Running Head: Improving CPAP Adherence in COPD/ Sleep Apnea Patients
Funding Support: The study was funded by the Patient-Centered Outcomes Research Institute (PCORI) contract PPRND-1507-3166.
Date of Acceptance: July 4, 2022 │ Published Online Date: July 7, 2022
Abbreviations: obstructive sleep apnea, OSA; chronic obstructive pulmonary disease, COPD; continuous positive airway pressure, CPAP; proactive care, PC; reactive care, RC; National Patient-Centered Outcomes Research Network, PCORnet; Patient-Centered Outcomes Research Institute, PCORI; overlap syndrome, OVS; Patient-Powered Research Network, PPRN; Confirmation of Eligibility, COE; home medical equipment, HME; apnea-hypopnea index, API; respiratory therapist, RT; Functional Outcomes of Sleep Questionnaire, FOSQ; Patient-Reported Outcomes Measurement Information System, PROMIS; Pittsburgh Sleep Quality Inventory, PSQI; COPD Assessment Test, CAT; Epworth Sleepiness Scale, ESS; patient-reported outcomes, PROs; Functional Comorbidity Index, FCI; confidence interval, CI; standard deviation, SD; total sleep time, TST; total sleep period, TSP; mean wake after sleep onset, WASO
Citation: Martinez S, Sullivan J, Pasquale C, et al. Effect of two interventional strategies on improving continuous positive airway pressure adherence in existing COPD and obstructive sleep apnea patients: the O2VERLAP study. Chronic Obstr Pulm Dis. 2022; 9(3): 394-412. doi: http://doi.org/10.15326/jcopdf.2022.0293
Introduction
Overlap Syndrome Overview
Chronic obstructive pulmonary disease (COPD) is a group of progressive and debilitating respiratory conditions affecting 15 to 25 million Americans1 and more than 300 million people worldwide.2 COPD is the fourth leading cause of death and a leading cause of disability in the United States.3 Each year, COPD results in as many as 800,000 hospital admissions and 1.5 million emergency department visits.4 Obstructive sleep apnea (OSA) is a prevalent chronic medical condition characterized by repeated stops (apneas) and near stops (hypopneas) of breathing during sleep, due to the closure or partial closure of the upper airway.5 Apneas and hypopneas cause repeated sleep arousals and oxygen desaturations that lead to significant consequences, including daytime sleepiness and an increased risk of cardiovascular problems. OSA affects 17% of adults and more than 25% of older adults,6 with rates increasing over the last 2 decades likely due in part to the obesity epidemic.7 OSA requires immediate and ongoing therapy because of its psychosocial consequences (impaired cognitive performance,8,9 health-related effects, decrement to quality of life,10,11 anxiety and depression,12 daytime sleepiness,13 and motor vehicle crashes14,15); medical consequences (hypertension,16 stroke,17,18 and impaired glucose metabolism19); and increased mortality.20,21 Untreated OSA has a significant economic and societal burden.22
OSA is prevalent in 10% to 15% of patients diagnosed with COPD.23 The prevalence of COPD is similar in patients with OSA as in those in the general population. When COPD and OSA co-exist in a patient, it is commonly referred to as the overlap syndrome (OVS).24 OVS is generally considered distinct from either condition alone. Patients with OVS have a worse prognosis than patients who have only COPD or only OSA for several reasons that have important implications for diagnosis, treatment, and outcome.25 Studies that have examined CPAP therapy for OVS have shown that higher levels of CPAP use are associated with better treatment outcomes,23 improved walking capacity,26 and longer survival in patients with COPD who are hypercapnic.27 Individuals with OVS who do not use CPAP therapy have an increased risk of death and more hospitalizations from acute exacerbations of COPD, demonstrating the importance of CPAP therapy in OVS patients.28
Of the approximately 80% of patients who initially accept CPAP therapy, most patients fall into a partial use pattern of only 3 to 5 hours/night.29 Adherence to long-term oxygen use has a parallel story; it is beneficial the more it is used, but adherence is less than optimal,30 ranging from 45% to 70%. CPAP is prescribed to be used whenever asleep. When CPAP is only used for part of the night, the full benefits of therapy are not attained. This evidence highlights the importance of providing the OVS patient population with the information and support necessary to improve adherence to CPAP therapy to maximize therapeutic benefits.
Methods
The O2VERLAP study was a Research Demonstration Project within the National Patient-Centered Outcomes Research Network (PCORnet) to support the patient-powered research networks (PPRNs) in conducting comparative clinical effectiveness research on questions that are important to patients and other key stakeholders. As such, the main scientific focus of the project was informed by pilot work that was done prior to the main study.31
O2VERLAP study aims were to:
- Aim 1: Compare the effectiveness of proactive care (PC)—web-based peer-coaching education and support to reactive care (RC)—education and support based on limited scheduled interactions and patient-initiated contacts on improving adherence to CPAP therapy in OVS patients. The hypothesis was that participants in the proactive care group would have higher CPAP adherence levels compared to those in the reactive care group.
- Aim 2: Compare the effectiveness of the 2 intervention groups on patient-centered outcomes, including daytime functioning, sleep quality, and daytime symptoms. The hypothesis was that the proactive care group would have better patient-centered outcomes compared to reactive care group.
