Running Head: Heart Rate Variability and Risk of Acute Exacerbation
Funding Support: The Beta-Blockers for the Prevention of Acute Exacerbations of COPD trial (BLOCK COPD) was supported by a grant from the Department of Defense (W81XWH-15-1-0705). DMM was supported by the University of Minnesota T32 Training in Lung Science training grant (2T32HL007741-26A1). This material is also the result of work supported with resources and the use of facilities at the Minneapolis VA Medical Center, Minneapolis. The views expressed in this article are those of the authors and do not reflect the views of the United States Government, the Department of Defense, the Department of Veterans Affairs, or any of the authors’ affiliated academic institutions.
Date of Acceptance: April 2, 2022 │ Published Online: April 9, 2022
Abbreviations: chronic obstructive pulmonary disease, COPD; acute exacerbation of COPD, AECOPD; heart rate variability, HRV; standard deviation of normal RR intervals, SDNN; root mean square of successive RR interval differences, RMSSD; electrocardiograms, ECGs; Beta Blockers for the Prevention of Acute Exacerbations of COPD trial, BLOCK COPD; forced expiratory volume in 1 second,FEV1;confidence interval, CI; forced vital capacity, FVC; long-acting muscarinic antagonists, LAMAs; St George’s Respiratory Questionnaire, SGRQ; locally estimated scatterplot smoothing line, LOESS; standard deviation, SD; interquartile range, IQR; adjusted hazard ratio, aHR
Citation: MacDonald DM, Mkorombindo T, Ling SX, et al. Heart rate variability on 10-second electrocardiogram and risk of acute exacerbation of COPD: a secondary analysis of the BLOCK COPD trial. Chronic Obstr Pulm Dis. 2022; 9(2): 226-236. doi: http://doi.org/10.15326/jcopdf.2021.0264
Online Supplemental Material: Read Online Supplemental Material (481KB)
Note: Portions of these data were published in abstract form at the CHEST 2021 Virtual Conference.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common progressive respiratory disease with an estimated worldwide prevalence of 13.1% (95% confidence interval [CI] 10.2% to 15.6%).1 COPD has a high burden of morbidity and mortality, much of which is caused by periods of worsening respiratory symptoms, known as acute exacerbations of COPD (AECOPD).2,3 Established risk factors for AECOPD include worse lung function, a history of prior AECOPD, tobacco smoking, and a greater burden of respiratory symptoms.2,4 Autonomic nervous system dysfunction is common in COPD, and may be an additional marker of AECOPD risk, but has not been well investigated.5,6
Heart rate variability (HRV) measures the variation in time between heart beats and is one measure of autonomic function. HRV is influenced through multiple pathways but is, in part, a marker of the balance between the sympathetic (i.e., fight or flight response) and parasympathetic (i.e., rest and digest) components of the autonomic nervous system. Part of the parasympathetic input to HRV is a result of pulmonary stretch receptors, which synapse in the central nervous system before acting on the sinoatrial node, a phenomenon best known as respiratory sinus arrhythmia.7 HRV can be measured in the time domain, which measures the variability in time between successive heart beats, or in the frequency domain, which measures the distribution of heart rate variability into frequency bands. HRV measurements typically require longer (typically 2 to 10 minutes, but up to 24 hours) electrocardiogram (ECG) recordings, but 10-second measurements of the standard deviation of normal RR intervals (SDNN) and root mean square of successive time differences between normal heartbeats (RMSSD), which are both time domain HRV measurements, have moderate to strong correlation with 5-minute recordings (Pearson’s correlation 0.76 [95% CI 0.74 to 0.77] for SDNN and 0.85 (0.84 to 0.86) for RMSSD).8,9 In population-based cohorts, lower values of SDNN and RMSSD on 10-second ECG were associated with increased mortality.10,11
Greater HRV reflects a greater entropy of the autonomic nervous system, providing a positive ability to respond to changes in environmental or physiologic stimuli.7 Time domain HRV measures are decreased in stable COPD, but little is known about the relationship between time domain measurements of HRV and AECOPD, and we are aware of no studies analyzing HRV as a risk factor for AECOPD.5
In this post-hoc analysis, we used data from the Beta-Blockers for the Prevention of Acute Exacerbations of COPD (BLOCK COPD) study to test the hypotheses that higher SDNN and RMSSD on 10-second ECG are markers of decreased risk of AECOPD, and that these relationships would be impacted by random assignment to metoprolol versus placebo that occurred in BLOCK COPD.12
Methods
Participants
Beta-Blockers for the Prevention of Acute Exacerbations of COPD Trial: The details of the BLOCK COPD trial were previously published.12,13 Briefly, BLOCK COPD randomized participants with COPD and at least moderate airflow obstruction (forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC]ratio < 0.7 and FEV1 < 80% predicted) to metoprolol succinate versus placebo for the prevention of AECOPD. Participants were started on metoprolol succinate 50 mg once daily (or matching placebo) at randomization and dosage was titrated over 42 days based on heart rate, systolic blood pressure, changes in FEV1, and possible beta-blocker side effects to a final dose of 25, 50, or 100 mg once daily. BLOCK COPD recruited participants at increased risk of AECOPD by requiring at least 1 of the following: (1) receipt of corticosteroids or antibiotics for a breathing problem within the last year, (2) an emergency department visit or hospitalization for AECOPD within the last year, or (3) receipt of a prescription for supplemental oxygen for home use for COPD. Participants could not be enrolled until at least 4 weeks from their most recent exacerbation. Exclusion criteria included heart failure with a known left ventricular ejection fraction of < 40%, tachyarrhythmias and bradyarrhythmias requiring treatment, medications that affect atrioventricular node conduction, pacemakers, and a history of myocardial infarction or revascularization within the last 36 months. Participants were followed until day 336, or until the time the trial was stopped early based upon conditional power analyses and safety concerns.
