Running Head: Treatable Traits in Misdiagnosed COPD
Funding Support: The Akershus Cardiac Examination 1950 Study is funded by 2 health trusts (Akershus University Hospital HF and Vestre Viken HF), and the South-Eastern Regional Health Authority, the University of Oslo, and the Norwegian Health Association. GE has received a grant (2015/FO7781) from Extrastiftelsen initiated in collaboration with the Norwegian Heart and Lung Association (LHL), was supported by funding grants from Akershus University Hospital, and received a grant from Trelasthandler A. Delphin og hustrus fund.
Date of Acceptance: February 10, 2022 │ Published Online: February 14, 2022
Abbreviations: chronic obstructive pulmonary disease, COPD; the Akershus Cardiac Examination 1950 Study, ACE 1950; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; lower limit of normal, LLN; bronchodilation, BD; European Coal and Steel Company, ECSC; modified Medical Research Council dyspnea scale, mMRC; electrocardiogram, ECG; body mass index, BMI; physical activity index, PAI; C-reactive protein, CRP; Global Lung Initiative, GLI; receiver operating area under the curve, AUC-ROC; standard deviation, SD; waist-hip ratio, WHR; short-acting beta2-agonist, SABA; long-acting beta2-agonist, LABA; long-acting muscarinic antagonist, LAMA; inhaled corticosteroid, ICS
Citation: Caspersen NF, Soyseth V, Lyngbakken MN, et al. Treatable traits in misdiagnosed chronic obstructive pulmonary disease: data from the Akershus Cardiac Examination 1950 study. Chronic Obstr Pulm Dis. 2022; 9(2): 165-180. doi: http://doi.org/10.15326/jcopdf.2021.0265
Online Supplemental Material: Read Online Supplemental Material (227KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death worldwide and a major cause of chronic morbidity.1,2 The disease is preventable and treatable, but misdiagnosis is common.3-5 Current guidelines advocate spirometry in individuals with respiratory symptoms, a history of risk factors for the disease, and/or recurrent lower respiratory tract infection.2,6 The diagnostic criteria of COPD is irreversible airflow limitation defined as a forced expiratory volume during 1 second (FEV1) to forced vital capacity (FVC) ratio <0.70 or below the lower limit of normal (LLN). However, population-based samples suggest that 60%-86% of COPD patients remain undiagnosed, while 30%-50% are overdiagnosed.3,5
The undiagnosed group is described as having better lung function and fewer respiratory symptoms than individuals with diagnosed COPD, but with an increased risk of death compared to individuals without airflow limitation.3,7-11 Overdiagnosis in COPD has been less studied. A population-based study reported that 50% of participants with self-reported COPD did not have irreversible airflow limitations,4 while studies from primary care suggest that one-third to one-half of patients with a COPD diagnosis have been overdiagnosed.4,5 Overdiagnosed individuals may receive inappropriate medication and have increased use of health services.4,5,8,12
Beyond a reduced FEV1/FVC, other clinical characterizations based on risk factors, symptoms, and imaging may increase precision in diagnostics and treatment of COPD.13-15,16 Phenotyping misdiagnosed COPD patients by assessing their treatable traits may help to optimize case-finding strategies and improve knowledge to better address this large group.17-21
Accordingly, the aims of the present study were to estimate the prevalence of over- and undiagnosed COPD in a middle-aged Norwegian population and to assess treatable traits in these groups. We also assessed diagnostic strategies for case finding based on our data.
Materials and Methods
Design and Data Sources
The ACE 1950 Study is a longitudinal cohort study of the 1950 birth cohort residing in Akershus County, Norway.22 The baseline visit was conducted between January 2012 and May 2015 with participants aged 62-65 years. It was designed to investigate the development of cardiovascular and other related diseases in the general population.22 The 1950 birth cohort had previously participated in a national population-based health examination survey, the Age 40 Program, conducted by the National Health Screening Service.23 The ACE 1950 study and linkage of data from the Age 40 Program was approved by the Regional Ethical Committee of Southeastern Norway (2011/1475). All participants gave written, informed consent.
Participants
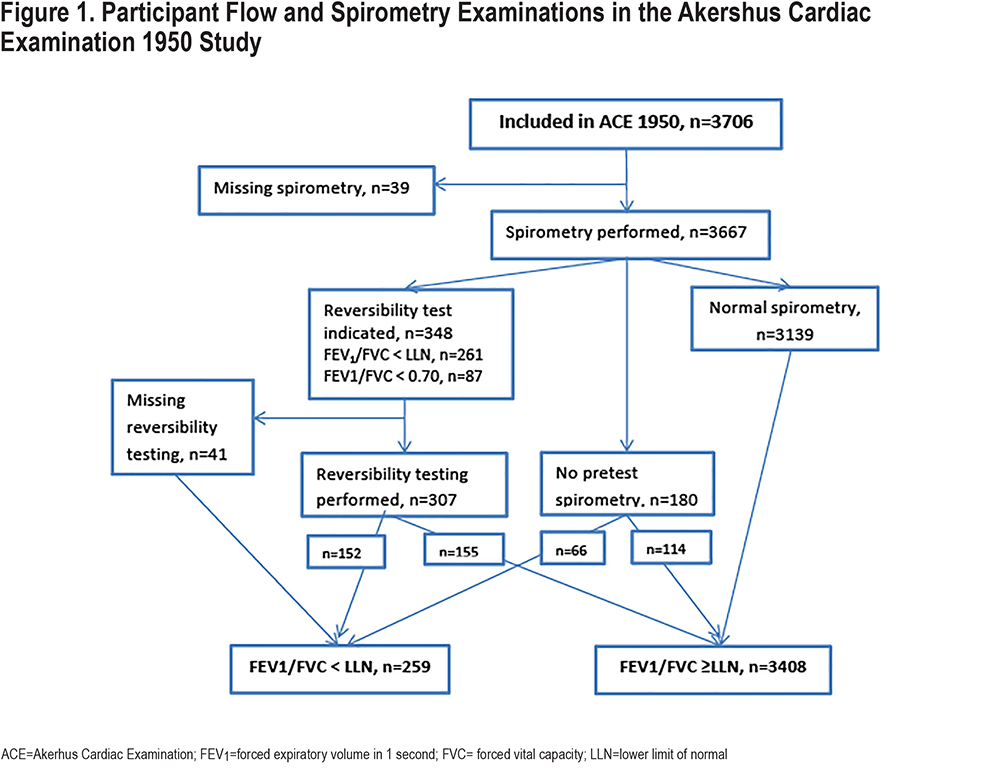
All inhabitants of Akershus County registered as residents according to the Norwegian National Registry as of November 2011 and born in 1950 (n=5827) were eligible for participation in the ACE 1950 Study. The participant flow in the ACE 1950 Study and the spirometry examinations are shown in Figure 1. In total, 3706 individuals (64% of the eligible, 51.2% male) signed an informed consent and participated in the study. Extensive information regarding the recruitment of participants and the data collection in the baseline ACE 1950 Study visit has previously been reported.22 In total, n=2742 (74%) of the participants in ACE 1950 had previously participated in the Age 40 Program.24
Data Collection in the ACE 1950 Study
The ACE 1950 Study was conducted at 2 hospitals located in Akershus County: Akershus University Hospital and Vestre Viken Hospital Trust, Bærum Hospital. The study personnel at both sites received the same training for the examination procedures.
