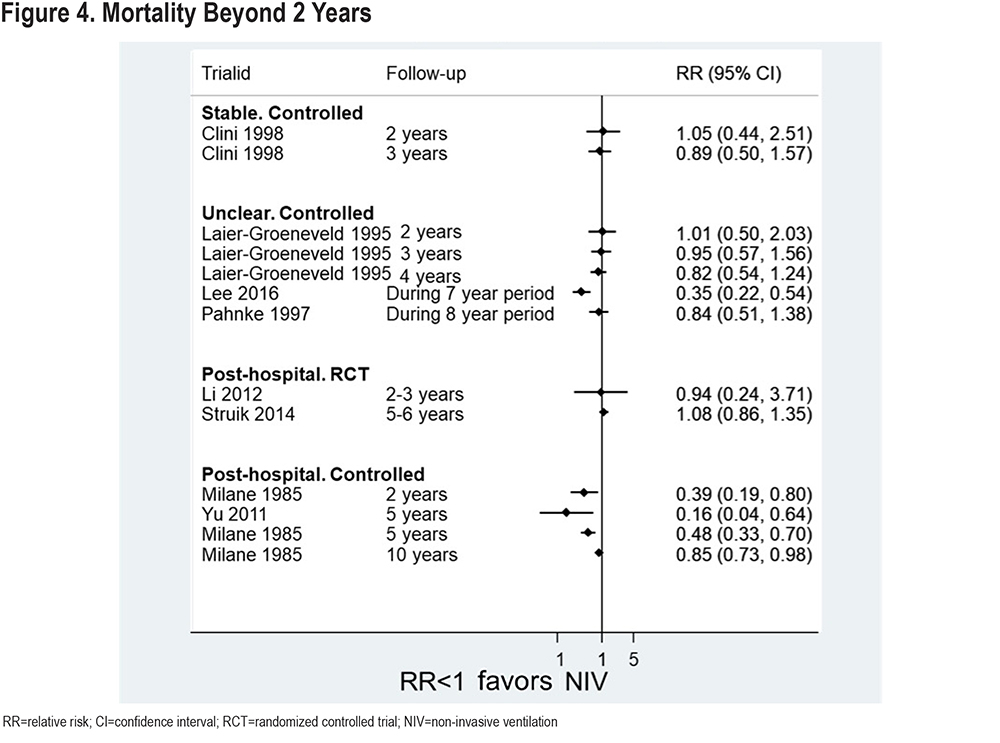
Results for all studies reporting mortality beyond 2 years are presented in Figure 4. Only 2 RCTs23,24 are included, and these find no significant difference in mortality at 2-3 and 5-6 years respectively (post-hospital population). The non-randomized studies (post-hospital population) appear to find a benefit in favor of NIV, though this diminishes at later time-points – this is based on limited data. All but 2 studies contributing data to this analysis are from a Western health care setting.
Hospitalizations: Figure 5 shows the pooled mean difference in number of hospital admissions per patient per year. Statistically significant differences in favor of NIV can be seen for both stable and post-hospital populations, and across RCTs and non-randomized studies (except for intensive care unit [ICU] admissions specifically where there is limited data). The reduction in number of admissions per patient per year is just over 1 for the stable population and around 2 for the post-hospital population. Limiting to non-Western studies does not substantially change findings, but slightly increases the reduction in admissions to closer to 2 in the stable population sub-groups (figure not shown).
