Running Head: COPD Symptom Variability and the E-RS COPD Instrument
Funding Support: The SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) was supported by contracts from the National Institutes of Health (NIH) / National Heart, Lung, and Blood Institute (NHLBI) (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune, Bayer, Bellerophon Therapeutics, Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici S.p.A., Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Novartis Pharmaceuticals Corporation, Nycomed GmbH, ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi, Sunovion, Takeda Pharmaceutical Company, and Theravance Biopharma and Mylan.
Dr. Krishnan is supported by the NIH T32 HL134629.
Date of Acceptance: April 7, 2022 │ Date Published Online: April 9, 2022
Abbreviations: SubPopulations and InteRmediate Outcome Measures In COPD Study, SPIROMICS; Evaluating Respiratory Symptoms in COPD, E-RS; within-subject standard deviation, WS-SD; St George’s Respiratory Questionnaire, SGRQ; patient-reported outcome, PRO; Exacerbations in Chronic Pulmonary Disease Tool, EXACT; square root of the wall area of a hypothetical airway with an internal perimeter of 10 mm, Pi10; health care resource utilization, HCRU; interquartile range, IQR; modified Medical Research Council, mMRC; Pittsburgh Sleep Quality Index, PSQ; hospital anxiety and depression scale, HADS; parametric response mapping, PRM; Global initiative for chronic Obstructive Lung Disease, GOLD; confidence interval, CI; correlation, corr; Body mass index-airway Obstruction-Dyspnea-Exercise capacity, BODE
Citation: Krishnan JK, Ancy KM, Oromendia C, et al. Characterizing COPD symptom variability in the stable state utilizing the Evaluating Respiratory Symptoms in COPD instrument. Chronic Obstr Pulm Dis. 2022; 9(2): 195-208. doi: http://doi.org/10.15326/jcopdf.2021.0263
Online Supplemental Material: Read Online Supplemental Material (508KB)
Introduction
Patients with chronic obstructive pulmonary disease (COPD) experience respiratory symptoms including breathlessness, cough, and sputum production that necessitate comprehensive assessment.1 This assessment, and hence, therapeutic decision-making, has not adequately incorporated symptom variability. Prior studies have examined the impact of morning symptoms and have correlated these to more frequent exacerbations and greater impact on health-related quality of life.2-7 Weaknesses of studies assessing symptom variability during the stable state include cross-sectional design and surveys dependent on participant recall of symptoms.2-11 Two prospective studies that examined symptom variability were limited by small sample size and a short follow-up period.9-12 The more recent prospective study evaluated 2669 participants over 1 week using the Night-time and the Early Morning Symptoms of COPD instruments and found more severe disease was associated with fluctuation in symptom number and intensity.12 A metric to define and compare individual symptom variability has not been developed.
Given correlation between morning symptom differences, quality of life, and exacerbation risk, the development of a symptom variability metric using prospectively collected data over a longer time in a highly phenotyped sample could contribute to comprehensive patient assessment. The SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) cohort provides a unique opportunity to evaluate the variability of respiratory symptoms prospectively. In the Exacerbation sub-study, a subset of SPIROMICS participants completed the Evaluating Respiratory Symptoms in COPD (E-RS™:COPD or E-RS),13,14 an 11-question patient-reported outcome (PRO) diary administered as part of the 14-item Exacerbation in Chronic Pulmonary Disease Tool (EXACT). The E-RS has been shown to be a reliable, valid, and responsive measure of respiratory symptom severity in stable COPD and is sensitive to the treatment effects of drug therapies.14-17 Measuring fluctuation in E-RS scores provides an opportunity to characterize day-to-day symptom variability as an additional dimension of symptom burden. A standardized measurement of symptom variability could in turn be tested for therapeutic responsiveness in future studies. Standard deviation is a common statistical measure to quantify variation and dispersion of biomedical data.18 We hypothesized that the within-subject standard deviation (WS-SD) of E-RS scores can serve as a potential metric to quantify variability of respiratory symptoms in patients with COPD.
Our objectives were as follows: (1) to evaluate the performance of the WS-SD variability metric compared to other statistical measures of variation, (2) to identify differences in baseline characteristics between individuals with higher versus lower WS-SD, and (3) explore the extent to which baseline symptom variability predicted future exacerbations. Preliminary results have been reported in an abstract.19
Materials and Methods
Study Cohort
SPIROMICS is a prospective cohort study that enrolled 2981 participants across 4 strata (never-smokers, smokers without COPD, mild/moderate COPD, and severe COPD) with the goals of identifying new COPD subgroups and markers of disease progression. The SPIROMICS protocol details full inclusion and exclusion criteria and assessments performed at baseline.13 In addition to these assessments, the main SPIROMICS study obtained additional imaging phenotypic markers including the square root of the wall area of a hypothetical airway with an internal perimeter of 10 mm (Pi10) and parametric response mapping.20
Data for these analyses were derived from the subgroup of participants with COPD participating in the SPIROMICS Exacerbation sub-study; this was incremental to the previously published SPIROMICS-wide exacerbation data.13 To be representative of patients at a baseline stable state, sub-study eligibility criteria included being free of a health care resource utilization (HCRU)-defined exacerbation in the 30 days before enrollment. Exclusion criteria included a primary diagnosis of asthma or visual or cognitive impairment preventing completion of the daily diary using a personal digital assistant. Institutional review boards at each center approved SPIROMICS, and all participants provided informed consent.
Symptom and Exacerbation Assessment
Sub-study participants completed the E-RS as part of their daily study diary. The E-RS total score ranges from 1 to 40 with higher scores indicating more severe symptoms, overall.21 Consistent with E-RS scoring guidelines, participants with at least 4 days of diary data per week and 80% completion rate of daily diary data for the first 5 weeks were included in the analysis; participants who did not meet this diary completion rate were excluded.