Spirometry
For measurement of lung function, a dry bellow spirometer (Vitalograph Alpha III) was used in accordance with the European Respiratory Society/American Thoracic Society recommendations.25 Calibrations were performed daily, including registration of temperature and atmospheric pressure in the laboratory. A supervising pulmonologist (VS) performed the theoretical and practical training of all personnel conducting spirometry. The quality of the spirometry efforts was assessed consecutively by readings of the printed volume/time curve by the personnel conducting the spirometry.
Participants were seated, wore nose clips, and completed 3 consecutive performances of full inspiration followed by forced expiration for a minimum of 6 seconds while receiving instructions from the technician. The FEV1 and FVC were registered by manual readings on the printed time-volume curves, as the best of the 3 performances. If the 2 best performances varied by >150 ml or 5%, further performances were attempted until the 2 best performances varied by <150 ml or 5%. Lung volumes were converted to body temperature and pressure saturated volumes.26,27
By protocol, there were no exclusion criteria for spirometry. Registered explanations for not performing spirometry (n=39, 1.1%) included technical difficulties (equipment or patient related, n=6), medical reasons (including cough, facial paralysis, rib fracture, epistaxis, jaw surgery, Alzheimer’s disease, syncope, n=13), refusal to perform (n=2), or unknown (n=18). All current analysis included only participants with valid spirometry (n=3667).
Administration of inhaled medication on the day of examination was registered. If any short-acting or long-acting beta-adrenergic or muscarine receptor blockers had been inhaled within 4 hours, lung function measurements were considered post-bronchodilatory (bronchodilation [BD]).
All participants with pre-BD FEV1/FVC <lower limit of normal (LLN) according to the European Coal and Steel Company (ECSC) reference values were offered reversibility test by 0.4mg salbutamol on an inhalation chamber. New spirometries were performed within 30 minutes following drug administration and the best of the repeated performances was registered as post-BD lung function.
We performed a double reading of the spirometry results on a randomized sample of 125 participants. Cohen’s κ was run to determine if there was agreement between the 2 readings in categorizing FEV1/FVC<LLN. We found an acceptable agreement between the 2 rater judgements (κ=0.873, 95% confidence interval [CI] 0.77–0.98, p<0.001).
Demographic and Medical History and Clinical Examination
The participants answered a questionnaire, either electronically or as a printed version.The questions that participants received were previously used in other Norwegian population-based cohorts, i.e., the Age 40 Program23 and the Trøndelag Health Study.28 Self-reported obstructive pulmonary disease was assessed by the question “Have you ever been diagnosed with asthma, chronic bronchitis, or COPD/emphysema?” and “If yes, which diagnosis” with asthma, chronic bronchitis, and COPD/emphysema as alternative answers. Chronic bronchitis and COPD/emphysema were both interpreted as COPD. Respiratory symptoms were assessed by unique questions regarding regular cough, expectorate, and/or wheezing. Self-reported dyspnea was evaluated by the modified Medical Research Council dyspnea scale (mMRC). Coronary artery disease was defined as self-reported history of myocardial infarction or revascularization procedure. Atrial fibrillation was defined as self-reported disease or atrial fibrillation on electrocardiogram (ECG) at inclusion and was validated by re-evaluation of ECG and investigation of patient records.29 History of heart failure and obstructive sleep apnea was self-reported. Diabetes was defined as self-reported diagnosis, use of anti-diabetic medication, or both elevated fasting blood glucose (≥7.0mmol/L) and a hemoglobinA1c (≥6.5%).29 We measured arterial blood pressure, weight, and height, and calculated body mass index (BMI). Hypertension was defined as blood pressure ≥140mmHg systolic and/or ≥90mmHg diastolic and/or use of antihypertensive medication. Self-reported physical activity level was evaluated by a previously validated physical activity index (PAI) and deconditioning was defined as PAI 0 (no physical activity) or 1 (low physical activity).28 All current medication was registered, and participants were asked if they had been treated with antibiotics for bronchitis or pneumonia during the last 3 years.
Tobacco History
Tobacco consumption was registered in both ACE 1950 and in the Age 40 Program as current daily, former, or never smoking. Current or previous daily number of cigarettes (or cigarillos), and time point for start and quitting was registered. For former smokers in ACE 1950 who reported a lower cumulative tobacco exposure in ACE 1950 than in the Age 40 Program, their report from the Age 40 Program was used in the present analyses. For current smokers in ACE 1950 who reported a lower cumulative tobacco exposure in ACE 1950 than in the Age 40 Program, their report from the Age 40 Program was added to estimated pack years between the Age 40 Program and ACE 1950 based on their report from ACE 1950. Finally, participants in ACE 1950 claiming to be never smokers who responded as current or former smokers in the Age 40 Program were recoded as former smokers in ACE 1950.
