Running Head: Alpha-1 Patients and the COVID-19 Vaccine
Funding support: OJMcE received support from the Elaine Galwey Memorial Research Bursary.
Date of Acceptance: March 31, 2022 │ Date Published Online: April 9, 2022
Abbreviations: alpha-1 antitrypsin deficiency, AATD;chronic obstructive pulmonary disease, COPD; coronavirus disease 2019, COVID-19; alpha-1 antitrypsin, AAT; forced expiratory volume in 1 second, FEV1; adverse events, AEs
Citation: McElvaney OJ, Cleary B, Fraughen DD, et al. Attitudes towards vaccination for coronavirus disease 2019 in patients with severe alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis. 2022; 9(2): 266-273. doi: http://doi.org/10.15326/jcopdf.2022.0288
Online Supplemental Material: Read Online Supplemental Material (307KB)
Introduction
Effective vaccines against coronavirus disease 2019 (COVID-19) have been developed with unprecedented speed, but uptake in some regions has been slow. To maximize vaccine coverage, policymakers must appeal to unvaccinated individuals by targeting personal factors that influence decision-making. Assessing attitudes towards vaccination in different patient populations is, therefore, essential since individuals with certain conditions may prioritize issues differently than the general population.
Alpha-1 antitrypsin deficiency (AATD) is an autosomal codominant disorder resulting from mutations in the SERPINA1 gene.1-3 The normal, non-mutated Pi*M allele is associated with the healthy Pi*MM genotype and serum alpha-1 antitrypsin (AAT) concentrations of 20-53µM.4 Patients who are homozygous for the pathologically mutated Pi*Z allele display a severe functional AAT deficiency with circulating levels substantially below the putative protective threshold5 of 11µM, a pro-inflammatory phenotype,6-9 and a significantly increased risk of developing early-onset emphysema, particularly if they smoke.10-12
Although vaccination against COVID-19 is indicated as a priority for patients with chronic lung disease, no AATD-specific data regarding this essential public health measure are available. We prospectively investigated 170 Pi*ZZ genotype individuals to determine the factors influencing patients with severe AATD to proceed with vaccination.
Methodology
Ethical approval for the study was granted by the Beaumont Hospital Ethics Committee. Patients with Pi*ZZ AATD (n=179) and Pi*MM COPD (n=164) were consecutively identified from the Irish Alpha-1 Patient Registry, Alpha-1 National Targeted Detection Programme, the Irish National AATD Clinic at Beaumont Hospital and Beaumont Hospital Respiratory Outpatient Department and offered vaccination with ChAdOx1 nCoV-19 (Astra Zeneca) following the categorization of AATD patients as high-priority candidates for vaccination by the Irish government. Non-lung disease controls were selected at random from the list of patients due to attend the vaccination clinic during the same week as AATD and/or COPD patients. Individuals who had previously been diagnosed with COVID-19 or had already been vaccinated were excluded. Patient AAT protein phenotypes were previously determined by immunofixation of glycoforms via isoelectric focusing gel electrophoresis with confirmatory genotyping, following a serum AAT level. Patients in the non-lung disease control group were deemed eligible following review of their medical records and serum AAT levels. Patients accepting the offer of vaccination were then referred to a hospital-based vaccination clinic overseen by the Health Service Executive of Ireland. Non-pulmonary patients attending this vaccination clinic included patients referred from other medical clinics, non-clinical hospital staff, and community referrals from family practitioners.
Patients who accepted the offer of vaccination were included in the study analysis and matched with non-lung disease controls (n=140) attending for vaccination in the same week. Patient attitudes towards vaccination, motivations for getting vaccinated, and pre-vaccine concerns were assessed using predetermined questionnaires at the time of the first vaccine being offered. Patients who accepted the offer of vaccination were surveyed immediately after accepting the offer of vaccination, before they attended for their vaccination appointment. The time between vaccine offer and vaccine appointment was approximately 5 days. In order to ensure the survey was taken under circumstances that were as comparable as possible, non-lung disease controls were also surveyed 5 days before their scheduled appointment. Similarly, the study groups were matched for date of vaccination so as to minimize the potential confounding effects of differences in extrinsic factors such as media coverage on patient attitudes. For each of the groups studied, participant surveys were conducted between March 10, 2021, and April 28, 2021. In the Pi*ZZ cohort, we subsequently assessed factors influencing the decision to accept a scheduled booster dose.