Exacerbations were defined by HCRU, i.e., an increase in respiratory symptoms that required a health care visit and were treated with antibiotics, systemic corticosteroids, or both.20 In addition, symptom-defined EXACT events were identified using the 14-item EXACT score, which ranges from 0-100 with higher scores indicating a worse health state. EXACT (symptom-defined) events, which are periods of acute sustained symptom worsening, are characterized by an increase from a stable baseline state in an individual’s EXACT score of >9 points for 3 consecutive days, or >12 points for 2 consecutive days.22,23
Study Design and Statistical Methods
We compared the performance of the WS-SD of E-RS total scores calculated from the first week (baseline or Week 0) to 5 other metrics for estimating variability: interquartile range (IQR), range, median absolute deviation, variance, and coefficient of variation. The week 0 value for each variability measure was compared with the week 4 value using Pearson’s correlation to determine reproducibility. We constructed Bland-Altman plots for each variability metric also comparing week 0 values to week 4 values. Our main analysis focused on the first 5 weeks because prior to sub-study enrollment, participants were free of HCRU exacerbation for at least 30 days. We were interested in symptom variability during a time period closest to this stable state. A schematic of our study and timing of assessments is presented in Figure 1. Finally, we examined the change in WS-SD over each of 13 weeks and used paired t-tests to compare changes.