Current Analysis: All participants in BLOCK COPD were included in this analysis. We excluded ECGs that had more than 3 premature atrial or ventricular complexes, any atrial or ventricular arrhythmia, poor data quality which precluded RR interval measurement, or < 4 normal RR intervals.
Heart Rate Variability
In BLOCK COPD, resting 10-second ECGs were performed at the screening visit prior to randomization, during dose titration, at the day 42 dose finalization visit, and during follow-up at the day 112 and day 336 visits. ECGs were obtained by ECG machines available at each site, and a scanned copy was transmitted to the data coordinating center. Baseline and day 42 ECGs were analyzed by 1 investigator using the EP Calipers app (EP Calipers, Inc.) to measure RR intervals. The RR intervals before and after a premature ventricular or atrial complex were excluded.10 This was then cross-validated by a different investigator on the first 48 ECGs. We excluded RR measurements less than 500ms and greater than 1500ms, corresponding to heart rates more than 120 beats per minute and less than 40 beats per minute, respectively, which were felt to represent measurement error, a conduction abnormality that was not obviously a premature atrial or ventricular contraction, or an acute alteration in heart rate making inclusion inappropriate for testing our hypothesis. SDNN was defined as the standard deviation of normal (in sinus rhythm and not before or after a premature complex) RR intervals. RMSSD was defined as the square root of the average of the sum of squares of differences between normal RR intervals.
Acute Exacerbation of COPD
In BLOCK COPD, AECOPDs were defined as an increase or new onset of 2 or more of the following: cough, wheezing, dyspnea, increased sputum production, or chest tightness that was treated with antibiotics or systemic glucocorticoids for 3 or more days. AECOPDs were classified as mild (home management), moderate (visit to an emergency department), severe (hospitalization), and very severe (mechanical ventilation). In this analysis, we analyzed AECOPD of any severity, and separately analyzed severe AECOPD (those defined as severe or very severe in BLOCK COPD).
Statistical Analysis
The agreement between ECG readers (DMM and TM) was evaluated using Spearman’s correlation coefficients.
Cox proportional hazards models were used to test associations between baseline HRV measures (SDNN and RMSSD) and time to first AECOPD (any AECOPD and severe AECOPD). Adjusted models included age, sex, race, FEV1 percent predicted, history of coronary artery disease, use of inhaled long-acting muscarinic antagonists (LAMAs), smoking status, and treatment assignment (metoprolol versus placebo) as covariates and were stratified by study site. Kaplan-Meier plots and log-rank tests were used to evaluate the probability of remaining exacerbation-free between groups defined by quartiles of SDNN and RMSSD. We additionally tested associations between SDNN and RMSSD quartiles and time to first AECOPD using Cox-proportional hazards models, including the same covariates as in our primary analysis.
We incorporated day 42 SDNN and RMSSD measures into the Cox proportional hazards models by treating the HRV measures as time-varying covariates. This analytic approach accounts for variation in HRV measures over time and incorporates all available data (in this case both baseline and day 42 HRV measures). Adjusted models were constructed similarly as in the baseline analyses except that FEV1 percent predicted was also treated as a time-varying covariate as it was measured at both time points.
We assessed whether the relationship between HRV measures (baseline and treated as a time-varying covariate) and AECOPD was influenced by treatment assignment by testing for interactions in the respective models. Interaction terms reflect the ratio of HRs in those assigned to metoprolol versus placebo.
