Running Head: Frequency of COPD Exacerbations on Quality of Life
Funding Support: This research was supported by federal funding from the National Heart, Lung, and Blood Institute, the Canadian Institutes of Health Research, and the National Institutes of Health. NCT01061671, NCT00325897.
Date of Acceptance: March 30, 2022 │ Published online: April 1, 2022
Abbreviations: chronic obstructive pulmonary disease, COPD;Simvastatin for the Prevention of Exacerbations in Moderate-to-Severe COPD, STATCOPE; Azithromycin for Prevention of Exacerbations of COPD, MACRO; acute exacerbation of COPD, AECOPD; St George’s Respiratory Questionnaire, SGRQ; forced expiratory volume in 1 second, FEV1; 6-minute walking distance, 6MWD; body mass index, BMI; Global initiative for chronic Obstructive Lung Disease, GOLD; forced vital capacity, FVC; American Thoracic Society, ATS; European Respiratory Society, ERS; standard deviation, SD; long-acting muscarinic antagonist, LAMA; long-acting beta2-agonist, LABA; short-acting beta2-agonist, SABA; minimal clinically important difference, MCID
Citation: Camac ER, Stumpf NA, Voelker HK, Criner GJ; COPD Clinical Research Network. Short-term impact of the frequency of COPD exacerbations on quality of life. Chronic Obstr Pulm Dis. 2022; 9(3): 298-308. doi: http://doi.org/10.15326/jcopdf.2021.0280
Note: Information from this study was presented in abstract/thematic poster format at the 2015 American Thoracic Society International Conference in Denver, Colorado and later published as an abstract (2015. Am J Crit Care;191)
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease defined by the presence of airflow limitation which may be punctuated by acute exacerbations. Acute exacerbations of COPD (AECOPDs) act as markers of disease severity and have important prognostic implications. AECOPDs are associated with clinical worsening including decline in forced expiratory volume in 1 second (FEV1),1 lower baseline FEV1,2 decreased 6-minute walk distance (6MWD),1,2 increased health care utilization,3 and higher cost of care.3
In the 2015 Simvastatin for the Prevention of Exacerbations in Moderate-to-Severe COPD (STATCOPE) trial, acute exacerbations were defined as “an increase in severity or new onset of 2 or more respiratory symptoms (cough, sputum, wheezing, dyspnea, or chest tightness) lasting for at least 3 days and requiring treatment with antibiotics or systemic glucocorticoids.”4 Exacerbations may be triggered by a bacterial or viral respiratory illness,5,6 among other causes.
Previous studies have found that patients with a history of 1 or more exacerbations have higher St George’s Respiratory Questionnaire (SGRQ) scores than those with no exacerbations,7 implying an effect of AECOPDs on quality of life. The SGRQ examines 3 categories—symptoms, activities, and impact—to evaluate quality of life in patients with airway disease.8 Our goal was to further elucidate the short-term effect of AECOPDs on quality of life.9
We hypothesized that patients with frequent exacerbations would experience a greater short-term decline in quality of life as measured by the SGRQ than those with infrequent exacerbations. We also predicted that FEV1 and body mass index (BMI) would decline to a greater degree in patients with frequent exacerbations than in those with infrequent exacerbations.