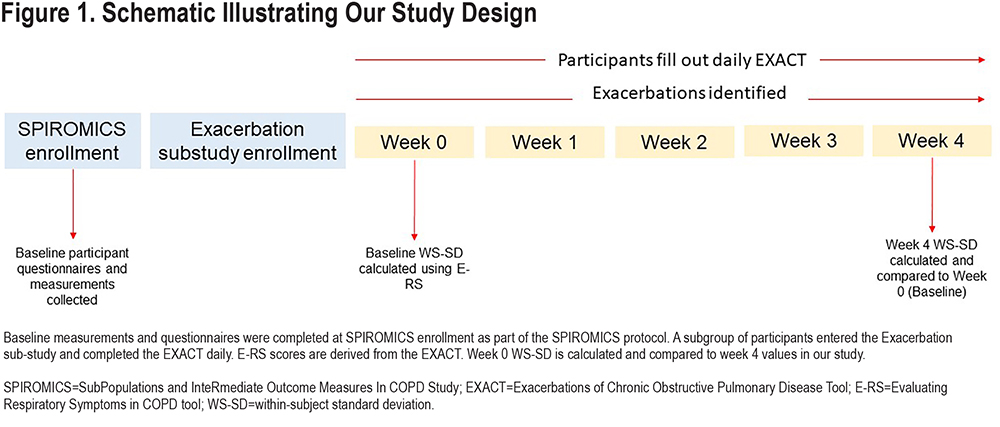
The week 0 median value of the E-RS total score WS-SD was used to dichotomize individuals into higher versus lower variability groups. Week 0 values were chosen as these were closest to the patient’s known 30-day exacerbation-free (stable) period prior to inclusion in the sub-study and reflect symptom variability at a stable state. Student t-test or Wilcoxon rank sum test was used for continuous variables and χ2 test or Fisher exact test was used for categorical variables for comparison of baseline patient characteristics between variability groups.
We explored differences in the number of participants experiencing HCRU-defined exacerbations over 5, 13, and 52 weeks in the higher versus lower variability group using Fisher exact test to compare proportions. We used multivariate logistic regression to explore the association between higher variability with an EXACT (symptom-defined) event during the 4-week post-baseline period. Statistical analysis was performed using R software version 3.6.3.
Results
Characteristics of Included Patients
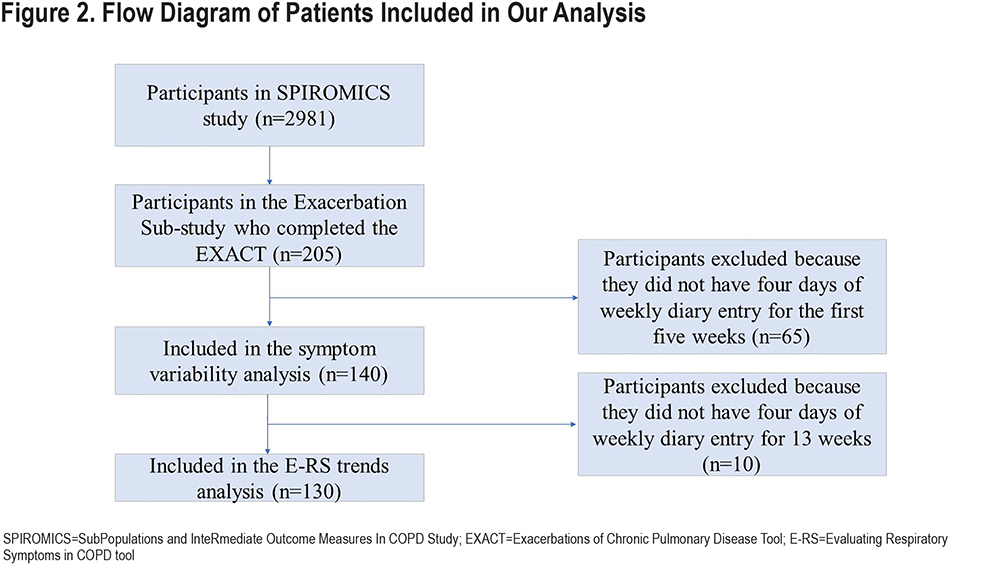
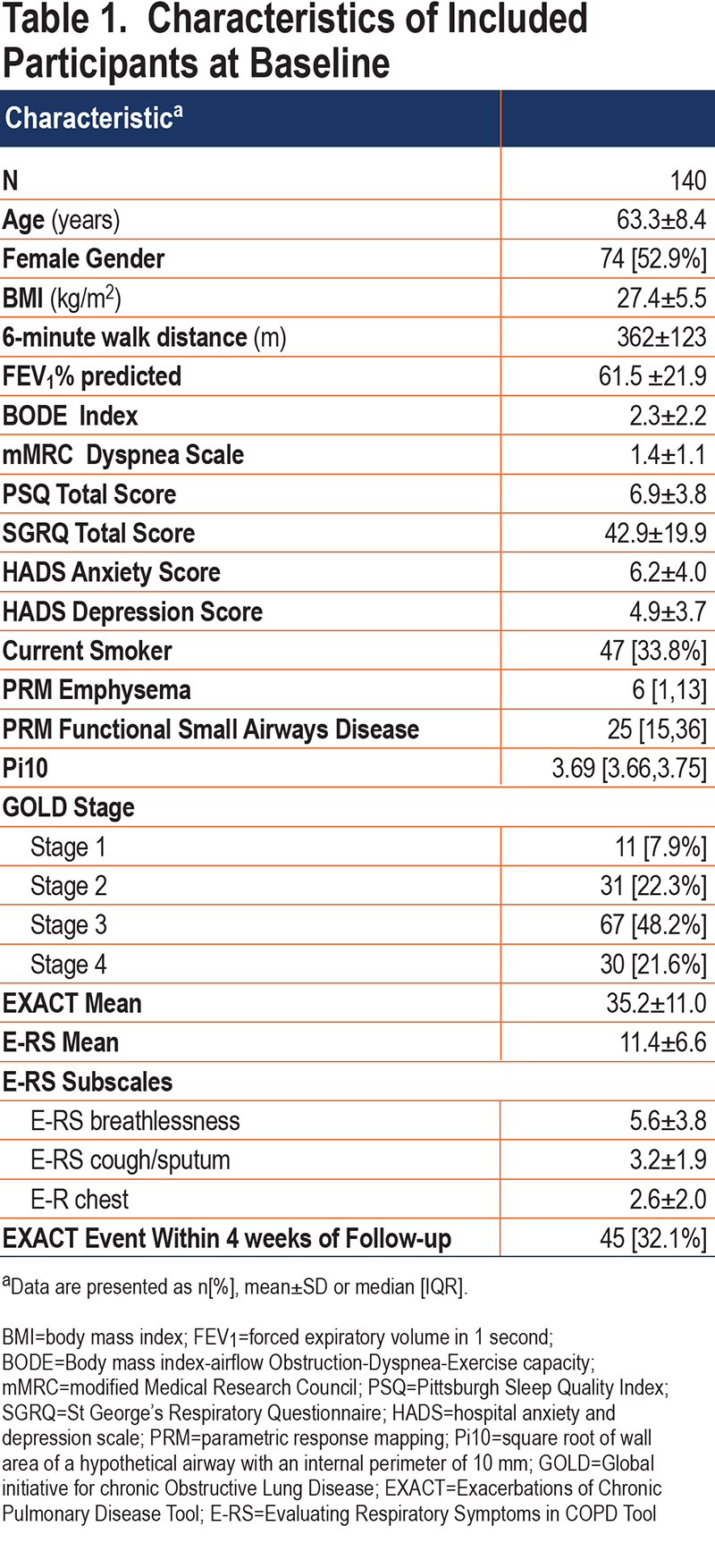