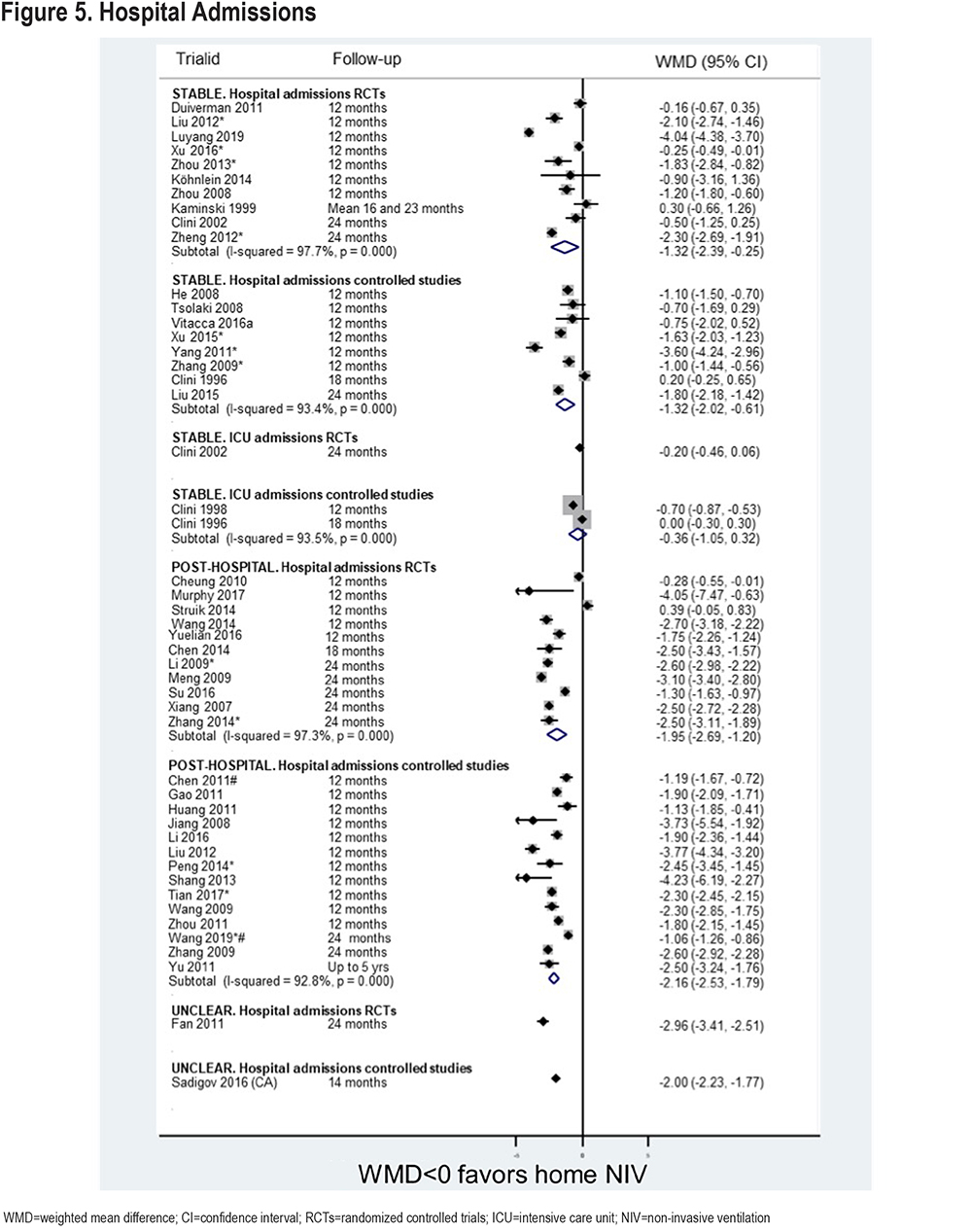
Significant differences are not seen when only Western studies are included (Figure 6). Data for a post-hospital population is limited to 2 RCTs.24,25 These find very disparate results, with 1 showing a statistically significant difference in favor of NIV (around 4 admissions per year less), the other finding no difference. The driving factor behind this disparity is most likely to be the minimum criterion for persistent hypercapnia for enrollment; 48 hours of persistent hypercapnia for the study by Struik et al24 and 2 weeks for the study by Murphy et al25 such that patients in the former study were still recovering when they started the study. When stabilized, patients in the latter study had more severe hypercapnia than those in the former, which may have predisposed the patients to better outcomes with NIV.