We compared the relationship between baseline HRV measurements (SDNN and RMSSD) and baseline FEV1 percent predicted and total St George’s Respiratory Questionnaire (SGRQ) score using Spearman’s correlation coefficient. Trends were visualized using a best fit line and locally estimated scatterplot smoothing (LOESS) line.14
Finally, we tested whether changes in SDNN and RMSSD from baseline to day 42 were affected by assignment to metoprolol versus placebo using Wilcoxon rank sum tests. Statistical analyses were conducted using R version 3.6.0 (https://www.R-project.org/).
Results
Participants
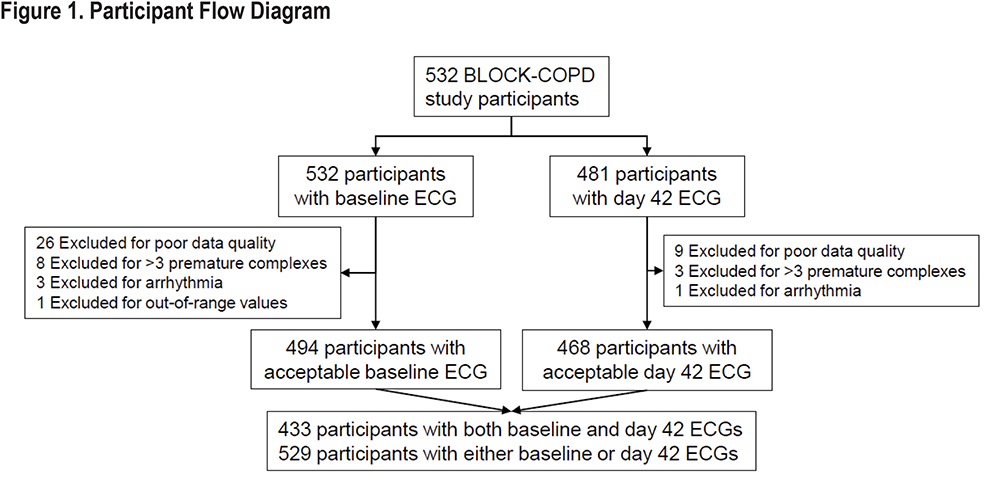
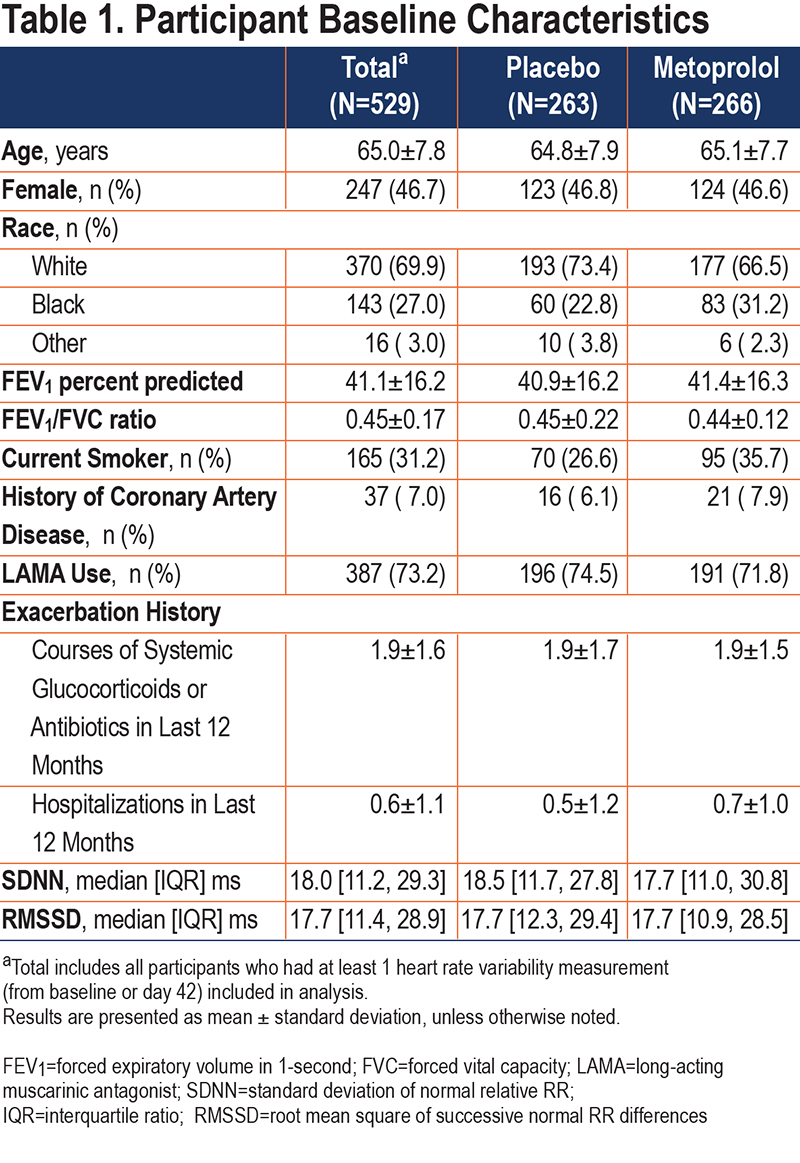
Of 532 BLOCK COPD participants, 529 (99.4%) were included in this analysis. See Figure 1 for participant flow diagram and reasons for exclusion. Baseline characteristics of included participants are shown in Table 1. Included participants had a mean (standard deviation [SD]) age of 65.0 (7.8) years and 46.7% were female. The mean FEV1 percent predicted was 41.1 (16.2) and 73.2% were on LAMAs. With the exception of current smoking status, baseline characteristics were similar in the placebo and