The E-RS was completed daily by 205 participants; data from 140 (68%) met criteria for this analysis with sufficient diary completion rates in the first 5 weeks of follow-up (Figure 2).Included participants had a mean age of 63.3±8.4 years and 53% were women. Mean E-RS total score at baseline, representing respiratory symptom severity overall, was 11.4±6.6. Further participant characterization is found in Table 1. Differences between the included and excluded participants were fewer EXACT events in the excluded group (anticipated because lower completion rates would result in fewer detected events), lower modified Medical Research Council (mMRC) Dyspnea Scale scores, and lower St George’s Respiratory Questionnaire (SGRQ) scores (Table E1 in the online supplement). Our extended analysis of E-RS scores through week 13 included a smaller sample size of 130 patients, as fewer participants met diary completion criteria (Figure 2).
Performance of Within Subject-Standard Deviation as a Symptom Variability Metric
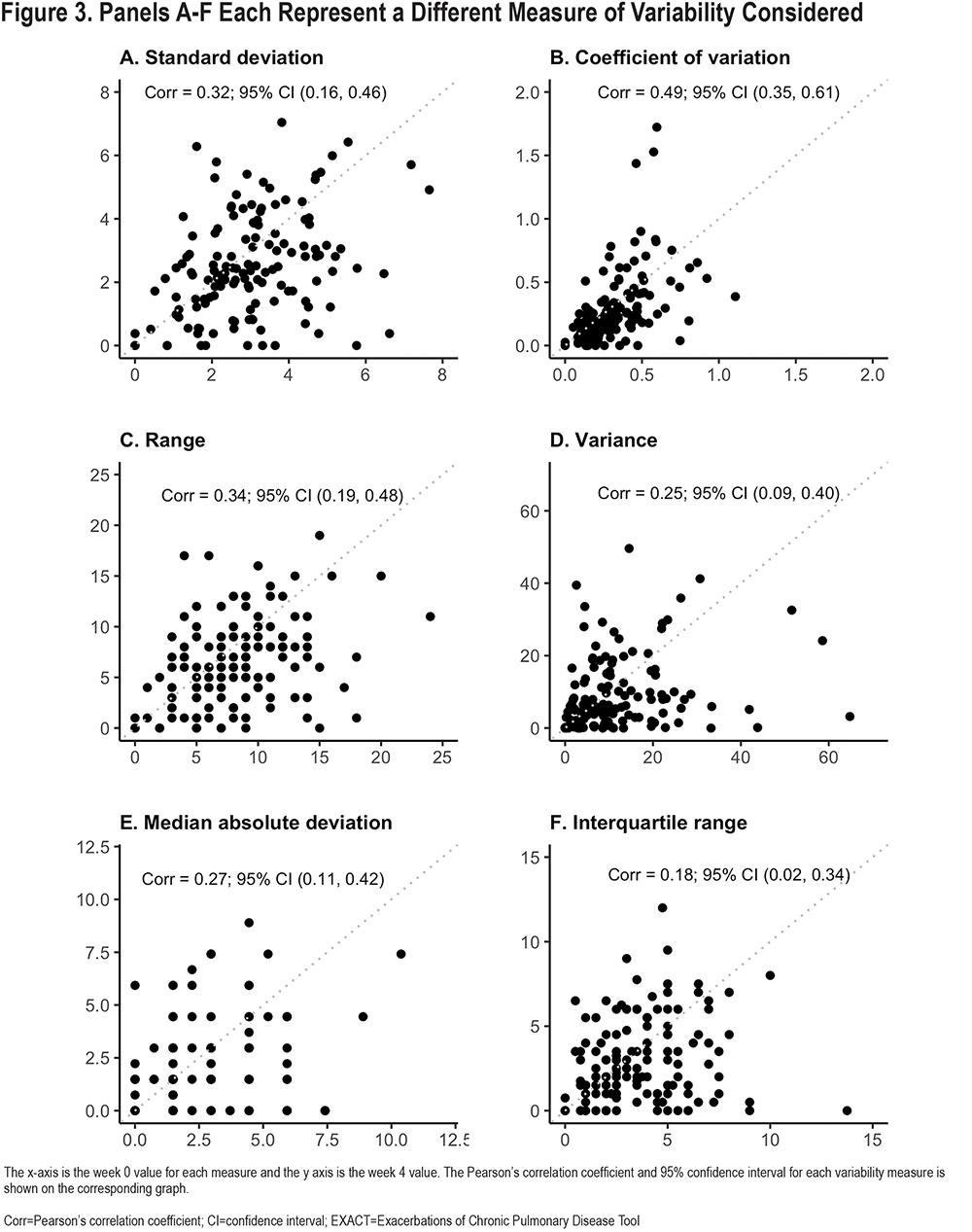
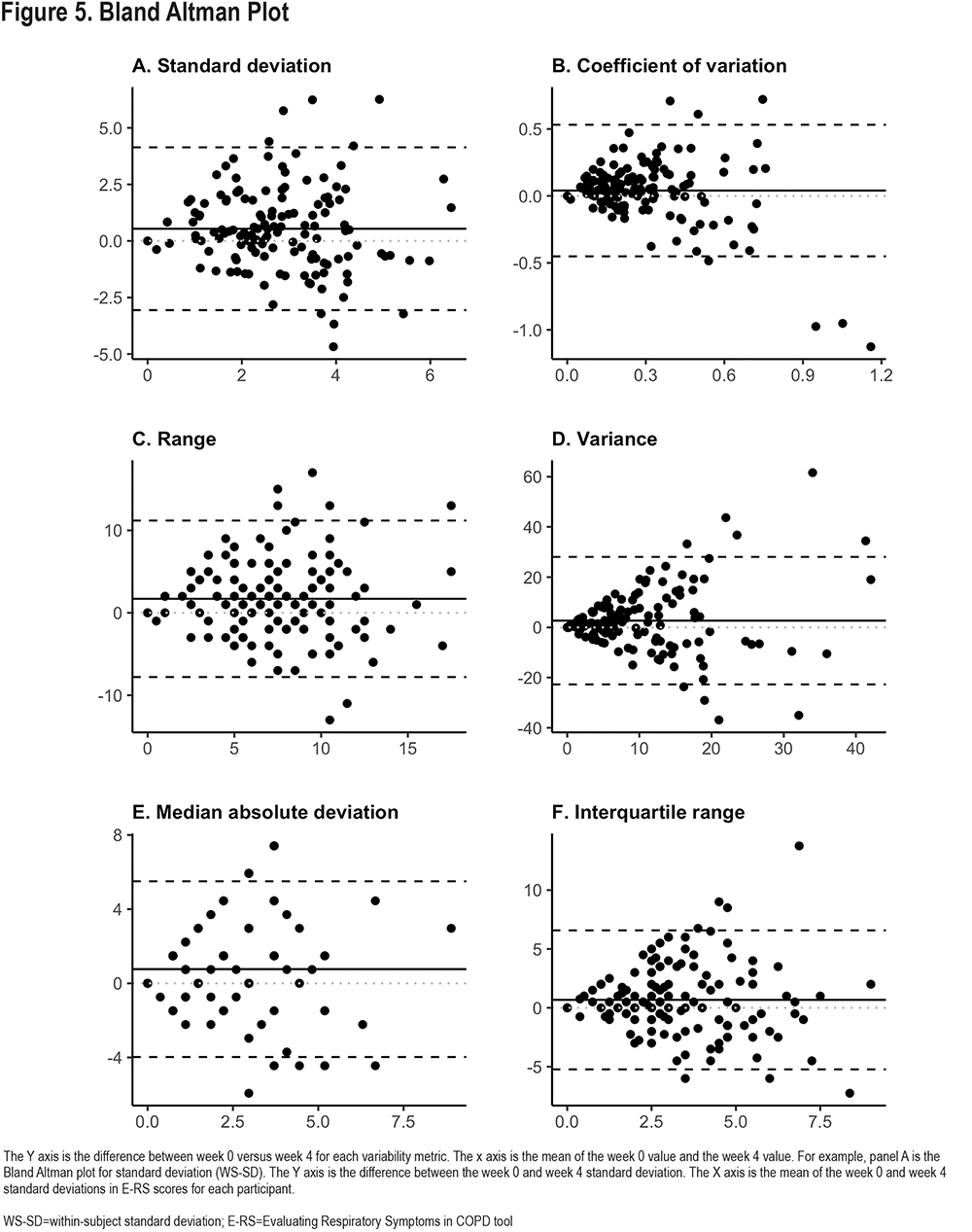
Reproducibility of the 6 different variability metrics between Week 0 and Week 4 were relatively low, with Pearson-correlation coefficients (r), ranging from 0.18–0.49 (Figure 3). By contrast, mean E-RS total scores for each participant were more constant [r=0.78; Cohen’s kappa=0.66 (95% CI: 0.53–0.78)] (Figure 4). Coefficient of variation (r=0.49, 95% confidence interval [CI] 0.35–0.61), WS-SD (r=0.32, 95% CI 0.16–0.46) and range (r=0.34, 95% CI 0.19–0.48) had the highest Pearson correlation coefficients. Bland Altmann plots were constructed for each of our variation measures and are shown in Figure 5.