Blood Sampling and Laboratory Analyses
Fasting venous blood samples were obtained during the examination day. Hematological analyses were performed by standard automatic cell counts on the same day, and the number of eosinophil cells/µl was registered. Systemic inflammation was assessed by C-reactive protein (CRP) (Vitros 5.1 FS, Ortho Clinical Diagnostics, Raritan, New Jersey, ). Detection limits for CRP were changed during the conduct of the study (3mg/L, 2.9mg/L, and 1mg/L). We used a cutoff of CRP ≥3mg/L (the highest detection limit throughout the study) to address the issue of elevated CRP as a marker of systemic inflammation.
Defining COPD and Treatable Traits
We defined COPD as post-BD FEV1/FVC ratio below the LLN according to ECSC reference values; FEV1/FVC ratio <0.64 for men and <0.66 for women.27 We did not include respiratory symptoms as obligatory for the COPD diagnosis. Many patients with established respiratory disease had already taken their medication on the day of examination and their spirometries were considered post-BD. Undiagnosed COPD was defined as spirometry-confirmed COPD at the ACE 1950 visit without self-reported history of COPD. Overdiagnosed COPD was defined as self-reported COPD and a spirometry within normal limits at the ACE 1950 visit. During the conduction of ACE 1950, a new set of reference values from the Global Lung Initiative (GLI) 2012 was established. Accordingly, we performed sensitivity analyses applying the GLI 2012 reference values.30
We assessed clinical characteristics as traits relevant to evaluation and treatment of chronic airway disease.16, 17,31 Pulmonary traits included lung function, eosinophilic inflammation (blood eosinophil cell count ≥300/µL), respiratory symptoms (dyspnea, cough, sputum, and wheezing), and prevalence of exacerbations (lower airway infections requiring treatment with antibiotics the last 3 years). Extrapulmonary traits included inactivity/low physical activity (PAI 0 or 0.05–1.50), obesity (BMI ≥30kg/m2), underweight (BMI <18kg/m2),32 systemic inflammation (CRP ≥3mg/L), and comorbidities including coronary artery disease, heart failure, atrial fibrillation, hypertension, obstructive sleep apnea, and diabetes mellitus. Among modifiable life-style factors, we assessed current and cumulative tobacco exposure.
Statistical Analyses
Comparisons of clinical characteristics between groups were performed by chi-square tests, Fisher’s exact test, t-tests or Mann-Whitney U tests and one-way analysis of variance analysis as appropriate. A significance level of 0.01 was used due to multiple comparisons. Univariate logistic regression was applied to determine associations between clinical characteristics and the presence of undiagnosed COPD. Variables that were significantly associated with undiagnosed COPD in univariate analyses were included in the multivariable logistic regression model. Sex was predetermined to be included in the multivariable analysis, but age was not included as all participants were from the same birth cohort. The diagnostic accuracy for undiagnosed COPD was assessed by receiver operating statistics' area under the curve (ROC-AUC). Separate AUCs were made for respiratory symptoms (dyspnea, cough, wheezing, sputum), current smoking, ≥20 pack-year history of tobacco use, and the use of antibiotics for respiratory infection during the last 3 years. Combinations of the individual variables with the highest AUCs were also examined and compared to a best-fit curve provided from the logit probabilities (optimal statistical model) from the final multivariable analysis. Finally, to assess the clinical utilities of early case finding strategies, 2x2 tables with previously undiagnosed COPD as the outcome were made to calculate the sensitivity, specificity, spirometries needed to detect 1 case (proportion of cases in screening positive), and the number of cases not detected. All statistical analyses in the present study have been performed in Statistical Package of Social Science 22 or STATA/SE 14.2.
Results
Baseline Characteristics
A total of 259 participants (7.1%) had a post-BD FEV1/FVC ratio below LLN. Only 72 ot these 259 participants (28.0%) reported a previously recognized COPD diagnosis, while 187 (72.2%) were undiagnosed. A total of 164 participants (4.5%) reported a history of COPD, of which 92 participants (56.1%) did not have post-BD FEV1/FVC <LLN. Demographic and clinical characteristics of the study population according to the presence of correctly diagnosed, undiagnosed, and overdiagnosed COPD are presented in Table 1. All 3 groups had more current smokers, a higher cumulative tobacco exposure, lower lung function, more respiratory symptoms, a higher prevalence of respiratory tract infections treated with antibiotics in the last 3 years, and more systemic inflammation than the group with no COPD. The correctly and undiagnosed COPD groups also had a significantly higher prevalence of inactivity when compared to the group with no COPD. The prevalence of obesity (BMI≥30) was lower in the undiagnosed COPD group compared to the group with no COPD.