The O2VERLAP study was designed to be national in scope and did not take place within any defined health care system. Primary study offices were located within the COPD Foundation and the University of California San Diego. The study was carried out via a secure web portal hosted by DatStat, Inc (Seattle, Washington). As a PPRN Research Demonstration Project initiative, an overarching goal of the project was to determine how a research study might be best carried out within PCORnet in conjunction with PCORnet partners and collaborators. The study was approved and conducted with oversight by the Western Institutional Review Board, in Puyallup, Washington (IRB# 20173014).
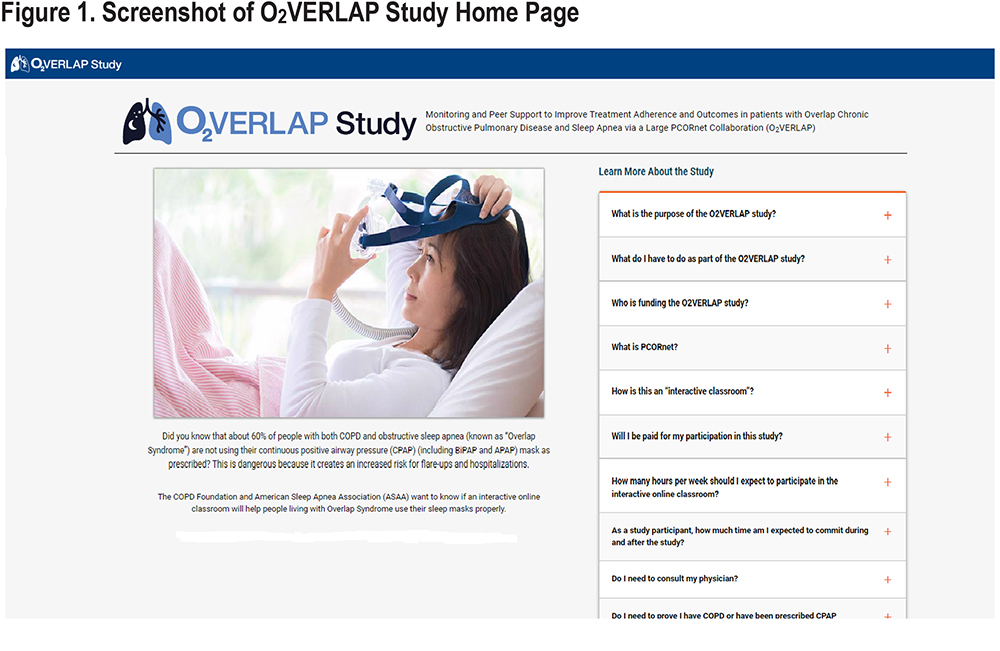
Figure 1 shows the online study home page, which provided information to potential participants about the study, obligations, inclusion criteria, and “frequently asked questions.” Content was carefully designed by the study team for interested potential participants. The study portal also housed digital e-consent and Health Insurance Portability and Accountability Act forms, as well as contact information for both the principal investigator and the project coordinator, so participants could reach out if they had any questions or concerns.
Recruitment
The O2VERLAP study relied almost entirely on electronic recruitment methods, including emails, social media posts, electronic newsletters, website home-page banners, and interactive platforms or forums. Some supplemental nonelectronic methods (i.e., in-person study promotional activities) were also used, including presenting at conferences, exhibiting at health fairs, and via word of mouth.
The DatStat Connect platform was also tied to Google Analytics and user flow was tracked with a Google Analytics Tracking ID. This provided generic information on the types of sites (e.g., social media, Google, direct URL) from which participants were referred. Google Analytics is a product feature; thus, it is tied to all major site functions, such as registration, log-in events, and pagination, but it was not customizable to specific implementations.
Participants
Inclusion and Exclusion Criteria: The inclusion criteria were: age ≥40 years, able to speak and read English, diagnoses of both COPD and OSA, currently using CPAP therapy, and access to the internet and a personal computer, tablet, or smartphone (to complete the online study activities). In addition, the CPAP device needed to have wireless connectivity. Exclusion criteria for the O2VERLAP study included being a non-English speaker and having a life expectancy of ≤6 months.
Onboarding: Signing up for the O2VERLAP study was a 2-step process comprised of: (1) registration and, (2) consent. Because the consent process was done remotely via the study platform, hereafter it is referred to as an “e-consent.” Those who registered but did not sign consent were contacted by study staff via phone and email to confirm they did in fact intend to stop and not continue with the consent process.
After an individual digitally signed the e-consent form, they were prompted to take a first survey which was a self-reported confirmation that they met the study’s eligibility criteria (≥40 years old, diagnosis of both COPD and OSA, and currently using CPAP). If an individual responded to a question in a way that indicated they did not meet the eligibility criteria, the study team was notified by email to schedule a call to review and confirm that the individual was in fact ineligible.
Completion of an e-consent also triggered a notification email to the study coordinator to conduct the first study phone call. The purpose of this first call was to complete the confirmation of eligibility (CoE) survey to verbally confirm study eligibility. The diagnoses of COPD and OSA were assessed based on several steps. First, potential participants completed the self-reported screening questionnaire that included these items: “Have you been diagnosed with chronic obstructive pulmonary disease (COPD)?” and “Have you been diagnosed with obstructive sleep apnea (OSA)?” Then each diagnosis was confirmed during the COE phone call. During this call, the year of the diagnosis was asked along with other relevant information, including who made the diagnosis, what symptoms were experienced, which therapy(ies) were prescribed and how they worked. If there was ever a question of COPD or OSA diagnosis, the obtained information was discussed with the study team and a decision to include or exclude was made.