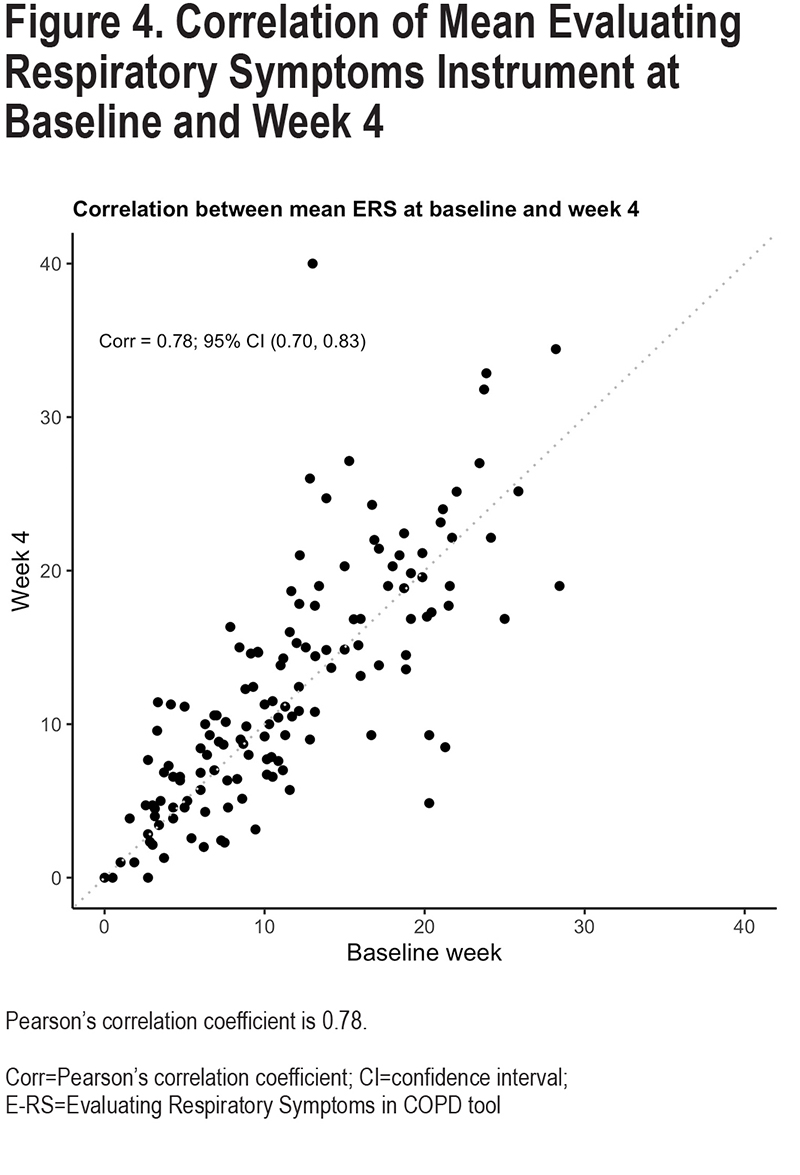
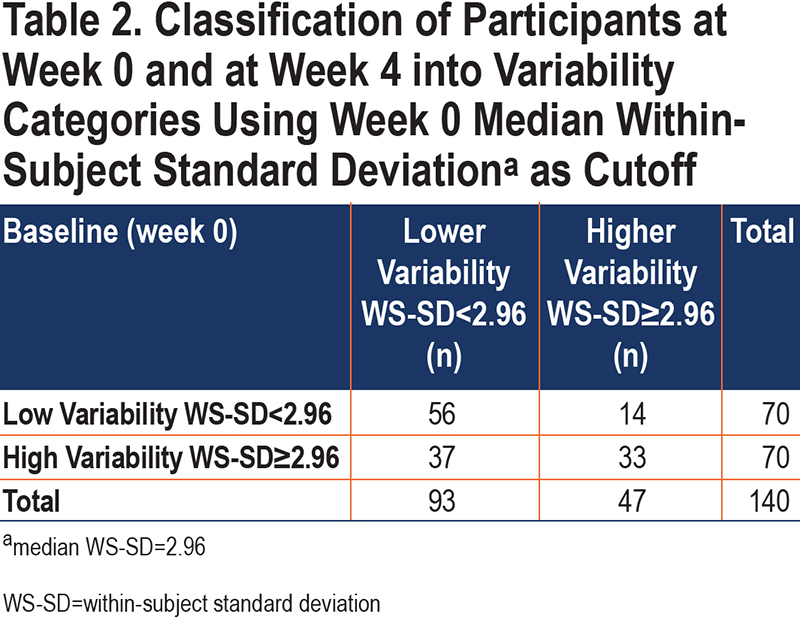
The median baseline (week 0) E-RS total WS-SD for all patients was 2.96. This value was used to dichotomize patients into lower variability versus higher variability groups for further study. There was poor agreement between Week 0 and Week 4 classifications, with a Cohen’s kappa of 0.27 (95% CI [0.12–0.42]). Between weeks 0 and 4, 36% of participants changed categories (Table 2) with the most common move from high variability at week 0 to low variability at week 4. Sensitivity analysis using a baseline period of 2 weeks rather than 1 demonstrated similar results.
We investigated overall trends in E-RS scores over the full 13-week study period, using mean E-RS total scores as a measure of overall symptom burden, and WS-SD as a measure of symptom variability. Mean WS-SD decreased by 11% (from 2.97 to 2.63) between Weeks 0 and 4 (p=0.002) (Figure E1 and E2 in the online supplement). This decrease attained statistical significance by Week 3 and was maintained throughout follow-up. When comparing Week 0 to Week 12, the mean WS-SD decreased 22%, to 2.23 (p<0.001). A large portion of the decrease occurred by Week 3, and almost no changes were detected between Weeks 6-12 (Table E2 in the online supplement).