Materials and Methods
Patient Population
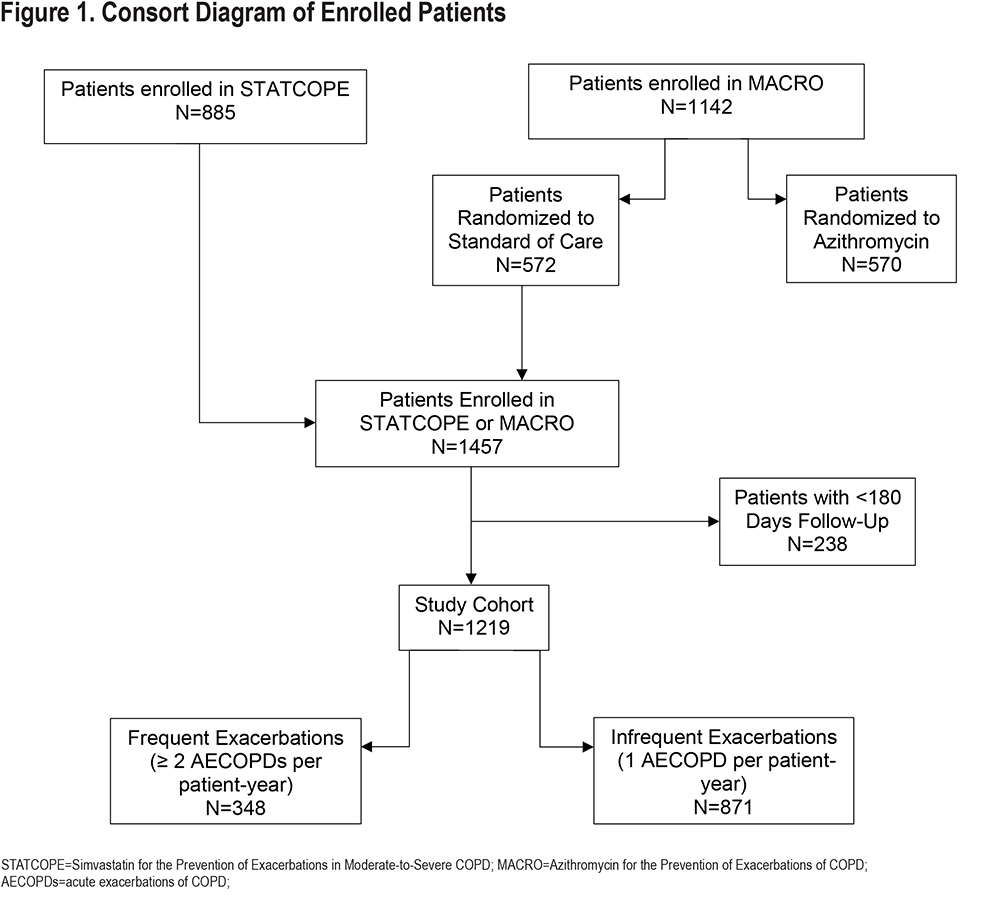
We analyzed data collected from patients enrolled in either STATCOPE or the Azithromycin for Prevention of Exacerbations of COPD (MACRO)10 trial, excluding patients randomized to the azithromycin group of MACRO. These cohorts were combined with confidence as simvastatin, the treatment arm intervention in STATCOPE, was not found to impact COPD outcomes.4 Of the 2027 total patients who were enrolled in STATCOPE or in MACRO, we identified 1219 patients who experienced at least 1 acute exacerbation and who were enrolled in either trial for at least 180 days. These patients were then divided into 2 groups: those with frequent exacerbations (≥ 2 AECOPDs per patient-year), and those with infrequent exacerbations (1 AECOPD per patient-year). Exacerbations in both trials were counted based on the above definition, and therefore, only when they were moderate-to-severe.
Inclusion criteria for the STATCOPE and MACRO trials included patient age between 40-80 years, a clinical diagnosis of Global initiative for chronic Obstructive Lung Disease11 (GOLD) 3-4 COPD, FEV1/ forced vital capacity (FVC) ratio of <70% post-bronchodilator by spirometry, former or current smoking history with at least 10 pack years of prior smoking, no incidence of AECOPD within the 4 weeks prior to enrollment, and one of the following in the past year: use of supplemental oxygen, a hospitalization or emergency department visit for AECOPD, and/or use of steroids or antibiotics for respiratory problems.
Differences exist in exclusion criteria for STATCOPE and MACRO. STATCOPE excluded patients with clinical indications for statin therapy, patients receiving drugs contraindicated for use with statins, and patients with alcohol use disorder or active liver disease. MACRO excluded patients with a recorded resting heart rate >100 beats per minute, prolonged corrected QT interval >450 milliseconds, hearing impairment as documented by audiometric testing, or use of medication known to prolong the QT interval or to provoke torsade-des-pointes with the exception of amiodarone. Figure 1 provides a consort diagram for patients enrolled in the study.