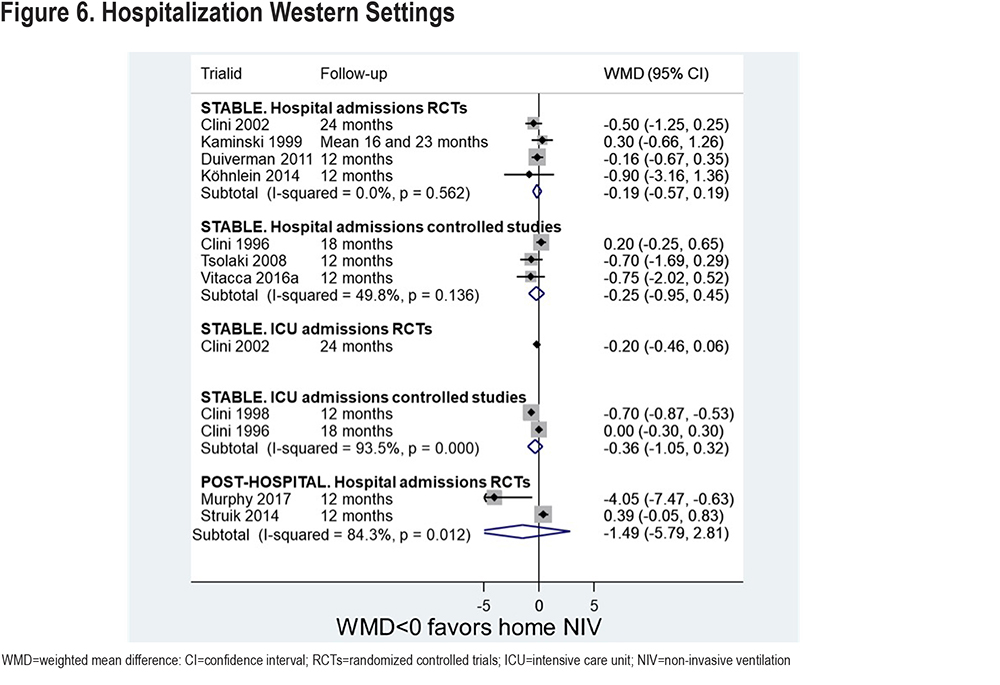
Days in the hospital were also measured in some studies (Figure 7). There is a statistically significant difference in favor of NIV for the stable population RCT sub-group (59 days difference), though not for the stable population sub-group of non-randomized studies.
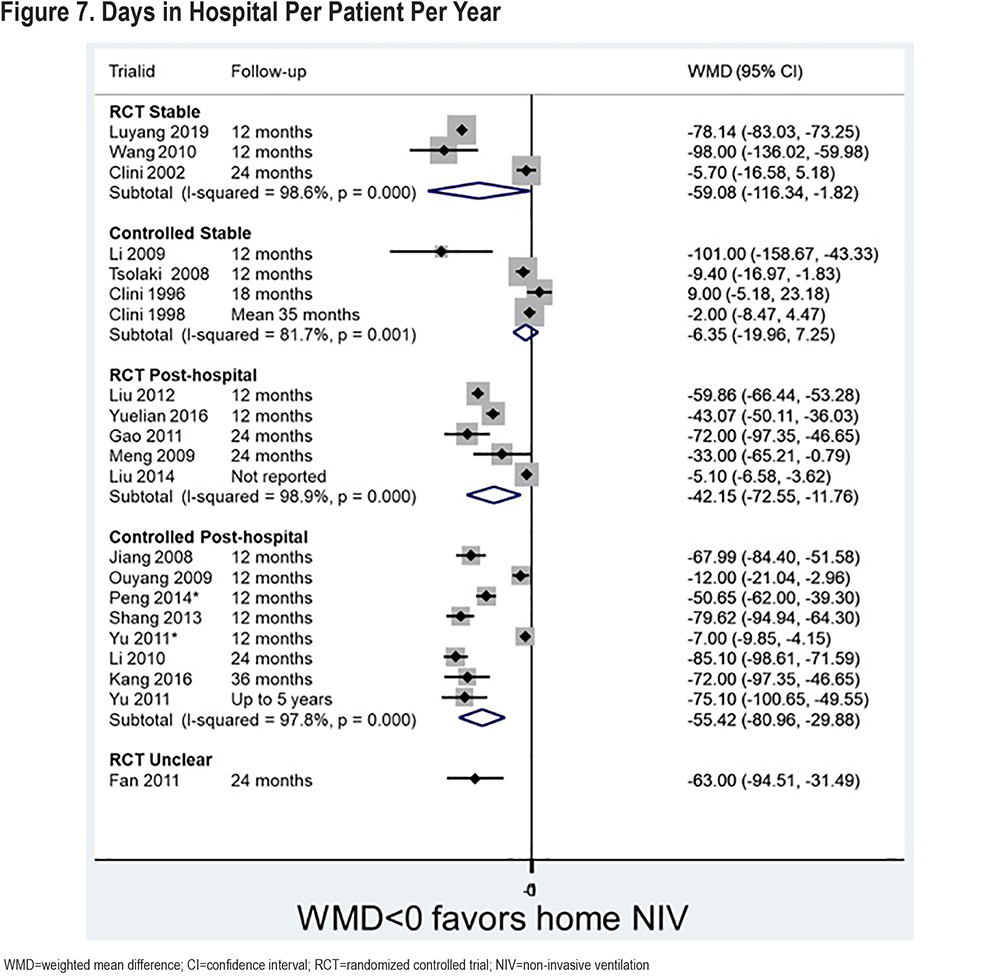
There are also statistically significant differences in favor of NIV for both post-hospital population sub-groups (RCTs and non-randomized studies, 40-and 60-days difference). These large differences in days in both the stable and post-hospital group, despite the difference in number of admissions being only 1-2 days, are likely a reflection of different health care settings. Most studies contributing data to this analysis were conducted in China; there is only limited data for Western studies (1 RCT26 and 3 non-randomized studies,27-29 figure not shown), and there are no significant differences.