Results
Baseline Characteristics of the Pi*ZZ Cohort
The mean age of the Pi*ZZ cohort (n=170) was 58 +/- 13 years; 86 were male, 84 were female.
Pi*ZZ patients had a mean forced expiratory volume in 1 second ( FEV1)of 61.7 +/- 31.6% of the predicted value and a diffusing capacity for carbon monoxide of 57.4 +/- 25.3%. A total of 39 patients (23%) had known hepatic involvement on ultrasound. Six were active smokers, 99 were ex-smokers, and 4 were active vapers. Twelve were receiving supplemental oxygen and 13 were receiving augmentation therapy with plasma-purified AAT. By comparison, the mean FEV1 (pp) in the Pi*MM COPD group (n=150, 71 male, 79 female) was 57.5%. The 3 study groups were matched for age, sex, and other known comorbidities.
Patient Views Prior to Vaccination
At the time of study commencement, 2 vaccines were in use in Ireland – ChAdOx1 nCoV-19 and the mRNA vaccine BNT162b2 (“Comirnaty,” BioNTech/Pfizer), with only the former available to AATD patients under the age of 80 years. Of the 179 Pi*ZZ individuals initially surveyed, 9 declined the opportunity to receive ChAdOx1 nCoV-19 while 14 out of 164 Pi*MM COPD patients turned down the vaccine (Table S1 in the online supplement). However, Pi*ZZ patients who accepted the offer did not necessarily have full confidence in the vaccine (Figure S1 in the online supplement). When asked which vaccine they would choose if both options were made available, 90% stated they would instead select BNT162b2. This pattern was reflected in the Pi*MM COPD and non-lung disease groups, where BNT162b2 was favored by 81% and 88%, respectively. This suggests that even though ChAdOx1 nCoV-19 was not the vaccine of choice for many individuals, it was viewed as a better option than remaining unvaccinated until access to BNT162b2 was extended to all groups and did not deter patients from attending for vaccination.