If the study coordinator confirmed eligibility, the outcome was documented in the study portal through the corresponding CoE survey, and the study coordinator then proceeded to complete additional forms that provided evidence of the participant’s eligibility (CPAP device information, medical history, and demographics). In addition to the portal automatically tracking all participants as they signed up, the study coordinator also kept a study screening log (i.e., a password-protected Excel spreadsheet) that documented all individuals who registered and e-consented and also noted the following scenarios: those who subsequently failed to meet inclusion criteria on the initial self-reported CoE survey, those who subsequently failed to meet inclusion criteria on the CoE phone call with the study coordinator, and all who were eligible for the study and would continue on to the next tasks.
Once eligibility was confirmed, study staff had to contact the participant’s home medical equipment (HME) company to request access to the participant’s CPAP data. This data sharing process involved the HME company adding the O2VERLAP study’s sleep lab to their patient’s integrator and physicians list on the Encore Anywhere (Philips Respironics data platform, Koninklijke Philips N.V.) or AirView (ResMed data platform, ResMed, Inc). This technique allowed the wireless flow of participant CPAP data from the corresponding data platform to the O2VERLAP study portal, which displayed the participants’ nightly CPAP data metrics (hours used, apnea-hypopnea index [AHI], and mask leak) in user-friendly graphs for the coordinator and participant to view.
Interventions
The study design was a comparison of 2 intervention groups: PC and RC. Once a participant’s eligibility was confirmed and connection to their CPAP data was established, they received their introductory phone call from their assigned research study respiratory therapist (RT) (note: not their personal RT). On this introductory call, study RTs would follow a scripted questionnaire to review the participant’s baseline CPAP adherence data and work with the participant to set 3 SMART (Specific, Measurable, Attainable, Relevant, and Timely) goals for improvement that would be reviewed with the PC group at the end of the intervention. Once the introductory RT call was completed, participants would be assigned the next available participant identification number from a preset randomization scheme spreadsheet that was tied to a specific randomized group assignment. This process was carefully handled only by the study coordinator and allocation was tracked in the password-protected Excel randomization scheme spreadsheet. Simple randomization to the 2 groups was accomplished with the use of a random number generator overseen by the study biostatistician.
PC Group: PC was considered the study intervention and included the following:
- Week 1:
– An introductory call from an RT and a COPD Information Line coach who acted as a peer coach on health topics covered in the curriculum.
– Access to module 1 of the online curriculum.
- Weeks 2 to 4:
– Weekly one-on-one peer coaching calls by COPD Information Line coaches.
– Access to modules 2 through 6 of the online educational curriculum, covering topics on COPD, OSA, and OVS.
- Week 5:
– Access to module 7, the final module in the curriculum.
– COPD Information Line coach call and RT follow-up call on completion of module 7.
Participants in the PC group also had access to their CPAP adherence monitoring data on the study portal to track their progress as they advanced through the study program, as well as access to a chat function in the portal to ask questions or contact the study team throughout the intervention period.
RC Group: The RC group of the study was given access to a study RT who made an introductory call during week 1. Participants in the RC group were given the phone number of the COPD Information Line that they could contact to seek advice about any aspect of CPAP therapy or information about general health topics related to OVS. RC group participants also had online access to their CPAP adherence monitoring data and to general informational COPD and OSA materials via the study portal. The primary characteristics of the RC group were that participants had access to the same educational materials as the PC group but were not required to go through them. They were provided with support contact information.
The perspective of the study team in designing the 2 interventional groups was that of a patient advocacy nonprofit organization providing this education and support outside of the health care system in an adjunctive way. From that perspective, the patient advocacy team was being proactive in providing education and support, and any study participants would be reactive if they took the initiative to seek out this information and support.
Online Curriculum: A previous online COPD educational curriculum developed by the COPD Foundation was used as the model for the O2VERLAP educational curriculum, both in terms of content and formatting. Two sleep education specialists affiliated with the American Sleep Apnea Association were chosen by the study team to write the OSA and CPAP content under the supervision of Carl Stepnowsky, PhD, who has developed several OSA- and CPAP-specific curricula for previous CPAP adherence studies. Table 1 provides the module titles and the lessons, divided by chapters, that make up each module. There was a total of 22 chapters, 1 of which was an introduction. Of the 21 topical chapters, 11 were focused on OSA/CPAP, 7 on COPD/oxygen, and 3 on both content areas.
Study Outcomes
The primary study outcome was CPAP adherence, which was objectively measured by the CPAP devices. All participants were assigned a baseline survey package. Based on our focus group findings, consultation with the study’s Stakeholder Advisory Board, and discussions with the study team, the patient-reported study outcomes included: (1) daytime functioning (as measured by the Functional Outcomes of Sleep Questionnaire [FOSQ] and the Patient-Reported Outcomes Measurement Information System[PROMIS] Sleep-Related Impairment scale); (2) sleep quality (as measured by the Pittsburgh Sleep Quality Inventory [PSQI] and the PROMIS Sleep Disturbance tool); and (3) symptoms (as measured by the COPD Assessment Test [CAT], Epworth Sleepiness Scale [ESS], and PSQI Daytime Dysfunction subscale).