Funnel plot asymmetry suggestive of bias was observed for mortality (non-randomized studies, post-hospital population) and hospital admissions (RCTs and non-randomized studies, post-hospital population). There was no obvious asymmetry in the funnel plot for mortality (non-randomized studies, stable population).
Exacerbations: Thirty-four studies (33%) reported exacerbations and indicated whether there was a statistically significant difference between NIV and usual care groups (Table 5 in online supplement). For a stable population, 4/7 (57%) RCTs and 5/6 (83%) non-randomized studies found a significant difference in favor of NIV. For a post-hospital population, this was the case for 6/8 (75%) RCTs and 13 (100%) non-randomized studies. No studies found a statistically significant difference in favor of usual care. Most of the evidence comes from non-Western studies; only the sub-group of RCTs in a stable population includes a greater proportion (57%) of Western studies.
Quality of Life: Forty-six studies reported quality of life (45%). A total of 14 quality of life assessment tools (where stated) were used 63 times across the studies (Table 6 in online supplement); most commonly the St George’s Respiratory Questionnaire (24 studies), the Severe Respiratory Insufficiency questionnaire (SRI) (8 studies), the COPD Assessment Test (6 studies) and the Chronic Respiratory Disease Questionnaire (5 studies).
Most studies found a statistically significant difference in quality of life favoring NIV, particular for post-hospital populations (RCTs and non-randomized studies) and the stable population (non-randomized studies). Some RCTs conducted in a stable population found a benefit from NIV based on 1 assessment tool but not others, or only for some subscales, time-points, or population sub-groups. Nonetheless, only one study30 of 15 RCTs with a stable population did not find any statistically significant difference in favor of NIV. No studies found a benefit in favor of usual care.
Most of the evidence is from non-Western studies. Only the sub-group of RCTs in a stable population includes a greater proportion of Western studies (53%).
Adverse Events and Adherence: Only 28% of studies (29/103) reported on specific AEs with NIV use, with a further 5% (5/103) reporting more generally on an inability to adapt to NIV or discomfort (Table 7 in online supplement). Commonly reported AEs were mucosal dryness, facial rash or injury, bloating, mask intolerance/leak, fear/anxiety, and inability to sleep. There was 1 suspected barotrauma/pneumothorax,31 and 1 further study32 specifically stated that there were no such cases.
Twenty studies (19%) reported discontinuation rates due to AEs with NIV (between 0% and 43%). Higher discontinuation rates were seen in older studies (pre-2000) compared with more recent studies (rates between 0% and 17%). Discontinuation rates were 5% or lower in 12/20 studies (60%). Twenty-two studies (21%) also reported mean hours use/night. These were commonly between 5 and 7 hours (range 3–9 hours), mainly from machine-based recordings of use. However, this does not give us any information on how many patients used NIV for a minimum time-period and is, therefore, of limited use in terms of gauging adherence.
Discussion
This systematic review highlights a considerably larger evidence base for home NIV in COPD than previously identified, emphasizing the importance of a global approach to evidence synthesis, which goes beyond the databases many Western reviewers typically consider. By including studies published in any language, including those undertaken in China and not indexed in standard medical databases (62% of studies included), the evidence base was increased substantially, while also reflecting the use of NIV more globally. It finds no significant effect on mortality based on RCTs for either the stable or post-hospital population based on all studies; a significant difference was found for Chinese studies only (RCTs) and for all non-randomized studies in a post-hospital population. For the latter group, bias from known and unknown confounders cannot be ruled out. This is in line with our previous findings,9 but in contrast to recent findings by Wilson et al8 which were based on fewer studies and inappropriately combined RCTs and non-randomized studies in a single analysis. Results from different study designs are likely to differ systematically and should not be combined in a meta-analysis.33 Further, pooled odds ratios are presented in Wilson et al,8 which are not directly comparable to our pooled RRs or HRs.