Motivations for Accepting Vaccination Among Patients with Pi*ZZ Alpha-1 Antitrypsin Deficiency
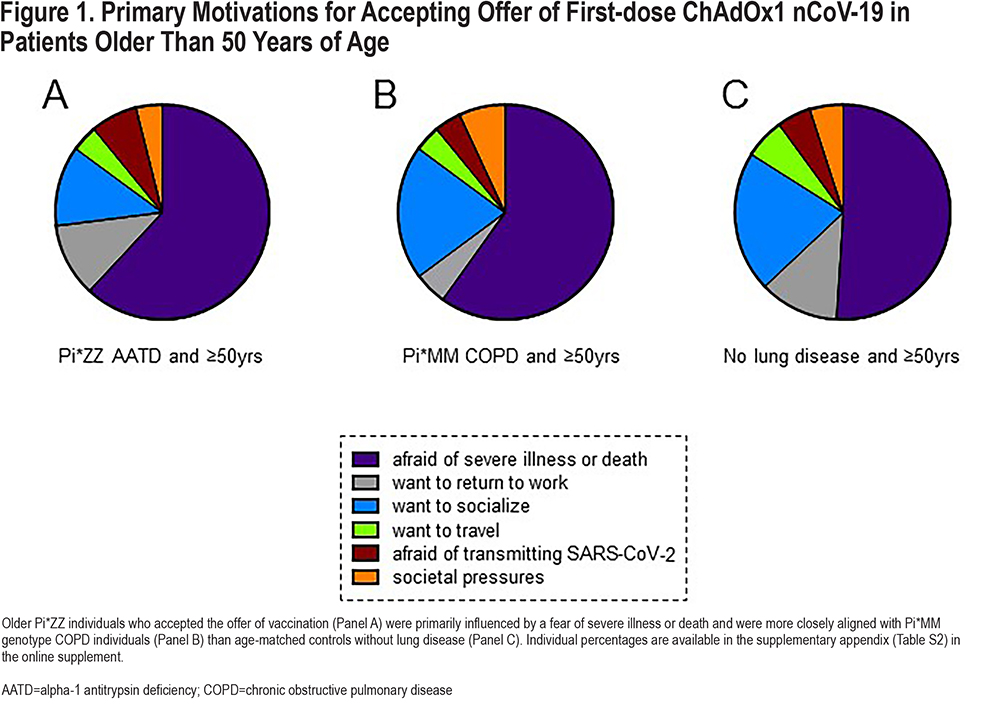
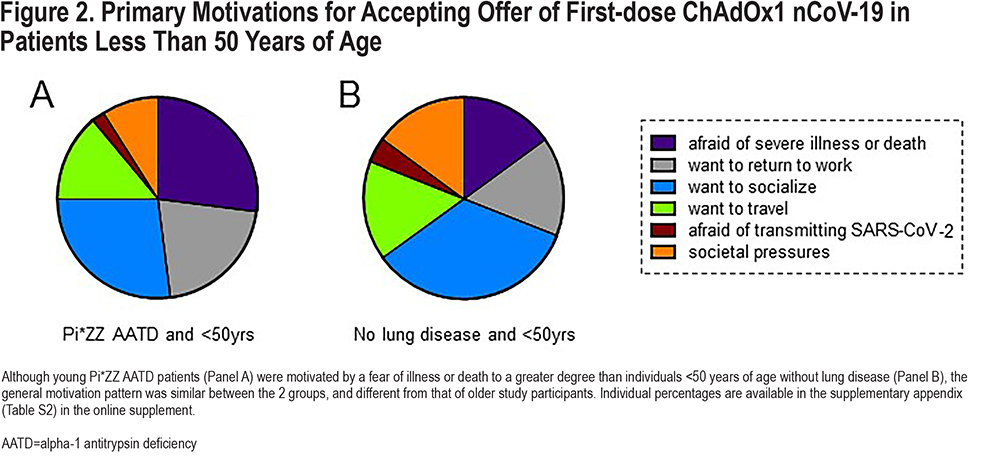
Motivations for accepting vaccination with ChAdOx1 nCoV-19 varied within groups, most notably according to age. In the Pi*ZZ AATD group, the most common reason for getting vaccinated in people over the age of 50 was a fear of serious illness or death, which was cited as the primary motivation for accepting vaccination by 62%, followed by an increased freedom to socialize (12%) and a desire to return to work (11%, Figure 1A). In contrast, only 27% of younger Pi*ZZ individuals listed protection against illness as their main consideration – the same percentage of younger people as accepted based on an eagerness to socialize (Figure 2A). A similar percentage (60%) of Pi*MM COPD patients ≥50 years were driven primarily by the threat of illness (Figure 1B), but this figure was lower (51%) in non-lung disease controls of comparable age (Figure 1C), consistent with the concept that a respiratory virus might be expected to impact those with pre-existing lung disease more severely. Societal pressures – predefined as direct perceived pressure from peers, perceived pressure from social media and news outlets, and/or a fear of peer judgment, – did not markedly influence older study participants, but had a clear impact on younger people, serving as the top motivation for getting a vaccine in 15% of under 50-year-olds without lung disease (Figure 2B). The percentage of study participants who cited a fear of transmitting SARS-CoV-2 to a more vulnerable person as their main motivation was low, ranging from 7% in Pi*ZZ patients ≥50 years to only 2% in Pi*ZZ patients under the age of 50.