metoprolol groups, and also similar to the parent BLOCK COPD trial.12 The placebo and metoprolol groups had similar baseline measures of SDNN (median [interquartile range (IQR)] 18.5 [11.7 to 27.8] and 17.7 [11.0 to 30.8], respectively) and RMSSD (17.7 [12.3 to 29.4] and 17.7 [10.9 to 28.5], respectively). The correlation between ECG readers in 48 tests was 0.96 for SDNN, with IQR for differences between readers of -2.2ms to 1.2 ms, and 0.89 for RMSSD with IQR for differences between readers of -2.5ms to 2.4 ms. Bland-Altman plots are included in the online supplement (e-Figure 1).
Associations Between Standard Deviation of Normal RR Intervals and Acute Exacerbations of COPD
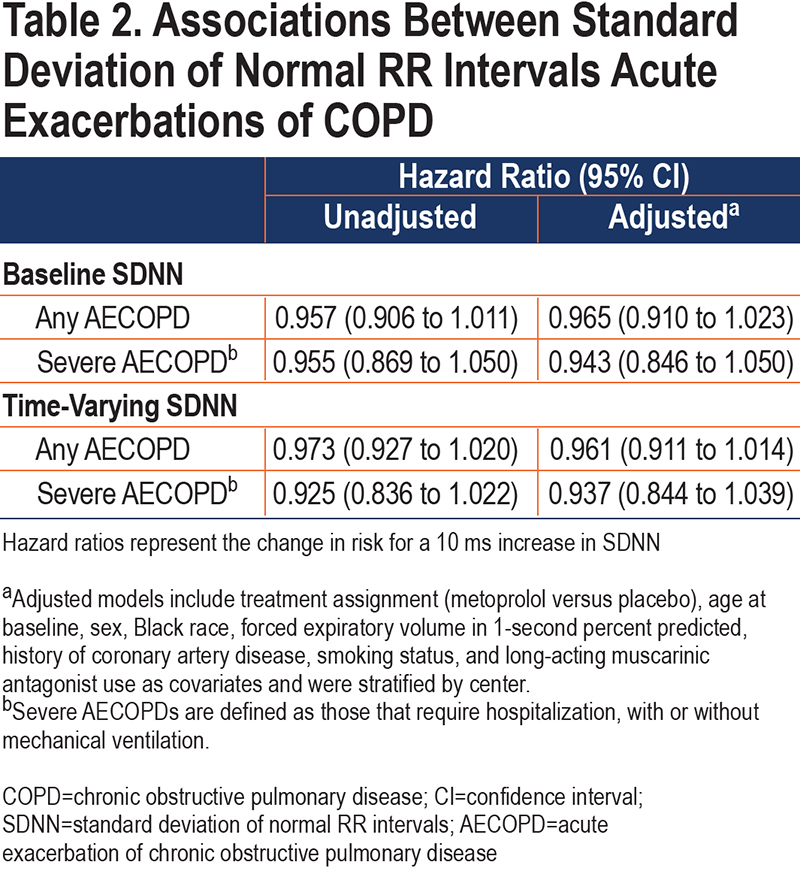
We did not find any significant associations between baseline or time-varying SDNN and time to any AECOPD or severe AECOPD (Table 2). Similarly, we did not find a significant relationship between SDNN quartile and time to any AECOPD or severe AECOPD. Kaplan-Meier curves, log-rank tests, and unadjusted and adjusted Cox-proportional hazards models for the association between SDNN quartiles and AECOPD are included in the online supplement (e-Figure 2).