We found a small but statistically significant reduction in number of hospital admissions across all sub-groups with NIV (between 1 and 2 days). Although the reduction is small, this may have substantial resource implications by preventing further hospital admissions, as the risk of admission remains higher after a previous admission, particularly in the first 3 months.34 In contrast, Wilson et al8 found no significant difference in hospital admissions, which is in line with our sensitivity analysis of Western studies. This may be due to the small number of studies contributing to the pooled effect estimate or may be a reflection of non-Western health care systems having a lower threshold for admitting patients to the hospital. We also looked at days in the hospital and found a substantial reduction largely driven by studies undertaken in China where patients may be more likely to stay in the hospital once admitted.
Findings need to be considered in the context of risk of bias in included studies. Most studies did not blind patients given the nature of the intervention; however, this is unlikely to have an effect on mortality or hospitalizations. The potential for selection bias between patients receiving NIV or standard care in the non-randomized studies remains a concern, as does the asymmetry observed in some funnel plots which could be suggestive of publication bias.
We found a clear trend towards improvement in quality of life and reduction in exacerbations with NIV. The size of effect on quality of life is difficult to estimate as studies used a variety of tools at different timepoints; some, such as the SRI, could be considered more appropriate in this population. The inability to blind patients to the intervention may have led to bias in the assessment of this outcome.
Despite the large number of studies, there was poor reporting of parameters which would allow further sub-group analysis, for example mean IPAP or level of hypercapnia. However, even more detailed reporting may be insufficient to fully evaluate which type of patient will benefit most from which type of NIV. Few studies had follow-up beyond 24 months and uncertainty, thus, remains around potential longer-term benefits.
Improving reporting in primary studies, particularly for patient characteristics, risk of bias parameters, AEs, and adherence, will be key in ensuring systematic reviews of those studies are informative. Future systematic reviews should consider searching the CNKI and Wanfang databases as standard, or risk missing large amounts of evidence. This entails resource implications in terms of including Chinese speakers to collaborate on reviews.
Given the large number of existing studies, further similar studies are unlikely to add much value. Future studies should consider targeting specific sub-groups of patients (e.g., those with pre-defined levels of hypercapnia) and the use of higher-pressure settings. Other useful future studies might be those exploring the role of NIV in weaning strategies, e.g., for patients who cannot be weaned in the hospital.35 Other patients who might benefit from NIV may be those with stable hypercapnic COPD and obstructive sleep apnea/obesity hypoventilation syndrome, and studies exploring the role of screening stable hypercapnic COPD patients to identify this group may also be of interest.36 Individual participant data analysis is likely to be needed to fully elucidate which patients are likely to benefit most from NIV. The role of tele-monitoring in potentially improving effectiveness of NIV has also not yet been well evaluated. We found only 1 study37 exploring this, but other studies are ongoing.38-40
Conclusion
This systematic review is based on a larger evidence base than previous similar reviews, driven by the inclusion of studies undertaken in China. Despite the large evidence base there was no evidence of a mortality benefit for stable populations (any analysis) or a post-hospital population (based on all RCTs). There was some evidence of a benefit from NIV for post-hospital populations (RCTs set in China only and non-randomized studies). There was also a potential reduction in hospitalizations, exacerbations, and improvement in quality of life. There is uncertainty associated with these findings, particularly where they are based on non-randomized studies, and further research is required to establish which type of patient may benefit most from which type of NIV.
Acknowledgements
Author contributions: JD undertook searches, screening, study selection, and data extraction of English language studies, undertook the analysis, and wrote the first draft of the report. JH undertook screening and study selection of English language studies and contributed to analyses. AMT managed the project, and contributed to design of the study, screening, and study selection, analyses, and writing the draft. DM and SJ contributed to the design of the study, project management, study selection, analyses, and writing the draft report. Both JD and AMT accessed and verified the (English language) data. JW undertook searches of Chinese language databases, study selection of Chinese language studies, and contributed to data extraction. MY, NG, EZ, and ML contributed to study selection and undertook data extraction of Chinese language studies. RM and DMu contributed to screening and study selection, clinical interpretation of findings, and writing the draft. All authors had full access to all the data in the study, had final responsibility for the decision to submit for publication including reviewing and approving the final, submitted manuscript.
The authors acknowledge Dr. Alain Al Helou for help with screening records for eligibility.
Declaration of Interest
AMT has received grant funding to her institution for the work contained in the manuscript from ResMed/Phillips, which funded JH and JD, as well as contributions for oversight from DM and SJ. AMT has also received grant funding and/or honoraria from several companies involved in the treatment of COPD, specifically AstraZeneca, Chiesi, GlaxoSmithKline, and Boehringer Ingelheim. JD, DM, JH, and SJ have no additional conflicts of interest. JW, MY, NG, ML, EZ, DMu, and RM have no conflicts of interest to declare.