Data Collection
The study received approval by the institutional review boards at each of the participating institutions, and the STATCOPE and MACRO steering committees.
Baseline pre-randomization data were collected for each included patient: demographics, respiratory medication use, smoking history, spirometry performed pre-bronchodilator as per American Thoracic Society (ATS)/ European Respiratory Society (ERS) guidelines, BMI, SGRQ, and presence of chronic bronchitis as measured by the ATS respiratory questionnaire.
For both groups, we recorded changes in BMI, spirometry, and SGRQ over time and compared results between patients in the infrequent exacerbator group and patients in the frequent exacerbator group.
Data Analysis
For this analysis, patients were divided into 2 groups: those with frequent moderate-to-severe exacerbations (≥2 AECOPDs per patient-year), and those with infrequent moderate-severe exacerbations (1 AECOPD per patient-year). Characteristics were compared between groups using Pearson’s chi-square test for categorical variables, with data expressed as a percentage, and using student’s t-test for continuous variables, with data expressed as mean±standard deviation. Analyses were completed using R version 3.32 or SAS (Cary, North Carolina.)12
Results
A total of 1219 patients were followed for at least 180 days. The infrequent exacerbation group included 871 patients, and the frequent exacerbation group included 348 patients.
Demographic data for the 2 groups are presented in Table 1. Several differences were identified between groups which were statistically significant. In total, 29.6% of infrequent exacerbators were current smokers compared to 20.1% of frequent exacerbators (p=0.005). Frequent exacerbators were more likely to bear a diagnosis of chronic bronchitis (53.2% versus 45.2%).
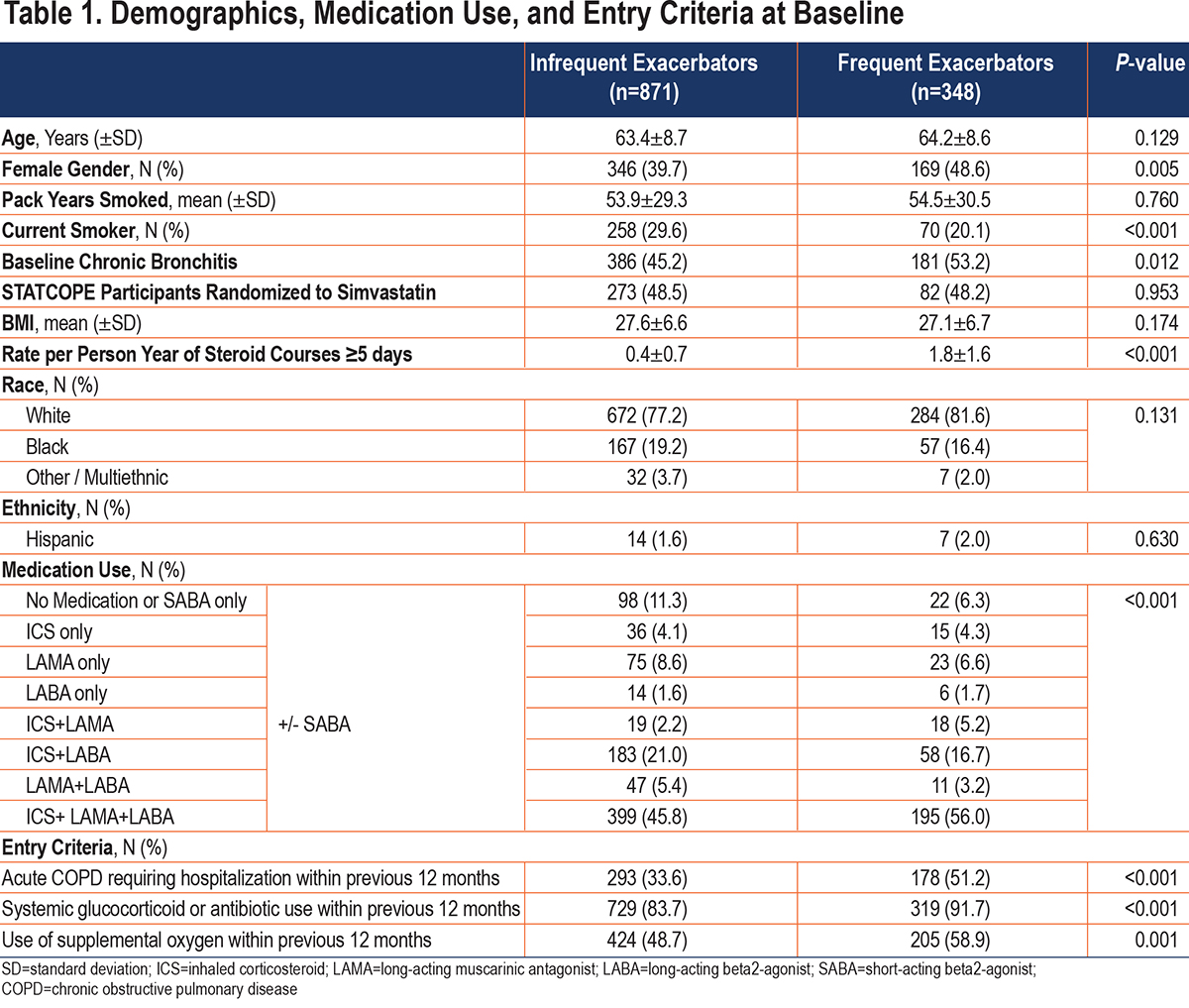
Frequent exacerbators at baseline had more impaired pulmonary function, but BMI was not different between groups. Figure 2 reveals the spirometric differences between groups. Frequent exacerbators had other markers of increased disease severity, including medication use. A total of 82.2% of frequent exacerbators were on a combined regimen including an inhaled corticosteroid (ICS), compared to 73.1% of infrequent exacerbators (p=<0.001). By contrast, infrequent exacerbators were more likely to take no therapies or to use only short-acting beta2- agonist (SABA) therapy. Frequent