Attitudes Towards Coronavirus Disease-2019 Booster Vaccination
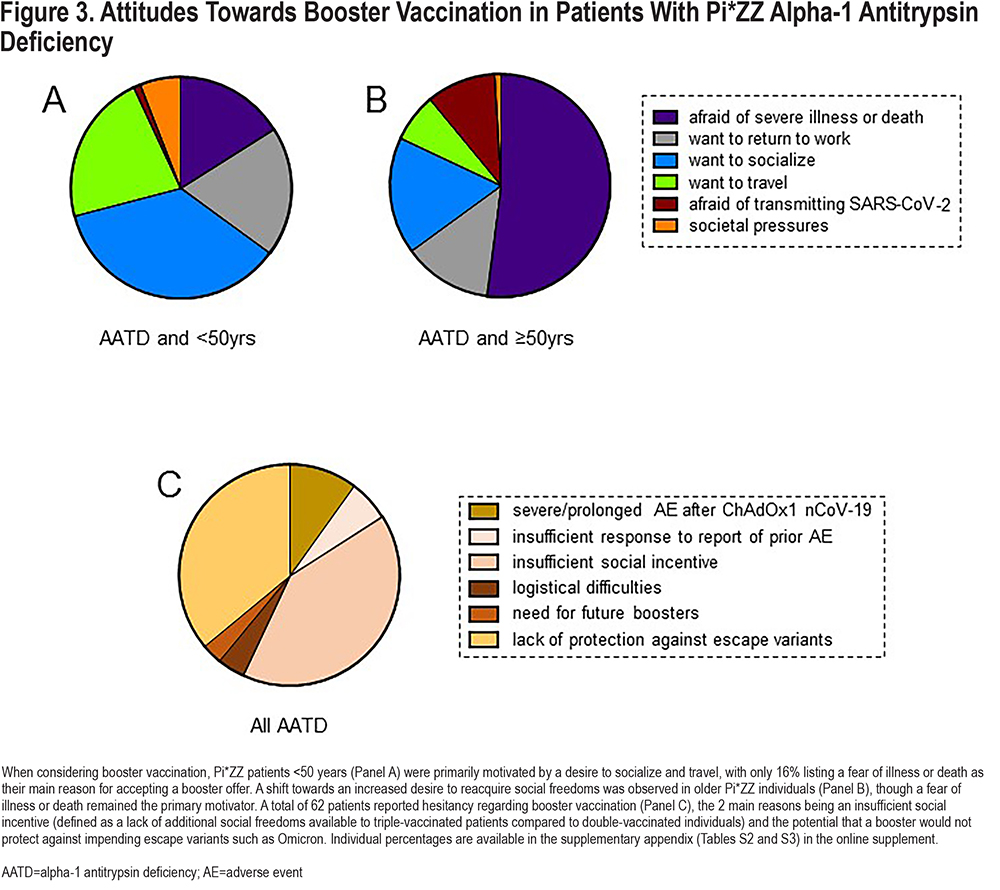
Following the October 2021 announcement by Irish public health officials that a COVID-19 booster vaccine program would be required, we revisited the Pi*ZZ cohort to see whether the key motivating factors for accepting a booster (Figure 3A, 3B) differed from those identified at the outset of the initial ChAdOx1 nCoV-19 vaccine course (Figure 1, Figure. 2). In both Pi*ZZ subgroups, fewer study participants considered a fear of illness or death to be their primary motivation, with patients instead influenced by the desire to socialize and travel (Figure 3A, 3B). This shift was most apparent in younger AATD patients, only 16% of whom cited a fear of illness or death as the primary reason they would undergo booster vaccination (Figure 3A).
Booster Hesitancy
A total of 62 Pi*ZZ patients (36%) expressed some degree of booster hesitancy, the most common reasons being uncertain coverage against escape variants such as Omicron, and a lack of incentive given that, in Ireland, no additional social liberties would be afforded to triple-vaccinated citizens compared to those who were double-vaccinated (Figure 3C). A total of 138 Pi*ZZ individuals who received ChAdOx1 nCoV-19 experienced local adverse events (AEs) following the initial vaccine dose, with 103 having systemic AEs and 55 having an AE duration of at least 3 days. Despite this, the large majority (91%) of Pi*ZZ participants who experienced AEs still planned to attend for a booster if offered one. Of the Pi*ZZ patients reporting booster hesitancy, only 6 (10%) cited a prolonged or severe vaccine-associated AE as the underlying cause.