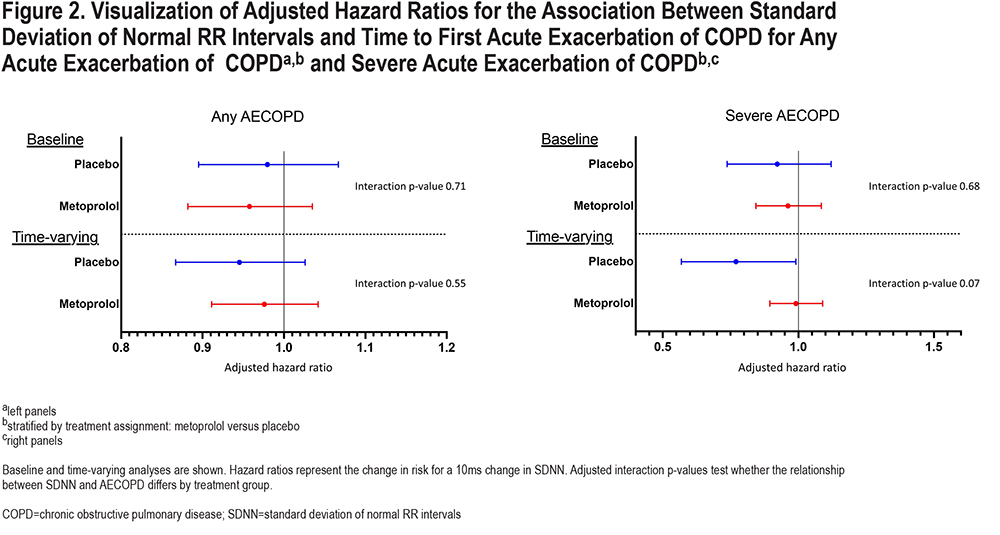
We did not find any statistically significant interactions between SDNN and assignment to metoprolol on time to any AECOPD or severe AECOPD. A visualizationis shown in Figure 2 and adjusted and unadjusted interaction terms, along with stratified analyses, are included in the online supplement e-Table 1.
Associations Between Root Mean Square of Successive RR Interval Differences and Acute Exacerbations of COPD
We did not find a significant association between baseline or time-varying RMSSD and time to any AECOPD, or severe AECOPD (Table 3). Similarly, there was no significant difference in the probability of remaining exacerbation-free between groups defined by quartiles in the online supplement (e-Figure 3).
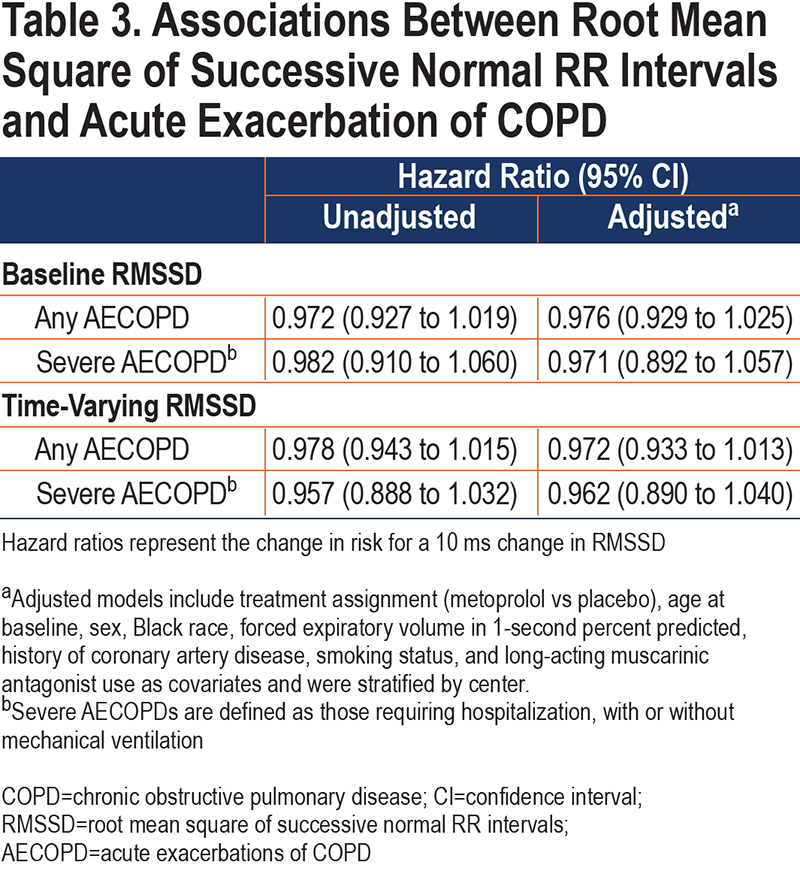
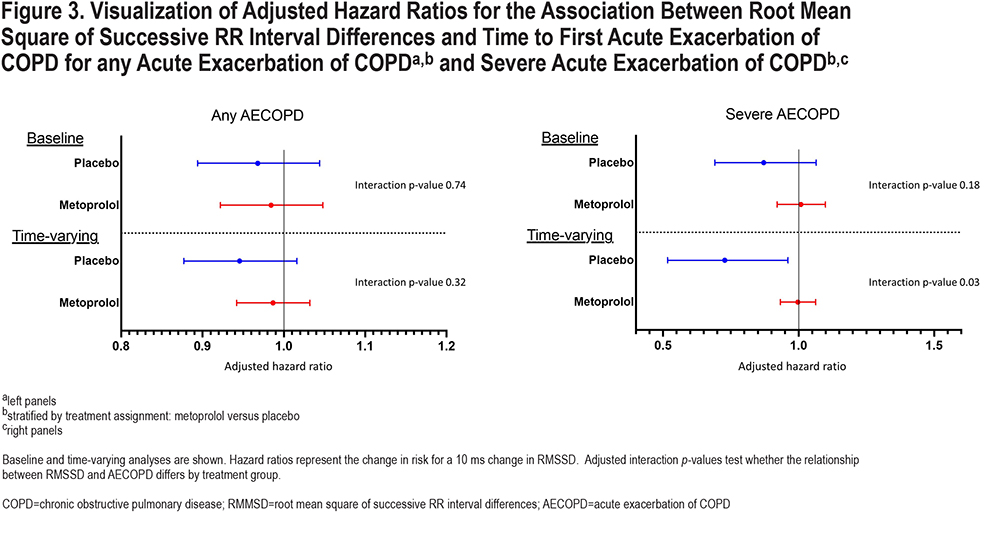
We did not find any significant interactions between RMSSD and assignment to metoprolol on time to any AECOPD or severe AECOPD using only baseline measures (Figure 3 and e-Table 2 in the online supplement). However, when day 42 measures were incorporated into the analysis, there was a significant interaction between treatment assignment and RMSSD on time to severe AECOPD (adjusted interaction p-value 0.032). Higher RMSSD was associated with lower risk of severe AECOPD in the placebo group (adjusted hazard ratio [aHR] 0.705 [95% CI 0.517 to 0.961]) but not in the metoprolol group (aHR: 0.996 [0.933 to 1.063]).