CPAP Adherence: CPAP adherence was operationally defined as the number of hours that CPAP was used at the prescribed pressure per day. In addition, the study included 2 measures of CPAP efficacy: mask leak (defined as the amount of air that escaped in liters per minute) and AHI (a measure of the number of apneas and hypopneas per hour of CPAP use). These metrics were available to the participants via the study portal, and the coaches used these metrics to provide feedback on how well CPAP was working to control OSA. For example, if mask leak was moderate to high, suggestions to improve the mask fit were discussed.
Measures of Daytime Functioning:
- FOSQ-10. Qualitative work with the OVS community of patients revealed that the most important outcome to patients is daytime functioning.31 The FOSQ-10 measures impact of sleepiness on activities of daily living and consists of 10 questions on a scale of 1 to 4 (1=extreme difficulty, 4=no difficulty).32,33 Scores range from 5 (maximum difficulty) to 20 (no difficulty). Change in FOSQ total score is calculated from baseline to endpoint, with higher (positive) values representing improvement.
- PROMIS Surveys. The PROMIS initiative of the National Institutes of Health was developed to advance the methodology and application of patient-reported outcomes (PROs) among patients with chronic diseases for use in research and clinical practice.34,35 The study used 2 related PROMIS 8-item sleep scales (sleep-related impairment and sleep disturbance), as well as the following additional PROMIS measures: global health (2-item), physical functioning (4-item), ability to participate in social roles and activities (4-item), anxiety (4-item), depression (4-item), pain interference and intensity (4-item), and cognitive functioning (4-item). All PROMIS measures are scored by: (1) summing the total and, (2) translating the total score to a T-score per PROMIS scoring instructions. A T-score is a standardized score with a mean of 50 and standard deviation of 10. Higher PROMIS scores represent more of the concept being measured.
- PSQI. The PSQI is a 19-item questionnaire used to assess sleep quality and disturbances over the previous ~1 month.36 The PSQI measures 7 areas of sleep: (1) subjective sleep quality, (2) sleep latency, (3) sleep duration, (4) habitual sleep efficiency, (5) sleep disturbances, (6) use of sleep medication, and (7) daytime dysfunction. Items are scored on a Likert scale, with anchors of 0 (better sleep) and 3 (poorer sleep). Individual items are then summed to produce a total PSQI score, which can range from 0 to 21. Higher scores indicate worse sleep quality.
- CAT. The CAT is an 8-item health status instrument for patients with COPD, which is highly practical,37 has good psychometric properties, and has been shown to be responsive to pulmonary rehabilitation and recovery from exacerbation.38-41 CAT scores range from 0 to 40, with higher scores representing a more severe impact of COPD on a patient’s life. The minimally important clinical difference score has been shown to be 2 points.42,43
- ESS. The ESS is an 8-item validated measure of daytime sleepiness and is the most widely used subjective measure of excessive daytime sleepiness in research and clinical settings.44,45 The ESS asks respondents to estimate how likely they are to doze in a variety of different situations, anchored by 0 (would never doze) and 3 (high chance of dozing). Individual items are summed, and the range of ESS scores is 0 to 24, with higher scores indicating a higher level of sleepiness. The ESS can be used to discriminate the sleepiness level of patients with OSA from that of healthy controls.46
Other Measures:
- Demographics. The sociodemographic information included age, sex, race, ethnicity, sexual orientation, and income. Additional participant characteristics included smoking status, geographic location, and years since OSA and COPD diagnoses.
- Functional Comorbidity Index (FCI). FCI is a comorbidity measure with the defining feature of having functional level as the outcome of interest.47 The FCI is composed of a list of 18 medical conditions that study respondents self-report as having or not having. Scoring is a simple summary with higher scores representing higher comorbidity.
- Oxygen Therapy Adherence. Oxygen therapy adherence was assessed by self-report. Several items asked about whether oxygen therapy was administered, as well as type and timing of oxygen therapy.
- Satisfaction. Participant satisfaction was assessed by self-report for each communication with the study staff (coach, RT, other) and by method (phone or online). Participants were presented with the following questions and response options: (1) Who did you have a study communication with? Response options: Information Line coach, RT, or other; (2) Was your communication by phone or portal messaging? Response options: video, phone, or online (chat); and (3) Communication Satisfaction score (based on a 1-10 scale, with higher scores indicating greater satisfaction). The purpose of the last question was to rate the participant’s satisfaction regarding their perceptions of the quality of communications with either an RT or a COPD Information Line coach. For the PC group, the satisfaction survey also appeared after communication via a chat function in the study portal.
Sample Size Calculations and Power
The power analysis was based on the primary hypothesis that CPAP adherence would be improved in the PC group in the first 6 weeks compared with the RC group. A sensitivity analysis was conducted by considering a range of sample sizes from 100 to 180 participants per group. Assuming a 2-sided type I error of α=.05, a standardized effect size (for the difference in CPAP adherence between the PC and RC groups) could be detected ranging from 0.296 to 0.398 with 80% power. These calculations indicate the study would have sufficient power to detect a small to medium standardized effect size (0.325) in adherence between the PC and RC groups when enrolling approximately 150 participants per group.