Discussion
Here we report the attitudes, motivations, and concerns of people with Pi*ZZ AATD regarding vaccination against COVID-19. ChAdOx1 nCoV-19 was not the preferred vaccine option for most of those included in the final analysis, with a sizeable number also expressing safety concerns in advance of being vaccinated. However, the overwhelming majority of people offered ChAdOx1 nCoV-19 accepted, rather than holding out for an alternative. Factors influencing the decision of Pi*ZZ patients to accept an offer of vaccination differed according to age. In Pi*ZZ individuals greater than 50 years, a fear of severe illness or death was, by a large margin, the primary motivation, in contrast to Pi*ZZ individuals under the age of 50, for whom the desire to socialize was equally as important. This trend was even more pronounced in the non-lung disease group, where the percentage of people motivated by a return to socializing was more than double that motivated by a fear of illness. Reducing the risk of transmitting SARS-CoV-2 was not a prominent consideration among Pi*ZZ study participants, particularly those less than 50 years old. This was somewhat surprising given that, at the time the study was conducted, many of these individuals were in regular contact with direct relatives affected by the same condition. In general, participants under the age of 50 were also more likely to be influenced by societal pressures and a fear of judgment by peers than their older counterparts.
While these results provide an insight into how patients with AATD and COPD interact with new public health policies and interventions, they should also be taken in context. The data reported here are based on the most important factor motivating respondents to accept an offer of vaccination. When designing the study, our priority was to identify the factors that most materially affected patient decision-making. We felt that, by compelling a given patient to commit to a single answer – as opposed to a collection of them – we stood a better chance of identifying the salient factor that ultimately determined their decision and informing more focused consultations and public health messaging.
Patients were surveyed in the first half of 2021, so it stands to reason that as the COVID-19 landscape evolved, so too did the factors motivating patients to attend for first-dose vaccination. Indeed, this is reflected in the responses of the Pi*ZZ cohort prior to the second vaccine dose. The availability of free vaccination and public health care services in Ireland also meant that financial concerns such as the potential cost of a COVID-19-related hospitalization were less likely to influence Irish patient decisions to the same extent as those made by patients from other jurisdictions. The cohort was limited to Ireland primarily because the variability in vaccine rollout schemes and dosing schedules between countries at the time the surveys were conducted made standardizing an international study unfeasible.
In broad terms, vaccine motivations in young patients with severe AATD were aligned with non-deficient individuals of comparable age, whereas older Pi*ZZ individuals were more closely aligned with Pi*MM COPD individuals and differed from age-matched healthy controls. When considering booster vaccination, lowering the personal risk of death or severe illness – or indeed the risk to others – influenced decision-making in younger Pi*ZZ patients to a lesser degree than anticipated. Although these results suggest that linking personal freedoms such as air travel to vaccination status may improve booster uptake, the corollary – that inclusion of triple-vaccinated individuals in future lockdown restrictions may reduce enthusiasm for accepting a vaccine – may also apply. These data may, therefore, inform policymakers attempting to promote vaccine uptake among younger people.
While this study is smaller than similar studies in more common chronic lung diseases, it is considerably larger than most observational studies of Pi*ZZ AATD published to date and is the first to examine COVID-19 vaccine behavior in patients with this rare disease. As the pandemic has evolved, so too has the concept of what makes vaccination valuable to patients. It is incumbent on health care providers and policymakers to involve patient-relevant goals in consultations and vaccination initiatives if they are to succeed.
Acknowledgements
Author contributions: OJMcE, TPC, and NGMcE conceptualized the study; OJMcE, DDF, GK, BC, OFMcE, PB, CG, and TPC recruited patients and collected data; OJMcE, DDF, MPM, TP, and NGMcE analyzed data; OJMcE and NGMcE wrote the manuscript. All authors reviewed and edited the manuscript prior to submission.
Declaration of Interest
OJMcE has been an investigator in clinical trials for Vertex and Chiesi, reports speaking fees – all outside the present unfunded study – from GlaxoSmithKline and Novartis and reports current funding from the Elaine Galwey Memorial Research Bursary. NGMcE has been an investigator in clinical trials for CSL Behring, Galapagos, Chiesi, and Vertex, and reports personal fees – all outside the present unfunded work – from CSL Behring, Grifols, Chiesi, and Shire. The remaining authors have no interests or funding sources to disclose.