Symptom Variability, Phenotypic Differences, and Exacerbations
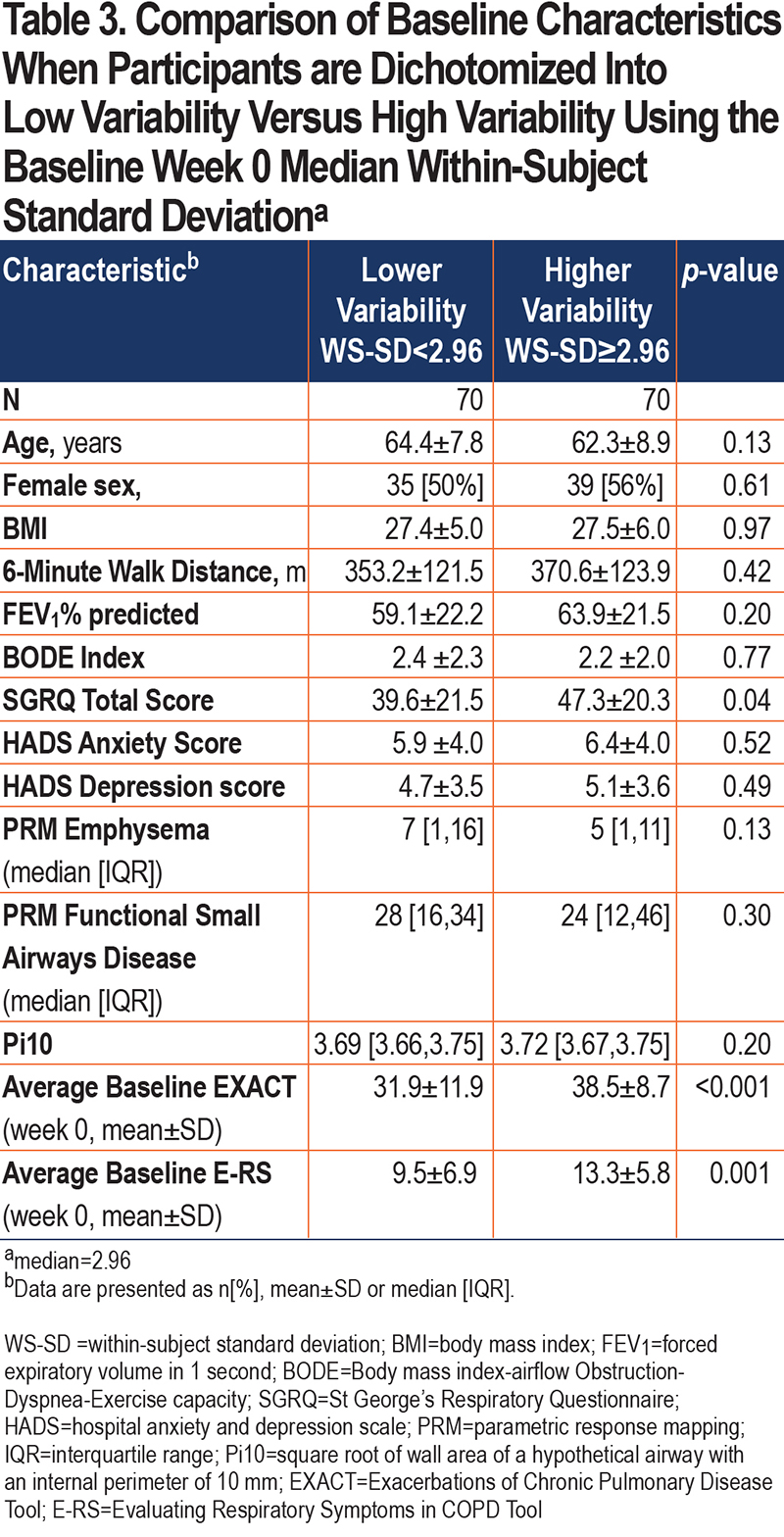
Patients in the high variability group had higher (worse) SGRQ scores (p=0.04) and higher (more severe symptoms) E-RS scores (p=0.001) during the baseline week (Table 3). Other phenotypic characteristics such as 6-minute walk distance, Body mass index-airway Obstruction-Dyspnea-Exercise tolerance (BODE) index, imaging characteristics, and comorbid anxiety and depression did not separate low versus high variability groups.
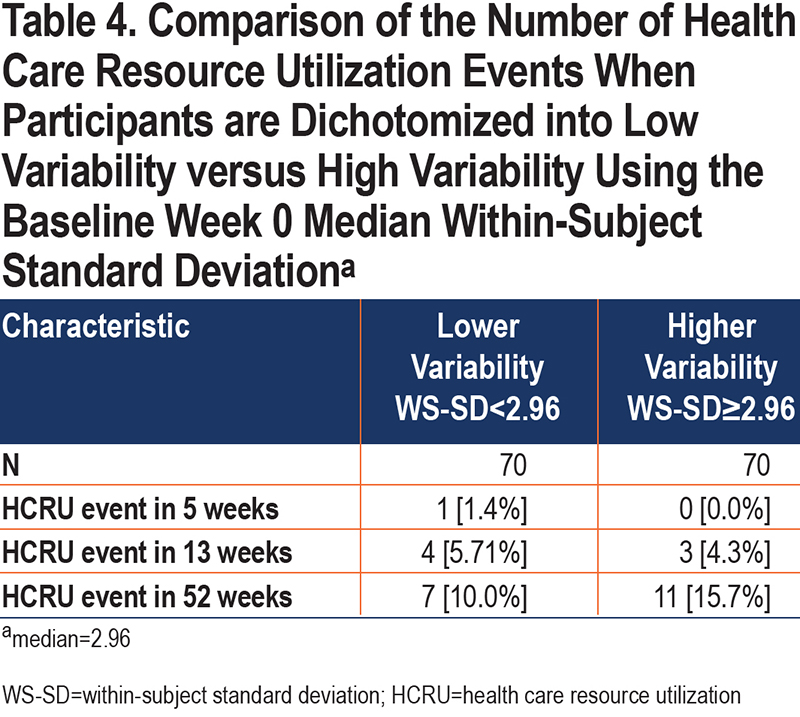
Additionally, we examined HCRU exacerbations in the participants included in our analysis. Only 1 participant experienced an HCRU exacerbation in the first 5 weeks of the study, 7 experienced an HCRU exacerbation in 13 weeks, and 18 in 52 weeks (Table 4). Participants were again divided into lower and higher variability based on an overall week 0 median WS-SD of 2.96. There were no differences in HCRU exacerbations between the low and high variability group at 13 weeks (5.7% versus 4.3%, p-value 1.0) and 52 weeks (10.0% versus 15.7%, p-value 0.45). In a sensitivity analysis, we also found that the mean baseline WS-SD was not different between those participants who experienced an HCRU versus those who did not (3.6 versus 3.0, p-value 0.44).