Data Collection and Sources
Questionnaires/Surveys: The study team collected all questionnaire/survey data electronically or by phone, using a Coordinator- and a Participant-facing portal. Both portals contained questionnaires for all users to complete that would become available according to a previously established time-sensitive workflow, starting with a Self-Report Eligibility questionnaire that was completed by the participant in the Participant portal after signing the e-consent. Completion of the Self-Report Eligibility questionnaire would then trigger subsequent forms for the coordinator to complete in the Coordinator portal (e.g., the CoE questionnaire, demographics, medical history).
Randomized participants then completed 3 main questionnaires that were available to them via the Participant portal. Those time-sensitive questionnaires were baseline, 6-week follow-up, and 12-week follow-up surveys. Each time point had a 2-week window during which the questionnaire was available to participants. Participants were offered an incentive of a $25 online gift card that was emailed to them upon completion of each survey. Reminder phone calls were made, and email reminders were sent to anyone who had a survey due to inform them of the approaching follow-up window closing date. Any surveys that were not completed were considered missing.
CPAP Data: CPAP data were included in the study in 2 ways. First, a data workflow integration was established such that data transfer calls were made twice a week (on Monday and Wednesday) to populate the O2VERLAP study portal. These data were used by both participants and interventionists to monitor progress and intervene as necessary. An intermediary, Corepoint Health (Frisco, Texas), was contracted to provide data integration services using middleware between the CPAP manufacturer servers and the study portal. Second, to ensure a comprehensive and accurate CPAP adherence data set, our research team engaged in an extensive quality assurance effort to verify every CPAP data point in our data set was consistent with the data at the source (the manufacturer’s servers).
Data Analysis
Overarching Approach to Analysis: The primary analysis was intention to treat (including all enrolled participants), and all analyses were performed using 2-sided tests with α=.05. Mean differences and 95% confidence intervals (CIs) were reported with the P values. Summary metrics were reported by mean±standard deviation (SD), unless otherwise specified. Analyses were conducted using R statistical software.48
Analyses for Study Primary Aim: It was hypothesized that CPAP adherence at the 6-week and 12-week time points would be improved in the PC group compared with the RC group. A random-effects model was used to compare the mean CPAP adherence over time between the PC and RC groups to account for the correlation among repeated measures within each participant. Interactions between study group and assessment time (baseline, week 6 and week 12) were included. If the interactions were not significant, we also removed the interactions and fit a model to examine the main effect of study group and assessment time. A multivariable random-effects model was used to assess the difference in CPAP adherence between PC and RC groups, with adjustment for potential covariates. Adjustments were made to correct for baseline imbalances across groups and to adjust for variables known to influence the outcome. Baseline demographics and other clinically important characteristics were also assessed for imbalance among the study groups, using Wilcoxon rank-sum test, Chi-square, or Fisher exact test, and their association with the outcome was assessed using a simple random-effects model. These variables were included as covariates in the multivariable model if found to be moderately associated with the outcome or unbalanced (P<0.15) across groups. All covariates significant at P<0.10 were kept in the final model. Since the results from the multivariable random-effects model with adjustment for baseline covariates were similar as those from the unadjusted analysis (univariable model), we reported the unadjusted results only.
Analyses for Study Secondary Aims: We hypothesized that improvement in patient-centered outcomes at 6 weeks and 12 weeks would be larger in the PC group than in the RC group. The change in patient-centered outcomes from baseline to week 6 and week 12 was compared between the PC and RC groups and was analyzed similarly as the primary outcome. The PRO measurements were divided into 3 categories: daytime functioning, sleep quality, and daytime symptoms. Daytime functioning was deemed the most important PRO per our focus group results. Daytime functioning was measured by the FOSQ, sleep quality by the PSQI, and daytime symptoms were measured by the ESS.
Exploratory Analyses: We conducted additional exploratory analyses that were unanticipated at study outset, examination of CPAP data use levels. CPAP adherence data, measured in duration of use per night, is most meaningful when compared to total sleep time and/or total sleep period (TSP). Because we did not have an objective measure of total sleep time in this study, we opted to use TSP from the PSQI. Descriptive statistics and Pearson correlation coefficients were used to examine the association between COPD severity as measured by the CAT score and mean CPAP adherence.