Associations Between Heart Rate Variability, Lung Function, and Respiratory Symptoms
We did not identify associations between baseline SGRQ scores and RMSSD or SDNN, and only found weak associations between baseline FEV1 percent predicted and RMSSD (Spearman correlation coefficient: -0.12, p-value=0.009) and SDNN (correlation coefficient: -0.11, p-value=0.011) (e-Figure 4 online supplement).
Effects of Metoprolol on Standard Deviation of Normal RR Intervals and Root Mean Square of Successive RR Interval Differences
We did not find any significant difference in the change (baseline to day 42) in SDNN between individuals assigned to metoprolol (median, IQR: 0.58, -8.4ms to 10.2ms) and individuals assigned to placebo (median, IQR: -2.3, -9.2ms to 6.1 ms; p-value for difference=0.11). However, metoprolol was associated with an increase in RMSSD compared to placebo (median, IQR: 1.2, -6.5ms to 10.8ms for metoprolol; -2.8, -10.1ms to 5.7ms for placebo; p-value for difference=0.002).
Discussion
In this hypothesis-generating analysis of exacerbation-prone COPD patients, we found no associations between SDNN or RMSSD and risk of AECOPD when all participants were analyzed together. However, when RMSSD was treated as a time-varying covariate we found that greater RMSSD was associated with a lower risk of severe AECOPD in participants assigned to placebo, but not participants assigned to metoprolol.