exacerbators were more likely to use oxygen. Before study enrollment, frequent exacerbators had more exacerbations and were more likely to have chronic bronchitis. In the 12 months before study enrollment, 51.2% of frequent exacerbators had an exacerbation compared to 33.6% in the infrequent exacerbators group (p=<0.001).
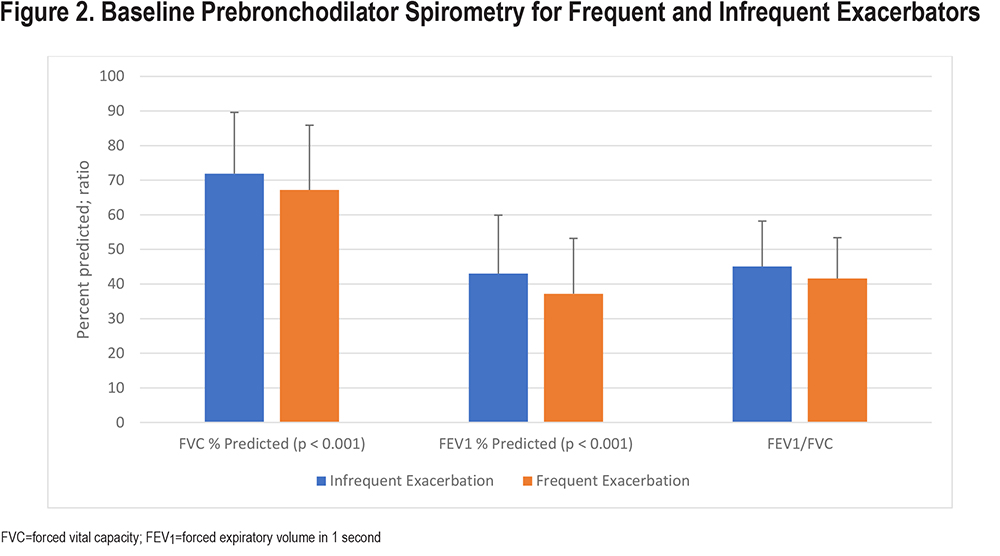
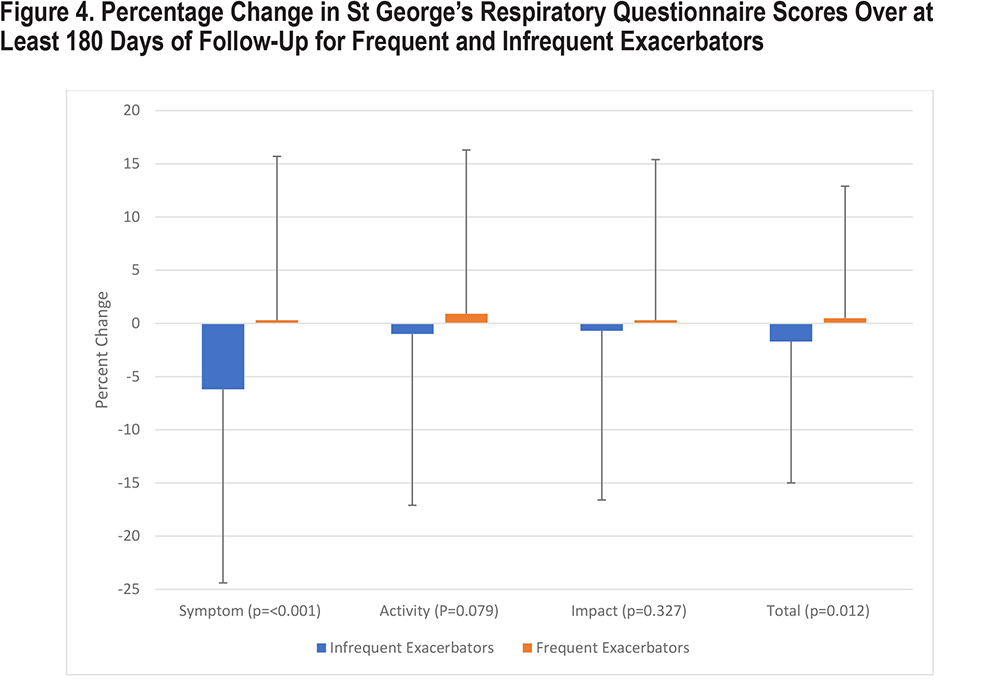
At baseline, frequent exacerbators had more severely impaired scores in all 3 domains of the SGRQ, including symptoms, activity, and impact (all p-values <0.001) (Figure 3). Over the course of the study, infrequent exacerbator scores experienced improvement, with a 6.2% improvement in symptom scores. Activity scores and impact scores were unchanged (Figure 4). By contrast, frequent exacerbator symptom scores worsened by 0.3%, activity scores worsened by 0.9%, and impact scores worsened by 0.3%. The difference in symptom scores between groups was statistically significant (p-value <0.001). Differences in activity and impact scores were not statistically significant.
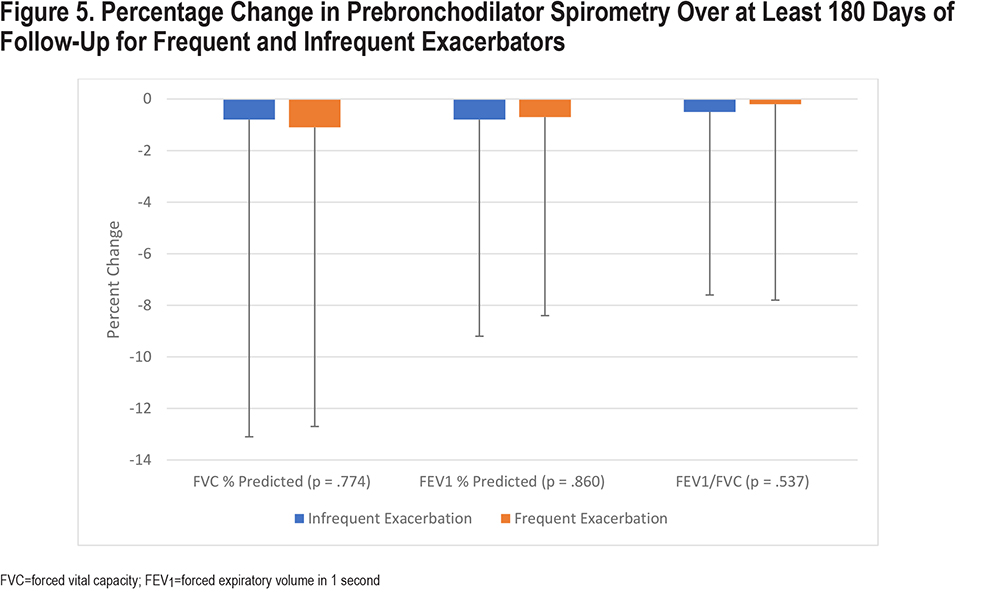
Change from baseline in spirometric values did not differ significantly between the 2 groups (Figure 5).