Results
Participant Flow
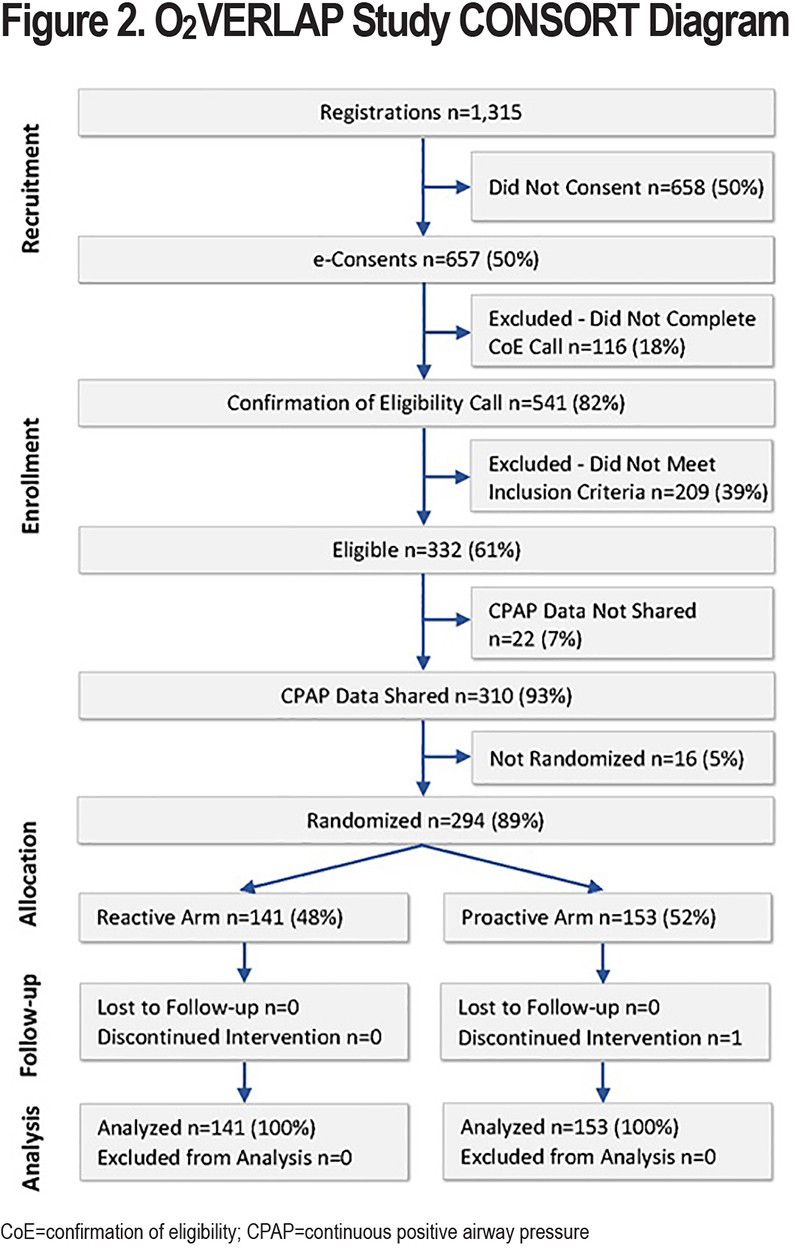
Figure 2 shows the study CONSORT diagram. Study recruitment efforts resulted in the registration of 1315 individuals on the O2VERLAP study home page. Of those who registered, 657 individuals (50%) proceeded to sign consent, whereas 658 did not. Of the 657 who consented, 541 participants (82%) completed the CoE phone call, and 116 (18%) were either not reached or were reached but decided they did not want to proceed with the study.
Study Ineligibility
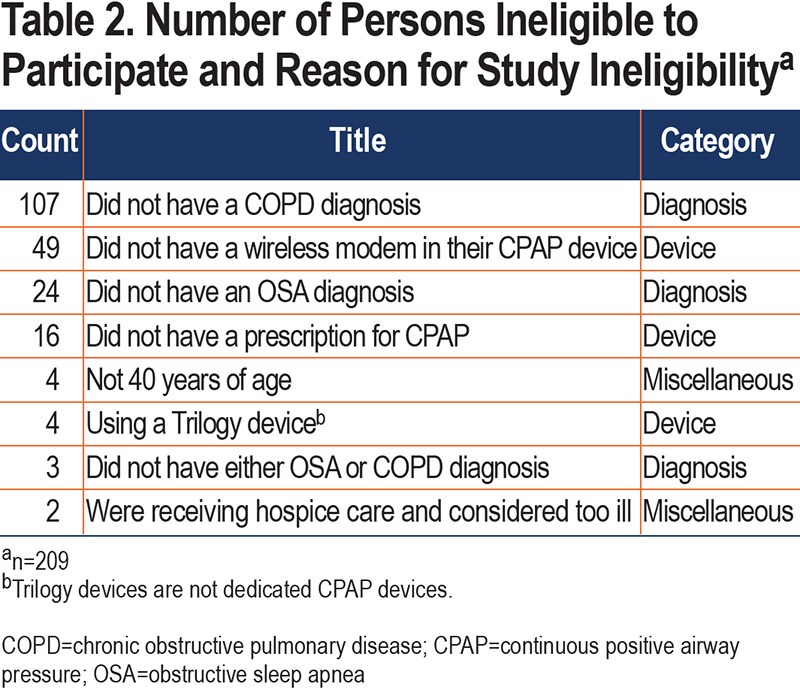
The O2VERLAP study goal was to enroll 330 participants and randomly assign 300 of them based on a 10% pre-randomization attrition rate. The study slightly exceeded its enrollment goal by enrolling a total of 332, which represented 61% of the 541 who had completed the CoE phone call. The remaining 209 (39%) were deemed ineligible for the study. Table 2 provides a breakdown of the reasons for ineligibility and categorizes them by diagnosis (n=134), device (n=69), and other miscellaneous reasons (n=6). The most common reason for ineligibility was “Did not have a COPD diagnosis” because of 2 factors: (1) the number of campaigns directed toward the OSA community; and (2) OSA is more common in people diagnosed with COPD than the reverse.