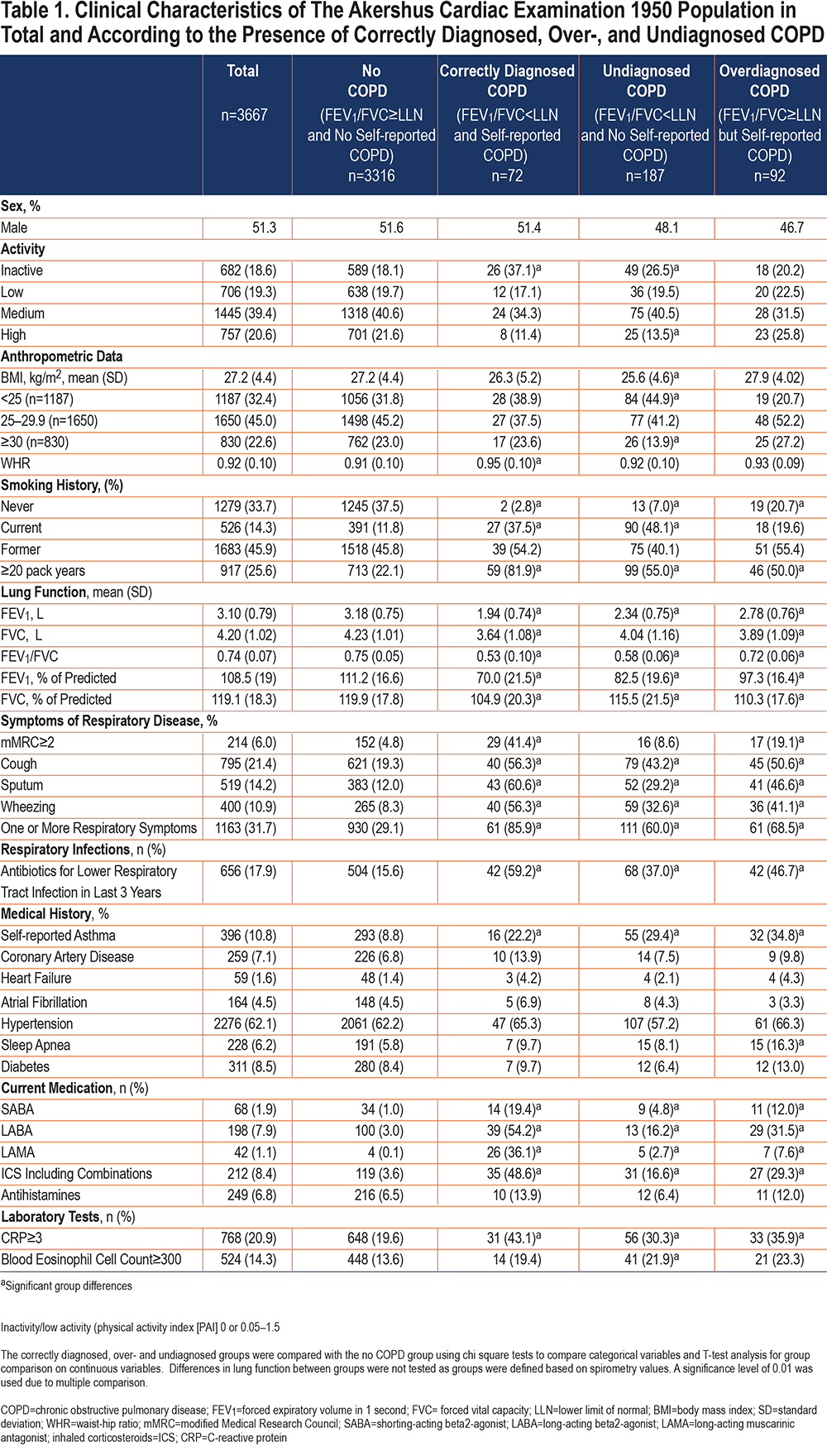
The overdiagnosed group had a lower prevalence of BMI <25kg/m2, more obstructive sleep apnea, more self-reported asthma, and a higher prevalence of eosinophilic inflammation than participants with no COPD. There were no differences in the physical activity level between the overdiagnosed and the no COPD group.
Clinical Characteristics of Participants with Undiagnosed COPD
The group with undiagnosed COPD had lower prevalence of cumulative tobacco consumption of≥20 pack years, less respiratory symptoms, and better lung function compared to the group with correctly diagnosed COPD (Supplementary Table 1 in the online supplement). However, there was no significant difference in the prevalence of current smoking, deconditioning, obesity, underweight, cardiovascular comorbidities, systemic inflammation, or eosinophilia between the groups. Notably, the prevalence of current smoking was numerically higher in participants with undiagnosed COPD (48.1%) than in participants with diagnosed COPD (37.5%), although not significant. The proportion of individuals with FEV1 <50% of predicted was higher in the diagnosed group than among undiagnosed, but the prevalence of moderate disease, defined as FEV1 between 50% and 80% of predicted, did not differ significantly between the groups.
In order to assess determinants of undiagnosed COPD, participants with correctly diagnosed COPD (FEV1/FVC <LLN and self-reported COPD, n=72) were excluded from analysis. The remaining 3595 participants included 187 participants with undiagnosed COPD. We found the prevalence of current smoking, dyspnea, cough, expectorate, wheezing, and respiratory infections to be higher in participants with spirometry results consistent with undiagnosed COPD (Table 2). Participants with undiagnosed COPD were also more underweight and deconditioned and had more systemic inflammation compared to participants not meeting the spirometry criteria for COPD. In contrast, comorbidities did not differ between the groups.
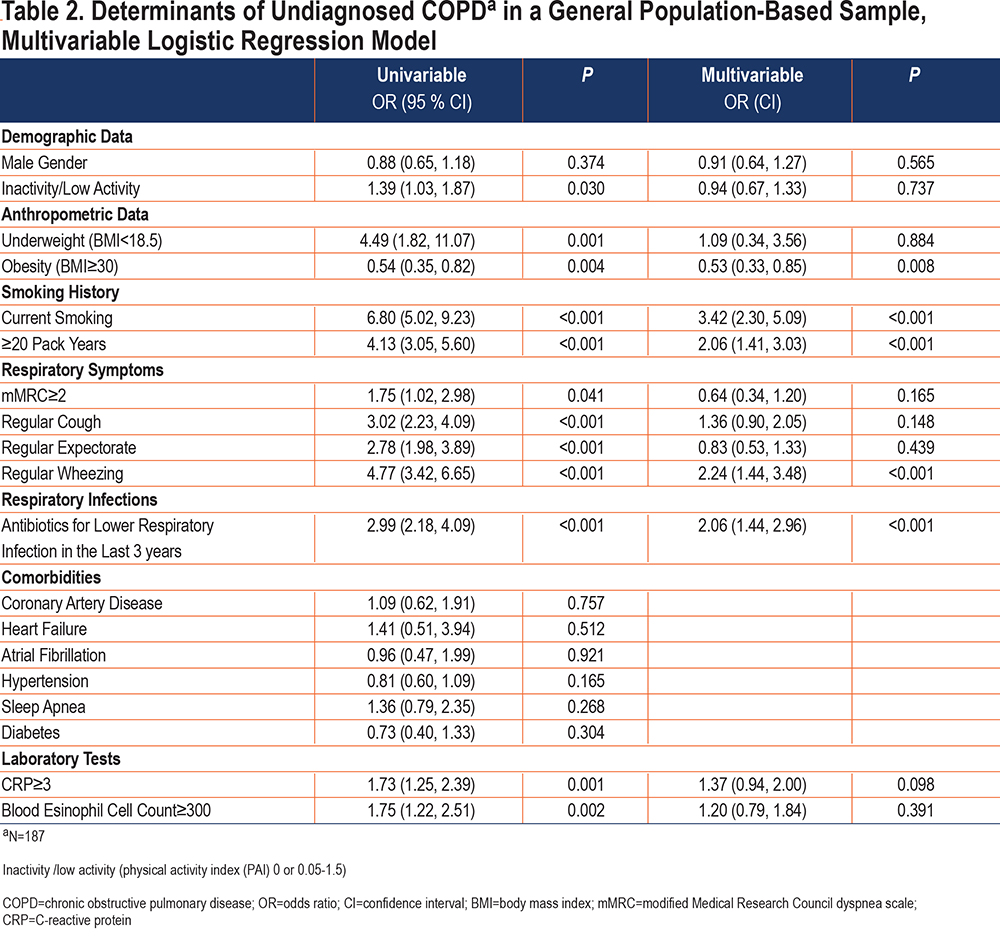
In multivariable analyses, obesity reduced the odds of having undiagnosed COPD while the presence of current smoking, cumulative smoking of ≥20 pack years, wheezing, and treatment with antibiotics for lower respiratory tract infections during the last 3 years were all independently associated with undiagnosed COPD (Table 2).