These findings suggest that lower RMSSD may be a marker of severe AECOPD risk in people with COPD who are not treated with beta-blockers. We found a significant association between RMSSD and severe AECOPD, but not any AECOPD. A potential interpretation of these findings is that RMSSD is not associated with risk of AECOPD, but when an AECOPD occurs, higher RMSSD is protective against hospitalization. This interpretation is consistent with the hypothesis that HRV reflects the ability of the body to respond to environmental or physiologic stimuli. Those with lower HRV have less ability to compensate for increased physiologic demands, such as AECOPD, and are more likely to require hospitalization, while those with greater HRV are more able to adapt (possess greater physiologic entropy) and require less intensive treatment. The protective effect of higher RMSSD was not present in those assigned to metoprolol, a beta-1 selective blocker which suppresses sympathetic activity.15 Though RMSSD is reflective of vagal/parasympathetic activity, and not thought to significantly reflect sympathetic activity, this finding suggests that those with higher RMSSD may respond to AECOPD with increased sympathetic activity, and that by blunting that response, metoprolol worsens the severity of AECOPD.7,16 Longer HRV recordings or more invasive testing, such as muscle sympathetic nerve activity, could help determine if participants with higher RMSSD also had evidence of higher sympathetic activity. Finally, we only found a significant interaction in analyses treating RMSSD as a time- varying covariate. This analytic approach evaluates the relationship between AECOPD and the risk factor measurement, in this case RMSSD, most proximal to the exacerbation and better accounts for variables that change over time than the analysis only including baseline RMSSD measures. By incorporating all available data, this approach increases power and may additionally suggest that RMSSD is a marker of short-term rather than longer-term severe AECOPD risk, and we only see significant associations when using more proximal measures of RMSSD.
While we found an interaction between RMSSD and metoprolol assignment on risk of severe AECOPD, we did not find a statistically significant interaction for SDNN. However, the trends in relationship between SDNN and RMSSD and risk of exacerbation were similar with higher levels of HRV being associated with lower risk of severe AECOPD in the placebo group but not in the metoprolol group (Figure 2). Though we need to be cautious when interpreting these 10-second ECGs, one possible explanation for this difference is that RMSSD primarily reflects parasympathetic activity, while SDNN reflects sympathetic activity, parasympathetic activity, and overall HRV.7,16,17 These findings may indicate that people with COPD who have impaired function of the parasympathetic arm of the autonomic nervous system are prone to AECOPD, and that impairment in the sympathetic arm is relatively less important or, perhaps more likely, not adequately captured in these short ECG recordings. Parasympathetic cardiac autonomic modulation is transmitted through the vagal nerve. The lungs have extensive vagal nerve innervation, including mechanosensitive and chemically sensitive afferent fibers, and this is one possible physiologic explanation for the association between RMSSD and AECOPD risk.18
Previous studies of participants without COPD found that metoprolol increased heart rate variability, including RMSSD and SDNN, though notably, in addition to longer recording times of 15-mintues to 24-hours, these studies also used higher doses of metoprolol than was used in BLOCK COPD.19-21 We did not find any statistically significant difference in the change in SDNN but found an association between treatment assignment and change in RMSSD with individuals in the metoprolol group having a larger median increase from baseline to day 42 which is consistent with those prior studies. Though we found that greater RMSSD was protective against severe AECOPD in participants assigned to placebo, metoprolol increased RMSSD and yet, RMSSD was not associated with risk of severe AECOPD in that arm of BLOCK COPD. It is possible that lower RMSSD is a marker of an underlying physiologic state that has adapted to respond to physiologic stress with sympathetic activation as opposed to parasympathetic withdrawal. In this case, treatment with metoprolol, which blocks sympathetic activation, would inhibit the ability to respond to physiologic stress, thus worsening the severity of AECOPD independent of an increase in RMSSD. It is also possible that metoprolol increased the risk of severe AECOPD on a pathway separate from RMSSD.
We tested 2 measures of HRV in the time-domain, as we were limited by the brief recording time of a standard 10-second ECG. HRV can also be measured in the frequency domain, where variability is assessed in 4 specific frequency ranges.7 Frequency domain measurements likely better reflect the status of the autonomic nervous system, and there are more data to suggest an association between frequency domain measurements and AECOPD than time-domain measurements. However, these measurements require much longer recording times (often up to 24 hours), and cannot be obtained from standard, 10-second ECGs.7 We are aware of only 5 previous studies analyzing HRV and AECOPD, and each had fewer participants than in the present study.22-26 To our knowledge no studies have analyzed HRV as a risk factor for AECOPD. Only 2 of these studies analyzed time-domain HRV measures. Kabbach and colleagues25 compared time-domain HRV measurements in 16 participants with stable COPD versus 16 participants with AECOPD. They found higher values of standard deviation of all RR intervals, (RMSSD), and 2 time-domain measures of overall HRV in participants with AECOPD compared to stable COPD.25 Castello-Simoes similarly found higher measures of mean RR intervals, SDNN, and RMSSD in 33 participants hospitalized for AECOPD versus 41 participants with stable COPD.26 These studies suggest that HRV is increased during the acute insult of AECOPD, but did not assess whether HRV in stable COPD may be associated with future occurrence of AECOPD. We do not have access to ECGs during AECOPD from BLOCK COPD, but our comparatively larger sample size and longitudinal design adds to the literature and provides additional evidence of an association between HRV and AECOPD that is deserving of further study.