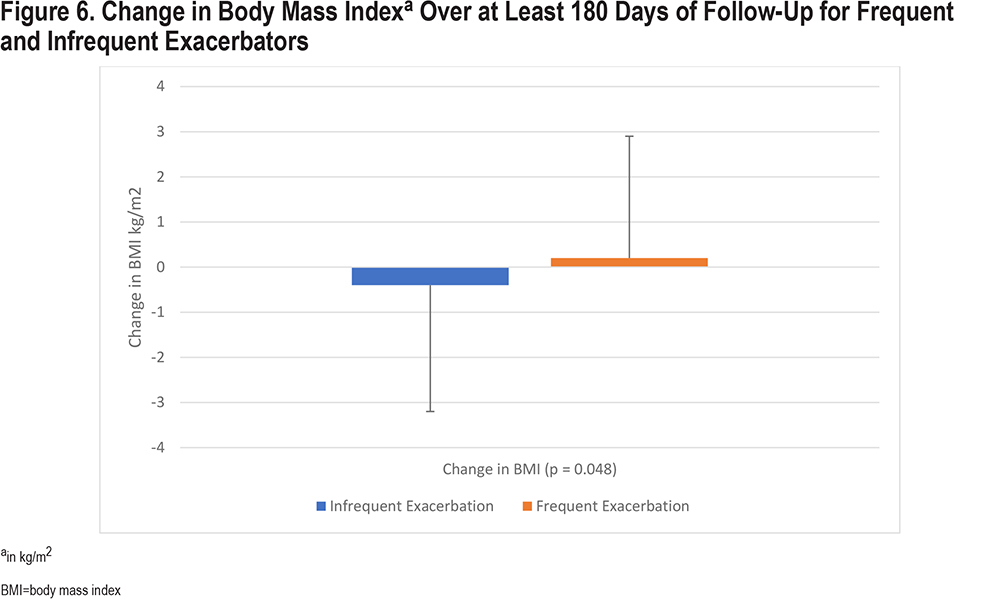
Over the course of the study, patients in the infrequent exacerbation group had a slight decrease in BMI, where patients in the frequent exacerbation group experienced a slight increase in BMI. This between-group difference was statistically significant (p=0.048) (Figure 6).
Discussion
The SGRQ was initially developed in 1991 by Jones, Quirk, and Baveystock at St George’s University of London for use as a measure of quality of life in airway disease and includes 3 subcategories: symptom, activity, and impact.8 Our analysis of the 1219 eligible patients enrolled in STATCOPE and MACRO over a minimum of 180 days found that those with 2 or more moderate-to-severe AECOPD events per patient year experienced a statistically significant worsening in SGRQ symptom scores compared to those with 1 or fewer moderate-to-severe AECOPD events.
This change was driven by improvement in the symptom subscores of the infrequent exacerbator group, which improved by -6.2±18.2%. During the same period, the frequent exacerbator group experienced a very slight worsening of +0.3±15.4%. These data suggest that the change in symptom scores between the 2 groups is predominantly driven not by a worsening in frequent exacerbator symptom scores, but rather by an improvement in infrequent exacerbator symptom scores over time. Although this difference was below the minimal clinically important difference (MCID) for SGRQ, we feel that it represents a valuable insight into the patient experience.13 In part, we feel this is true because patients included in this analysis were recruited in a randomized trial in which a placebo effect was evident.