Higher baseline WS-SD was associated with an increased likelihood of an EXACT (symptom-defined) event (O =2.52; p=0.02) during the subsequent 4 weeks. EXACT events from 2 participants are illustrated in Figure E3in the online supplement. Even after adjusting for symptom severity (using mean baseline E-RS total score) and other potential confounders (sex, dyspnea score, 6-minute walk distance, and forced expiratory volume in 1 second [FEV1]), participants classified as having high baseline symptom variability had 2.75 greater odds of experiencing an EXACT event during the 4 weeks than individuals with low symptom variability (OR=2.75 [1.13–6.86], p=0.02).
Discussion
We used a novel approach for characterizing symptom variability in patients with COPD by using prospectively collected E-RS data from participants in the SPIROMICS Exacerbations sub-study. Our analyses demonstrate that: (1) the WS-SD of the E-RS total score performed similarly to other variability measures in that baseline values are only weakly associated with variability at week 4, (2) mean WS-SD scores decreased significantly throughout a 12-week follow-up period, primarily during the first half of follow-up, and (3) participants with higher baseline variability have increased symptom severity, although they do not demonstrate differences in other phenotypic markers compared to low variability participants. These findings provide novel insights into the patient experience in COPD.
Day-to-day symptom variability is itself variable over time, as evidenced by relatively low correlations between weeks 0 and 4 in 6 different E-RS score variability metrics. Coefficient of variation has the highest correlation between week 0 and week 4 values and could be used as a metric by which to compare the magnitude of symptom variability to variation in other measures. The Bland-Altman plots for all variability metrics have a similar shape, with better agreement among patients with overall lower variability compared to participants with larger differences between week 0 and week 4 values. The changes to symptom variation within patients over time calls into question one component of the traditional HCRU exacerbation definition, namely what constitutes a given patient’s typical day-to-day symptom variation and what aspect of worsening informs a patient’s decision to seek care. Importantly, the fluctuation in symptoms we show may have important implications when evaluating a response to therapy, especially in the absence of a control group as in n-of-1 experiments. The median E-RS total score WS-SD was 2.96 in our study. To contextualize these findings, thresholds for symptomatic improvement using the E-RS tool were developed with clinical trial data examining subgroups of patients who demonstrated improvement using established measures including the SGRQ and the Breathlessness, Cough and Sputum Scale as anchors. An overall decline in E-RS score by 2 points has been proposed as the threshold to define symptomatic improvement.15 With a median WS-SD of 2.96 in our study, we show that patients can exhibit day-to-day variation higher than proposed thresholds of symptomatic change meaningful to patients. This makes the longitudinal analysis of E-RS daily scores even more important, with persistent improvements in severity of 2 points or more needed to demonstrate improvement over time, taking into consideration day-to-day variability.
Interestingly, throughout this 5-week study period, E-RS total scores remained stable over time, while WS-SD decreased significantly, suggesting symptom severity overall is stable, while day-to-day variability is not. The stability of mean E-RS scores as a measure of symptom severity has been demonstrated in the past.24 Participants who were in the high variability group at week 0 were not always in the same variability group at week 4. These findings should be interpreted in the context of the fact that participants were only known to be stable and exacerbation free in the weeks prior to study entry. It is possible that an intervening change in therapy during a routine clinic visit or an HCRU event resulted in less variability with no effect on overall severity, though this is unlikely. It is also possibly that participant complacency or “settling on an average” occurs over time, to facilitate diary completion. The Hawthorne effect, where the knowledge that one is being observed leads to a change in behavior, could result in increased introspection and impact variability as the study progresses. Periodic reminders to patients to complete the diary thoughtfully and accurately could address this potential issue. It is noteworthy that even with the flattening of variability scores over time, mean symptom severity levels were stable and EXACT events were detected during the latter weeks of the study, with baseline variability a predictor of these events.
Our analysis suggests that individual symptom variability may be associated with increased morbidity. Patients with higher variability also had higher mean E-RS scores, i.e., more severe respiratory symptoms. Elevated SGRQ, a marker of exacerbation risk,25 was also found in the higher symptom variability group. Notably, we were unable to distinguish individuals with higher versus lower symptom variability using other phenotypic markers, suggesting that symptom variability may be a construct that is different from other physiological or imaging metrics.
While the small number of HCRU exacerbations limited our ability to determine an association between symptom variability and exacerbation using the traditional definition, we were able to explore an association between baseline day-to-day variation in symptoms and risk of developing periods of sustained symptom worsening (EXACT events). Should these findings be confirmed in larger samples, symptom variability could be a promising component of comprehensive patient assessment.
Our cohort had a higher rate of EXACT events compared to what has been previously reported in the literature.26,27 We hypothesize that the principal reason for this is due to differences in the patient population. The majority of longitudinal EXACT data have been reported in the context of placebo-controlled trials in patients meeting strict inclusion and exclusion criteria. Our patient population reflects a different opportunistic cohort with limited inclusion/exclusion criteria, perhaps closer to real-world data. Differences in approach to data collection also may influence the number of EXACT events. For example, in the FLAME randomized control trial,26 participants completed an electronic daily diary in addition to the daily EXACT. Diary responses in turn triggered the participant to respond to a clinical center, which may have led to more HCRU events relative to the number of unreported EXACT events.26
Our study has limitations, including a relatively small number of participants and, for WS-SD stability, a short study period of 5 weeks. Additionally, we did not have data regarding changes to individual treatment between week 0 and week 4 which may have affected symptom variability at week 4. A larger dataset over an extended period may allow for better characterization of longitudinal trends in variability. Requiring a high completion rate of the E-RS/EXACT diary may have resulted in selection bias in this natural history study, with only the most adherent participants included. We also did not have daily criteria measures to assist with the evaluation of stability, such as global assessments, alternative symptom diaries, or activity monitors.
In summary, ostensibly stable COPD individuals experience wide temporal variability in respiratory symptoms. Using common statistical techniques such as WS-SD to quantify day-to-day variability can lead to further insight into the association between symptom fluctuation, symptom severity, and future exacerbations. Determining the utility of the E-RS variability metric to quantify day-to-day symptom variability in COPD will require study of more individuals over a longer follow-up period and using additional modes of symptom measurement.