Heart rate responses during exercise are another marker of autonomic function, and one commonly used measure is the chronotropic index, which measures the proportion of the expected heart rate increase attained during exercise.27 In BLOCK COPD there was no difference in time to first AECOPD between the metoprolol and placebo groups, but participants randomized to metoprolol had a higher risk of severe AECOPD.12 These results suggest that metoprolol does not affect the risk of AECOPD, but when an AECOPD occurs, it is more likely to be severe. In a previous secondary analysis of BLOCK COPD, we found a significant interaction between chronotropic index obtained from the 6-minute walk and assignment to metoprolol on risk of AECOPD, where participants who had a higher chronotropic index who were assigned to metoprolol had a greater risk of severe AECOPD than those assigned to placebo (aHR 0.74, 95% CI 0.58 to 0.95).28 This new analysis provides further evidence that metoprolol may be particularly harmful among people with COPD with preserved autonomic function.
Our study has several limitations. The primary limitation is the very short duration of ECG recording that was available from BLOCK COPD. Though prior studies showed moderate to strong correlation between 10-second ECGs and longer recordings, these studies were not done in people with COPD.8,9 People with COPD have high rates of autonomic dysfunction, and acute HRV changes may make these short recording times less reliable.5 Longer recording times would provide opportunity for a more rigorous analysis of heart rate variability, at various times of day and in various states of physical exertion, and for analysis in the frequency-domain. Additionally, we only found associations in the placebo arm of BLOCK COPD which limits the clinical significance of our findings. Longer recording times would also help to investigate if other HRV measures, or longer records of RMSSD, were associated with AECOPD risk in people with COPD who are on beta-blockers. Lastly, BLOCK COPD enrolled a population of people with COPD with a low rate of coronary artery disease (14.8%) and diabetes (15.8%), both of which are common comorbidities in people with COPD and can affect HRV.
Our study also has several strengths. The use of data from the BLOCK COPD trial allowed us to study a cohort of people with COPD who had rigorously collected ECGs and AECOPD data. We were also able to use the randomized design of BLOCK COPD to perform an unbiased analysis of interactions between heart rate variability measures and metoprolol use on AECOPD risk. Finally, the performance of serial ECGs in BLOCK COPD allowed us treat HRV measures as time-varying covariates in our analyses, which better accounts for change in HRV over time.
In conclusion, baseline HRV measures on 10-second ECG were not associated with risk of AECOPD. In time-varying analysis, greater RMSSD was associated with a lower risk of severe AECOPD in participants assigned to placebo, but not those assigned to metoprolol. Future studies should obtain longer ECG recording times for a more rigorous analysis of HRV in exacerbation-prone COPD patients.
Acknowledgements
Author contributions: Conceived the study: DMM and KMK; designed the study: DMM, KMK, SXL, and ESH; obtained funding: MTD; acquired the data: MTD, RC, WWS, and KMK; performed the primary statistical analysis: ESH and SXL; drafted the manuscript: DMM; critically revised the manuscript for important intellectual content and approved the final manuscript: all authors; take responsibility for the integrity of the data and the accuracy of the data analysis: all authors.
Declaration of Interest
SA has received research grants from Medtronic. RC has received grant support, advisory board fees, or lecture fees from GlaxoSmithKline, Boehringer Ingelheim, and AstraZeneca, consulting fees from Regeneron and Genentech, and reports owning stock in Inogen. HBR is supported by grants from National Institutes of Health (R01HL151452, R01HL153460, P50HD098593, R01DK122767, P2CHD086851), the Tobacco-Related Disease Research Program (T31IP1666), and the University of California, Office of the President. He reports consulting fees from Omniox Inc., and is involved in contracted clinical research with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Astellas, United Therapeutics, Genentech and Regeneron. He is a visiting Professor at the University of Leeds, United Kingdom. WWS has received grant support from AstraZeneca and Boehringer Ingelheim, consulting fees and fees for serving on a data and safety monitoring board from Allergan, and fees for serving on a data and safety monitoring board from Syneos Health. MTD has received grant support from the National Institutes of Health, the Department of Defense, and the American Lung Association; consulting from AstraZeneca, GlaxoSmithKline, Pulmonx, and Teva; and contracted clinical trial support from Boehringer Ingelheim, Boston Scientific, Gala, Nuvaira, and Pulmonx. KMK has received consulting fees from Nuvaria and Allergan. The remaining authors have nothing to declare.