Continuous Positive Airway Pressure Data Sharing
After participants were considered eligible and enrolled, 1 additional step (obtaining permission for CPAP data sharing) was required before random assignment to an intervention group occurred. Permission to share CPAP data was successfully obtained for 310 (93%) of the 332 enrolled participants. At study outset, data sharing was one of the most significant concerns for the study team. However, at study end, only 22 participants (7%) did not move forward in the CPAP data sharing process. Of those 22, seven were on hold or never provided their device serial number or HME information to make the connection; 6 withdrew during this time; 6 had data transmission issues; 2 were non-responsive; and 1 participant died during this time.
Eligible and Enrolled Participants Who Did Not Move Forward with the Study
Of the 310 participants who successfully reached the CPAP data sharing point of the study workflow, there were an additional 16 participants who did not move forward to the randomization phase for 2 reasons: (1) they either declined to move forward with the study (n=9); or (2) the study team was unable to contact them despite multiple attempts to reach them for randomization (n=7).
Randomization
A total of 294 (89%) of the 332 enrolled participants were randomly assigned to 1 of the 2 intervention groups: 153 to the PC group and 141 to the RC group. In comparing the 38 participants (who signed informed consent but were not randomly assigned) with the 294 participants (who met all study criteria and were randomly assigned), there were no statistically significant differences in any demographic characteristic.
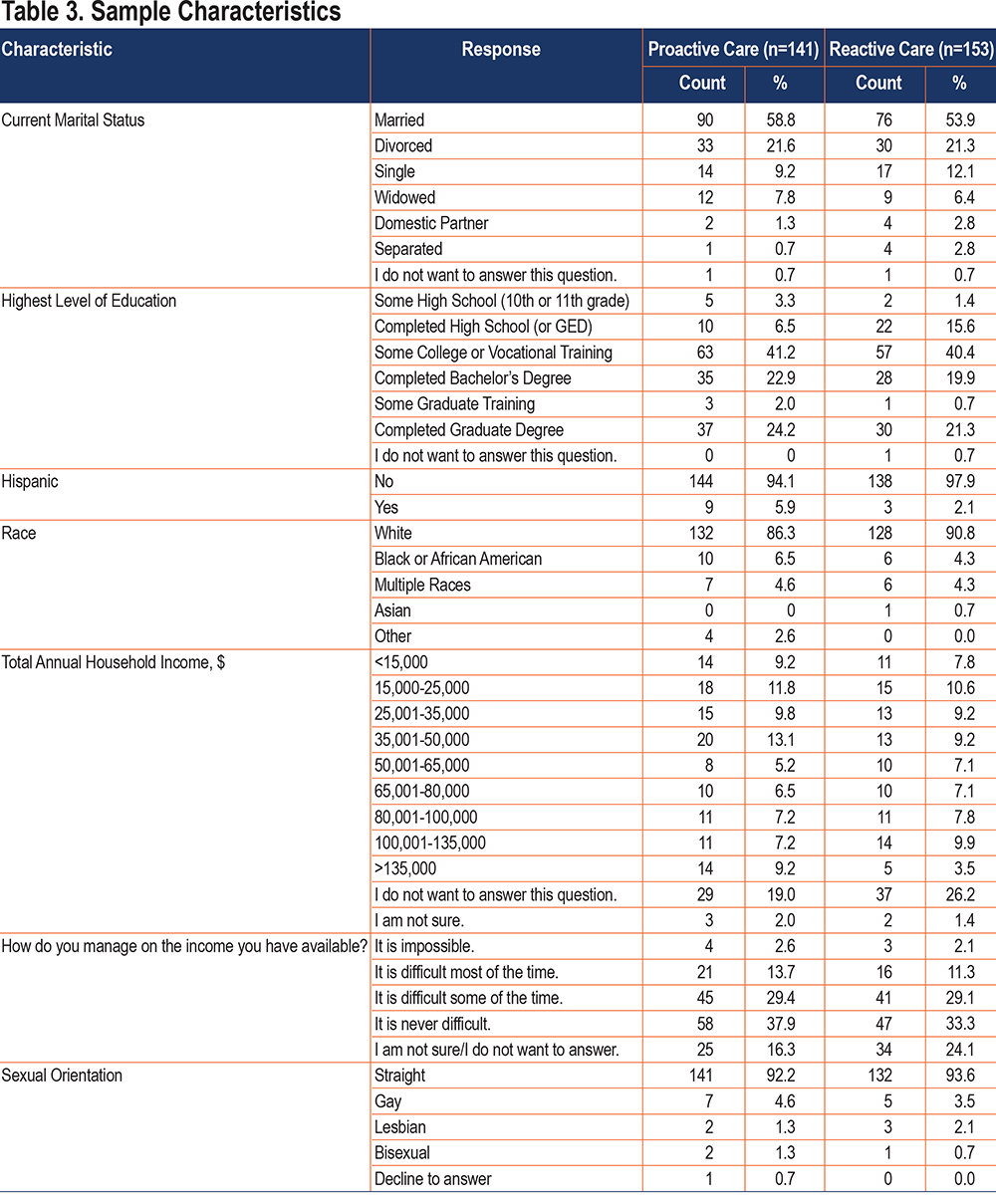
Sample Characteristics
Women and men comprised 47.3% and 52.7% of the randomized sample, respectively. The mean age of the sample was 64.0±9.6 (SD) years, ranging from 41 to 89 years (PC group mean age was 64.8±9.3 (range: 45–86); RC mean age was 64.1±9.8 (range: 41–89) years). For OSA diagnosis, 158 (54%) participants were diagnosed ≥6 years ago; 80 (27%) within the last 2–5 years; and 55 (19%) in the last 2 years. For COPD diagnosis, 170 (58%) were diagnosed ≥6 years ago; 93 (32%) within 2–5 years; and 30 (10%) in the last 2 years. Table 3 shows additional sample characteristics.