Clinical Characteristics of Participants with Overdiagnosed COPD
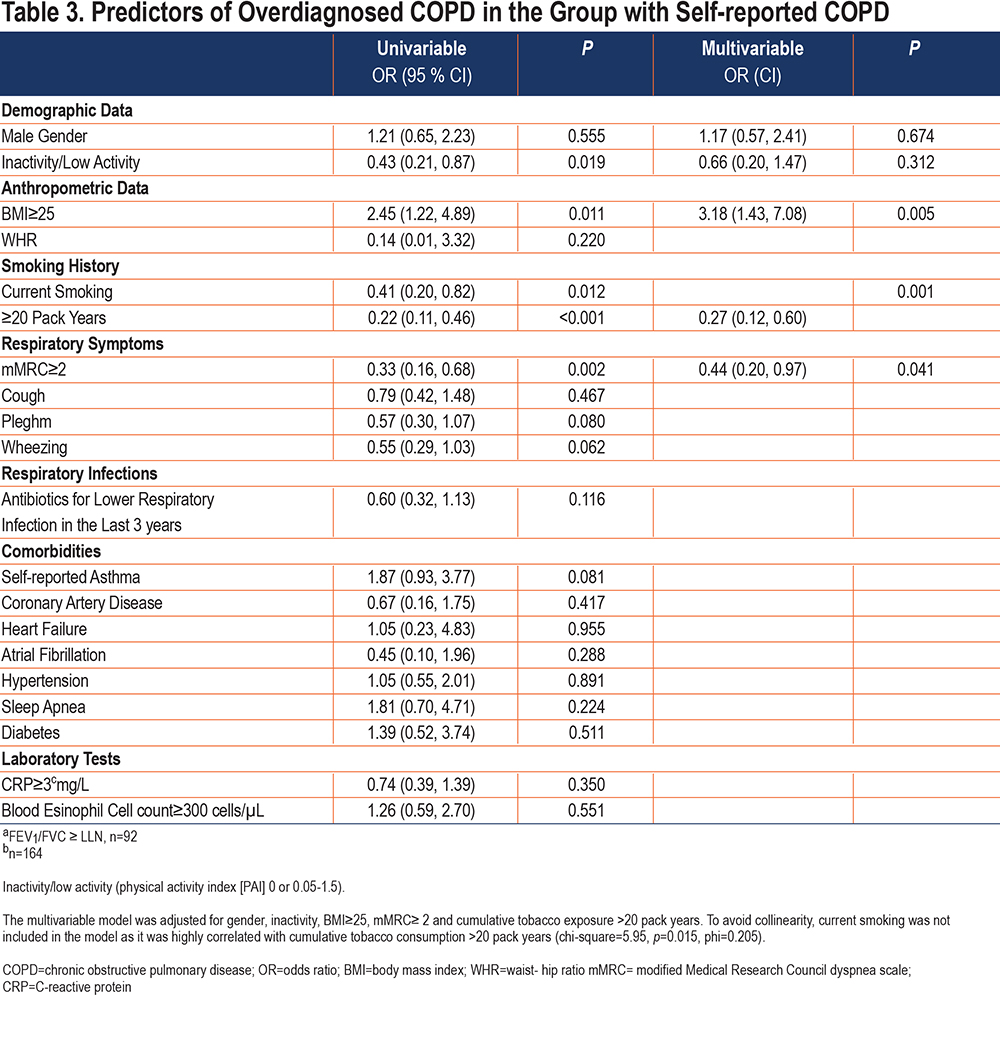
In Table 3 we assessed predictors of overdiagnosed COPD in the population with self-reported COPD (n=164) and found that overdiagnosed participants were more often overweight (BMI ≥25), had a lower prevalence of current smoking and tobacco consumption ≥20 pack years, and were more inactive and dyspneic compared to correctly diagnosed individuals.