The improvement experienced by patients with infrequent exacerbations is likely due to the adherence of patients in clinical trials to daily COPD therapies. The efficacy of the COPD therapies used by patients in MACRO and STATCOPE, in which most patients received daily inhaled therapies, has been well-established.4,10 Patients with frequent exacerbations were less likely to experience the improvement in symptom scores achieved by patients on therapy.
This study did not identify between-group differences in change in spirometric values, as was hypothesized. Similarly, contrary to our hypothesis, patients with frequent exacerbations were more likely to have an increase in BMI. Both findings may be impacted by the mean BMI of both cohorts beginning the study in the obese range. Obesity in patients with COPD has both an obstructive and restrictive impact on FEV1, which may limit the impact of subtle changes over time to the obstructive component.14 Patients with frequent exacerbations and obesity may have been more likely to gain weight as a result of several factors independent of disease severity including loss of muscle mass,15 inactivity,16 the impact of corticosteroid therapies,17 and the impact of increased baseline symptom burden on physical activity.18 This increase in BMI may have impacted symptom scores in those with frequent exacerbations.
Patients in the infrequent exacerbators group were more likely to be current smokers when compared to the frequent exacerbators group (29.6% versus 20.1%, p=0.005). This finding is similar to those in other trials, in which patients with more severe COPD by symptoms and spirometry were more likely to attempt smoking cessation and to succeed with quit attempts.19,20
The frequency of AECOPD events has previously been tied to a decline in patient-reported quality of life, though over longer durations7,9,21,22 as time from recovery to baseline from an index exacerbation is estimated at 91 days.23 These events are associated with low baseline patient-reported quality of life and lung function,24,25,26 as our study corroborates, and associated with the development of a frequent-exacerbator phenotype.27,28 One prior study reports similar findings, including the contrast between improvement in patients without exacerbations compared to a stepwise worsening in patients with an increasing frequency of exacerbations, but like other trials, this was observed over at least 52 weeks.29 Our study is the first, to our knowledge, to describe these effects over such a short timeline.
Although the current study included a large sample of patients enrolled in the STATCOPE and MACRO trials, it has some limitations. Patients were only eligible for STATCOPE and MACRO trials if they had moderate to severe COPD, GOLD 3-4, which could affect the applicability of the results to broader populations.11 Eligibility criteria also included at least 1 of the following: use of supplemental oxygen, a hospitalization or emergency department visit for AECOPD, and/or use of steroids or antibiotics for respiratory problems, criteria which further exclude patients with mild disease. All results may be impacted by the increased rate of systemic and inhaled glucocorticoid use in the frequent exacerbator group as well as the differences in other inhaled medication use between groups. Additionally, gender disparities between groups exist at baseline which may impact the results. Finally, study results may be limited as much as they are enhanced by the duration of follow-up, as 180 days may be an inadequate amount of time to elucidate some significant between-groups differences. Conversely, 180 days may be enough time that regression to the mean is possible in the infrequent exacerbators group.
Both the STATCOPE and the MACRO trials admitted patients with diverse phenotypes of COPD. This may impact the outcome of the interventions used in both studies, as well as analyses like this one. Patients with frequent exacerbations likely represent their own phenotype of moderate-to-severe COPD and are characterized by persistent severe symptoms less responsive to the placebo effect or medication treatment effect.
Conclusion
Our study examined short-term changes in quality of life, BMI, and spirometry in a large cohort of patients with moderate to severe COPD over the course of at least 180 days. We demonstrate that patients with frequent exacerbations of COPD experienced a slight worsening of severely impaired SGRQ symptom scores, while patients with infrequent exacerbations experienced improvement while on COPD therapies.
Acknowledgements
Author Contributions: EC and GC conceived of and refined the original research idea. EC and HV collected and analyzed the data. EC, NS, and GC wrote and edited the manuscript. All authors contributed substantially to the creation of the research project and this manuscript, and all authors approve of its publication.
Data Sharing Statement: Individual, de-identified participant data and related data are available upon request subject to and determined by the COPD Clinical Research Network and Canadian Institutes of Health Research policies. Please contact the COPD Clinical Research Network with questions and requests.
Declaration of Interest
The authors have no conflicts of interest to declare.