Acknowledgements
Author Contributions: KMA, JKK, CO, and FJM had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the analysis. KMA, JKK, CO, MKH, JAW, and FJM contributed to the conception and design of the study. KMA, JKK, CO, IE, KVB, MKH, and FJM contributed to data analysis and interpretation. EEC, SAC, DJC, GJC, JLC, MTD, MKH, NNH, RP, RPB, SPP, PGW, and FJM contributed to the acquisition of the data. KMA and JKK contributed to the drafting of the manuscript. KMA, JKK, CO, NKL, RPB, SAC, DJC, GJC, JLC, MTD, MKH, NNH, ASI, RP, SPP, JAW, PGW, KVB, and FJM contributed to revisions for critically important intellectual content. All of the authors approved this version of the manuscript to be published.
Availability of data: More information about the study and how to access SPIROMICS data is at www.spiromics.org.
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M. Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell, Jr, MD; Robert Paine, III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD and Lisa Viviano, BSN.
Declaration of Interest
JKK reports grants from NIH T32 HL134629, during the conduct of the study; non-financial support from GlaxoSmithKline, outside the submitted work. NKL reports other from Evidera, outside the submitted work. MKH reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, United Therapeutics, UpToDate, Medscape, and Integrity. She has received either in-kind research support or funds paid to the institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, Astrazeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association. She has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution. She has received stock options from Meissa Vaccines. RP reports grants from the NHLBI, grants from the COPD Foundation during the conduct of this study; grants from Department of Veterans Affairs, outside the submitted work. SAC. reports personal fees from AstraZeneca, personal fees from GlaxoSmithKline, personal fees from Amgen, personal fees from Glenmark, personal fees from Sunovion, personal fees from Genentech, personal fees from UpToDate, outside the submitted work. DJC reports grants from the NHLBI, grants from the COPD Foundation, during the conduct of the study. GJC reports grants and personal fees from GlaxoSmithKline, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Chiesi, grants and personal fees from Mereo, personal fees from Verona, grants and personal fees from AstraZeneca, grants and personal fees from Pulmonx, grants and personal fees from Pneumrx, personal fees from BTG, grants and personal fees from Olympus, grants and personal fees from Broncus, personal fees from EOLO, personal fees from NGM, grants and personal fees from Lungpacer, grants from Alung, grants and personal fees from Nuvaira, grants and personal fees from ResMed, grants and personal fees from Respironics, grants from Fisher Paykel, grants and personal fees from Patara, grants from Galapgos, outside the submitted work. JLC reports a grant from the NIH/NHLBI, during the conduct of the study; grants from the Department of Veterans Affairs and the Department of Defense, personal fees and non-financial support from AstraZeneca, and personal fees from Novartis, outside the submitted work. MTD reports grants from the NIH, during the conduct of the study; personal fees and other from Boehringer Ingelheim, personal fees and other from GlaxoSmithKline, other from Novartis, personal fees and other from AstraZeneca, other from Yungjin, personal fees and other from PneumRx/BTG, non-financial support and other from Pulmonx, other from Boston Scientific, personal fees from Quark Pharmaceuticals, personal fees from Mereo, grants from American Lung Association, grants from the NIH, grants from the Department of Veterans Affairs, other from Gala, other from Nuvaira, grants from the Department of Defense, outside the submitted work. NNH reports grants from the NIH, grants from the COPD Foundation, grants and personal fees from AstraZeneca, grants and personal fees from GlaxoSmithKline, grants from Boehringer Ingelheim, personal fees from Mylan during the conduct of the study. ASI reports grants from Agency for Healthcare Research and Quality and salary support from the University of Alabama at Birmingham Center for Outcomes and Effectiveness Research and Education, during the conduct of the study. SP reports grants from the NIH, the NHLBI, and the COPD Foundation, during the conduct of the study. JAW reports grants from GlaxoSmithKline, other from Novartis, other from Boehringer Ingelheim, other from Astra Zeneca, other from Chiesi outside the submitted work. PGW reports personal fees from Sanofi, personal fees from Regeneron, personal fees from Glenmark Pharmaceuticals, personal fees from Theravance, personal fees from GlaxoSmithKline, personal fees from NGM Pharma, from Amgen, personal fees from Genentech, outside the submitted work. KVB reports personal fees from Johnson and Johnson, personal fees from Janssen Oncology, personal fees from Eli Lilly, personal fees from Takada, outside the submitted work. FJM reports grants from the NHLBI, during the conduct of the study; personal fees, non-financial support, and other from AstraZeneca, other from Afferent/Merck, personal fees, non-financial support and other from Boheringer Ingelheim, other from Bristol Myers Squibb, other from Chiesi, personal fees and non-financial support from Canadian Respiratory Society, personal fees and non-financial support from CME Outfitters, personal fees and non-financial support from CSL Behring, personal fees from Dartmouth University, personal fees from France Foundation, personal fees from Gala, personal fees and non-financial support from Genentech, grants, personal fees, non-financial support, and other from GlaxoSmithKline, personal fees and non-financial support from Inova Fairfax, personal fees and non-financial support from MDMagazine, personal fees and non-financial support from NYP Methodist Hospital Brooklyn, personal fees and non-financial support from Miller Communications, personal fees and non-financial support from National Association for Continuing Education/Integritas, other from Nitto, personal fees and non-financial support from Novartis, personal fees from New York University, personal fees and non-financial support from Patara/Respivant, personal fees from Pearl, personal fees and non-financial support from Peer View, personal fees from Physicians Education Resource, personal fees from ProMedior, personal fees and non-financial support from Rare Diseases Healthcare Communications, personal fees from Rockpointe Communications, personal fees and non-financial support from Sanofi/Regeneron, other from Biogen, personal fees and non-financial support from Sunovion, personal fees and non-financial support from Teva, other from twoXAR, personal fees from University of Alabama at Birmingham, personal fees from UpToDate, non-financial support from Veracyte, personal fees from Vindico, personal fees and non-financial support from WebMD/MedScape, non-financial support and other from Zambon, non-financial support from ProTerrix Bio, personal fees from IQVIA, personal fees from Raziel, from Abvie, from Verona, outside the submitted work. CO, IE, and RPB report have no conflicts to disclose.