Diagnostic Strategies to Detect Undiagnosed COPD
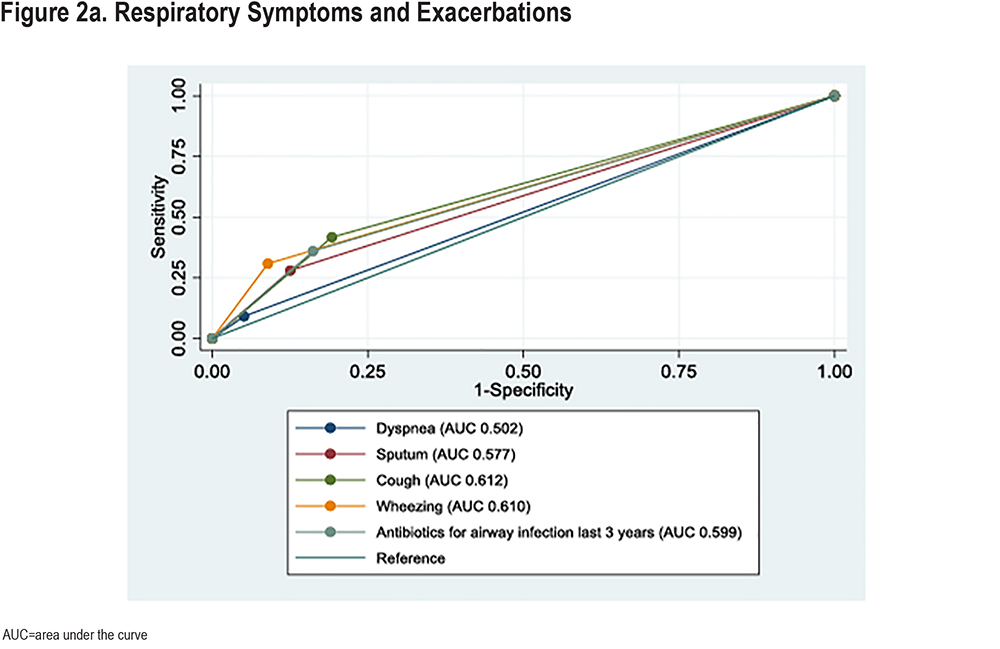
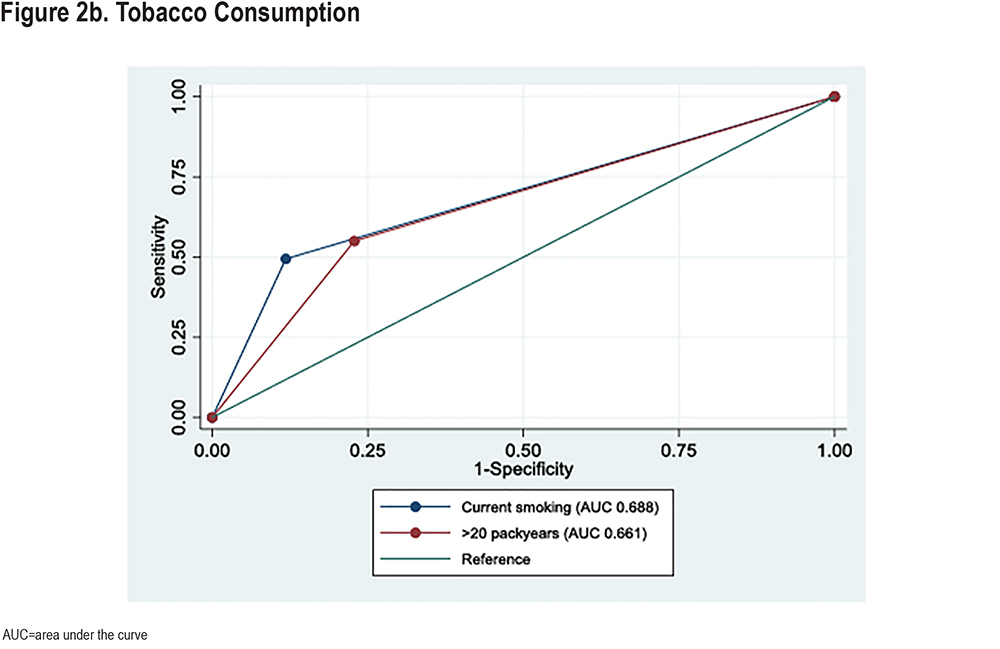
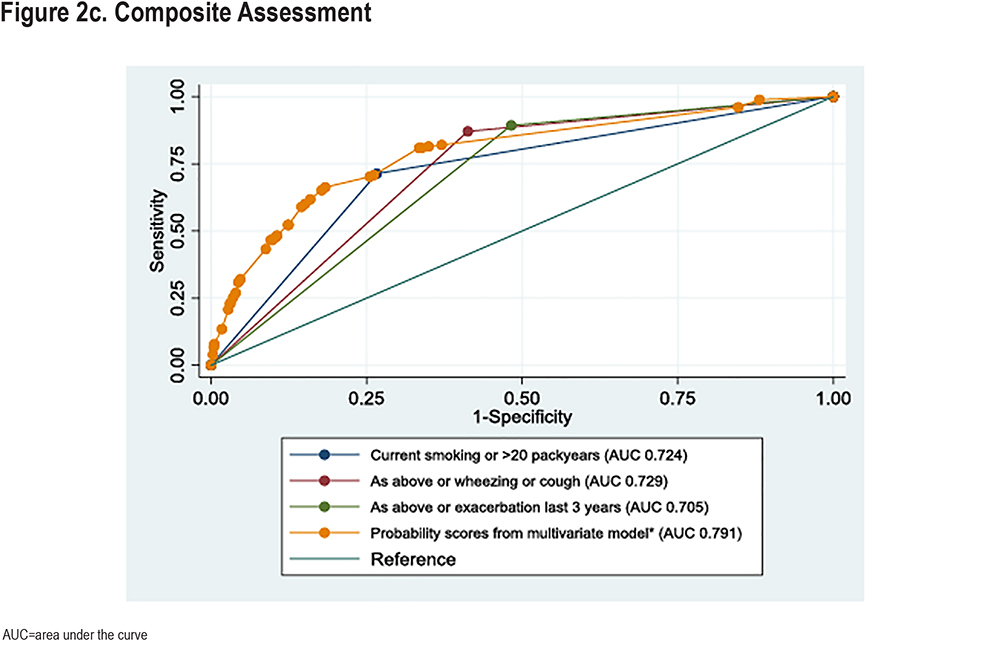
The diagnostic accuracy of clinical variables to detect participants currently unaware of their COPD diagnosis is demonstrated in Figure 2. Coughing and wheezing had the highest ROC-AUCs among respiratory symptoms, significantly higher than dyspnea (Figure 2a and Supplementary Table 2 in the online supplement). We also explored the diagnostic accuracy of the combination of variables. Both the combination of current smoking or≥20 pack years and the combination cough or wheezing had significantly higher ROC-AUCs than individual variables (Figure 2b and Supplementary Table 2 in the online supplement). Current smoking or >20 pack years had higher accuracy than cough or wheezing. Figure 2c presents combined variables. Adding wheezing or cough to current smoking or >20 pack years did not increase diagnostic accuracy (p=0.72). A model including wheezing or cough, current smoking or >20 pack years, or exacerbations in the last 3 years did not improve diagnostic accuracy compared to current smoking or >20 pack years alone (p=0.42). However, a statistical optimal model consisting of predicted individual probabilities based on the multivariable model in Table 3 had higher diagnostic accuracy than the clinical approach focusing on smoking history and symptoms (p<0.001).
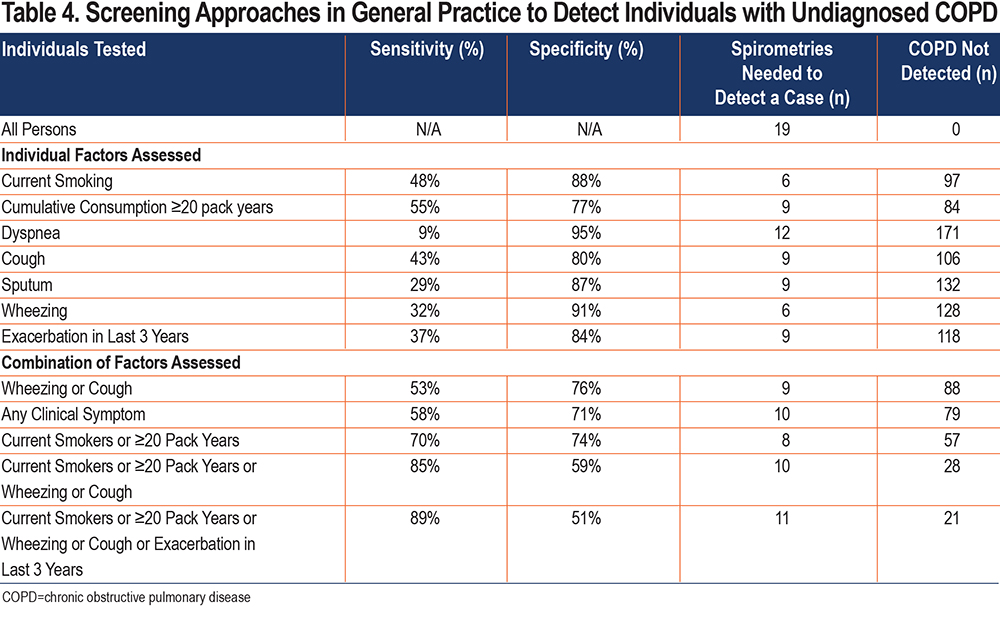
We also examined different strategies to detect undiagnosed COPD (Table 4). Using individual clinical variables as selection criteria for spirometry was generally associated with high specificities and low sensitivities. Performing spirometry in all current smokers would require spirometry testing in 6 persons to detect 1 person currently unaware of having COPD but would imply a large fraction of individuals remaining undiagnosed. By combining several individual clinical variables, the sensitivity in diagnosing COPD increased at the cost of lower specificity and a larger number of spirometries to be performed per diagnosed person. Table 4 shows that combining information on wheezing and cough with current smoking and ≥20 pack years has a sensitivity of 85% and specificity of 59%, thus requiring 10 spirometries to detect 1 undiagnosed case of COPD, with 28 of 185 individuals (15%) potentially remaining undiagnosed.
Applying the new reference values of GLI 2012 to define COPD increased the total prevalence of COPD to 307 (8.4%), with a fraction of 75% undiagnosed cases. In the sensitivity analyses similar results as in the main analyses were found, except that the diagnosed group also reported more cough and systemic inflammation than the undiagnosed group (Supplementary Tables 2 and 3 in the online supplement).
Discussion
In the present population-based study consisting of 3667 individuals aged 62-65 years, we found a prevalence of COPD of 7.1%. Moreover, 72.2% of the participants with an FEV1/FVC <LLN were not aware of having reduced lung function consistent with a COPD diagnosis, while 56% of participants with self-reported COPD did not have an FEV1/FVC <LLN. The undiagnosed group had better lung function and less respiratory symptoms, but similar rates of extrapulmonary clinical traits as diagnosed individuals. BMI≥25 was the main predictor of overdiagnosed COPD among the self-reported. We found that restricting spirometry to participants with current or past substantial tobacco consumption and self-reported wheezing or cough would have identified most undiagnosed COPD cases.
A Danish study analyzing data from the Copenhagen General Population Study found that 78% of participants with COPD were undiagnosed,9 while a multinational study with data from 27 countries and a total of 30,874 participants found that 81.4% of participants with COPD were undiagnosed.33 Although the overall prevalence of COPD varies in these and other similar studies, the prevalence of undiagnosed disease is universally high.9,11,33-35
In line with other studies, we find that the undiagnosed group has better lung function and fewer respiratory symptoms compared to individuals without COPD.3,8,9 A few findings and novel contributions in the current study merit mentioning. We have studied clinical characteristics in accordance with a recent focus on treatable traits, information relevant for prognosis, and treatment selections.16 First, despite a higher average pulmonary function in the undiagnosed group, the prevalence of moderate severity of COPD (FEV1 50%-70% of predicted) did not differ between the diagnosed and undiagnosed, and 7% of the undiagnosed participants had airflow limitation consistent with Global Initiative for Chronic Obstructive Lung Disease2 stages 3 or 4, likely to affect prognosis.36,37 Second, extrapulmonary traits such as deconditioning and systemic inflammation were more prevalent in the undiagnosed COPD group. The association between inflammatory markers, such as CRP or eosinophilia, and undiagnosed COPD has, to our knowledge, not been studied previously, and may describe a clinically relevant trait. Third, the undiagnosed group reported more frequent lower respiratory tract infections, compared to individuals without COPD. Unfortunately, we did not have data on short-term oral steroid use. However, we believe that more frequent use of treatment with antibiotics for lower respiratory tract infections in the undiagnosed group may indicate exacerbations. Fourth, persons with overlapping asthma/COPD may be prone to misdiagnosis. In our data, 29% of individuals in the undiagnosed group reported a previous diagnosis of asthma suggesting that some of the undiagnosed individuals may have progressed to chronic obstruction from asthma or represent an overlapping phenotype.
In summary, these findings indicate that patients unaware of having COPD have similar clinical traits affecting prognosis as those diagnosed with COPD.
More than 50% of participants with self-reported COPD did not have irreversible airway limitation. This group was recognized by intermediate level symptoms, and the main predictor of being overdiagnosed was a BMI ≥25. Two explanations are relevant for this finding: the difference between diagnostic cut-offs in the 2012 guidelines (FEV1/FVC <0.70) and FEV1/FVC <LLN explains 39% of the participants in this group. Second, overdiagnosis in overweight and obese individuals may reflect a clinical evaluation based on respiratory symptoms and/or risk exposure, but with preserved FEV1/FVC ratio caused by significant, but disproportionate reductions in FEV1 and FVC.12,38
The clinical relevance of increasing the proportion of individuals aware of having COPD depends on the potential for intervention and possible changes in outcomes. The rationale for early and active case-finding by physicians in primary care is strong.18,19,39,40 First and foremost, the undiagnosed group probably has the most to gain by early smoking cessation.
Another novel approach in the current study was to explore case-finding strategies based on these clinical characteristics in the undiagnosed population. We assessed how any of these clinical characteristics, easily obtained in primary care, could contribute to detecting individuals with undiagnosed COPD in our population-based cohort. When modeling the optimal case-finding strategy to detect those currently unaware of COPD, the highest diagnostic accuracy was found by including the 2 questions of current smoking and/or history of≥20 pack years. With those 2 questions, 30% of the participants would still remain undiagnosed, while adding questions of wheezing or cough would reduce this fraction to 15% at the cost of performing 2 more negative spirometries for each positive case.
We also report that the statistical optimal approach to detect previously undiagnosed COPD would include taking into consideration BMI and CRP in addition to tobacco consumption and symptoms. Obesity was the only factor that remained associated with lower probability of having undiagnosed COPD in multivariable analysis.It is commonly observed that patients with severe COPD have a lower BMI compared to the general population19 and we also now extend such observations to a cohort with mainly mild COPD. However, as the prevalence of obesity was comparable among diagnosed and undiagnosed COPD, this observation may have limited clinical implication for case-finding strategies in COPD.
Compared to previous epidemiological studies, a strength of the current study is the use of post-BD values in defining COPD, thereby, reducing the misclassification of asthmatic individuals. Furthermore, we expect no significant participation bias with regard to respiratory symptoms, as pulmonary disease was not a focus in the study invitations.
The participation rate of 64% from a general population is reasonably high, compared to other general population cohorts of today.41,42
A limitation is the cross-sectional study design with a very limited age range. Hence, our results may not be valid for other age groups. The study was initiated prior to the publication of the GLI 2012 reference values, and the main analysis is, thus, reported according to the ECSC reference set. Yet, the similar results of the sensitivity analyses using the GLI 2012 reference set suggest that our data are valid also for populations diagnosed from 2012 to the present day. Our data may also support that those few extra persons diagnosed with COPD by GLI 2012 compared to ECSC do not have highly symptomatic disease but share some traits with the more symptomatic group.
Factors that lead to miscommunication of a COPD diagnosis include different cutoffs defining chronic obstruction clinically and epidemiologically, spirometries performed without reversibility testing, or stigmas surrounding COPD. Medical health records were not available, and the use of self-reported COPD as an indicator of diagnosed COPD may not have been consistent with the diagnoses provided by each individual’s primary physician. This is most relevant for persons having overlapping asthma/COPD. Additionally, COPD and emphysema were combined in the questionnaire identifying previous respiratory disease. This may have led to misclassification of some participants with emphysema, but no COPD.
Conclusion
In the present study of a Norwegian general population sample, we found a COPD prevalence of 7.1%, of which 72.2% were undiagnosed. Individuals with undiagnosed COPD have better lung function, and fewer symptoms and exacerbations than individuals with an established COPD diagnosis, but similar extrapulmonary traits including physical inactivity, comorbidities, and systemic inflammation. We recommend that current smokers and smokers with cumulative tobacco consumption ≥20 pack years, cough, and wheezing should be offered spirometry with BD-test to uncover undiagnosed COPD.
The prevalence of overdiagnosed COPD was 2.5%. Individuals with overdiagnosed COPD have lower lung function, more respiratory symptoms, more self-reported asthma, more eosinophilic inflammation, and more sleep apnea than other individuals without COPD. The main predictor of being overdiagnosed was being overweight.
Acknowledgements
Author contributions: GE and NFC designed the present study. HR, MNL, TB, AT, and VS designed and organized the ACE 1950 Study including baseline examinations and data collection. NFC, MNL and GE analysed the data. NFC and GE edited the manuscript, VS, MNL, TB, IA, AT, and HR co-edited the manuscript. All authors approved the final version of the manuscript.
Availability of data and material: The data set used in this study is not publicly available as the Data Protection Authority approval and patient consent do not allow for such publication. However, the study group welcomes initiatives for cooperation, and data access may be granted upon application. More information at: https://ace1950.no/
The authors acknowledge the skilled staff at the Clinical Trial Unit, Division of Medicine, Akershus University Hospital and the Department of Medical Research, Bærum Hospital, Vestre Viken Hospital Trust.
Declaration of Interest
GE has received research funding from AstraZeneca and Boehringer Ingelheim for studies not related to the current study. TB has received speaker fees from Bayer, Boehringer Ingelheim, BMS, and Pfizer (non-related to COPD or the submitted work). The other authors declare no conflict of interest.