Study Outcomes
Primary Aim: The primary aim of the study was to examine the effect of the intervention (PC to RC) on CPAP adherence. Table 4 provides the adherence values by group and assessment time point (baseline, 6 weeks, and 12 weeks). The groups differed at baseline, with the RC group (7.3 hours/night) using CPAP slightly more than the PC group (6.1 hours/night; P<0.001) during the 30 days before study start.
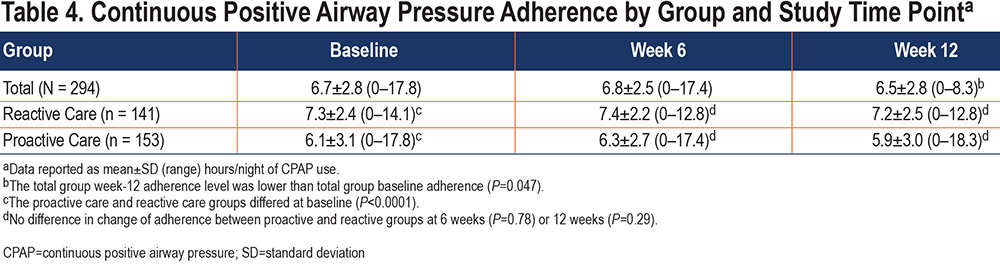
In an unadjusted linear random-effects model, the interaction between time point and intervention group was not statistically significant, which indicated no significant difference in change of CPAP adherence between the 2 study groups in either week 6 (difference: 0.18; 95% CI: −0.16 to 0.52; P=0.29) or week 12 (difference: −0.05; 95% CI: −0.39 to 0.29; P=0.78). Removing the interaction term from the model, we found that overall, the week-12 CPAP adherence level was significantly lower than at baseline (difference: −0.17; 95% CI: −0.34 to −0.002; P=0.047) while controlling for the group, and the PC group had lower CPAP adherence compared with the RC group while controlling for the time point (difference: −1.16; 95% CI: −1.75 to −0.58; P<0.001).
Secondary Aim: The secondary aim of the study was to examine the relationships between group assignment and the following PROs: daytime functioning, sleep quality, and daytime symptoms. Table 5 provides a summary of these measures by group and time point.
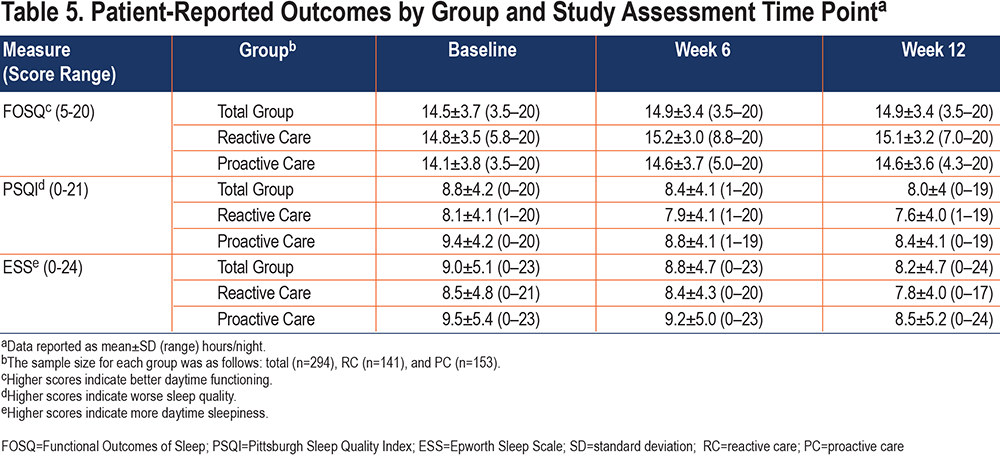
- FOSQ-10. Baseline scores on the FOSQ-10 did not differ between the 2 groups (P=0.16), with a mean score of 14.8 for the RC group and 14.1 for the PC group. The unadjusted linear random-effects model showed no significant difference in change in FOSQ-10 score between the 2 study groups in either week 6 (difference: 0.12; 95% CI: −0.53 to 0.77; P=0.72) or week 12 (difference: 0.16; 95% CI: −0.51 to 0.83; P=0.64). Removing interactions from the model, we found that the week-6 FOSQ-10 score was marginally significantly higher than at baseline (difference: 0.32; 95% CI: −0.01 to 0.64; P=0.06) while controlling for the group, and the PC group had a marginally significantly lower FOSQ-10 score compared with the RC group while controlling for the time point (difference: −0.64; 95% CI: −1.39 to 0.12; P=0.097).
- PSQI. Baseline scores on the PSQI were significantly different between the 2 groups (P=0.01), with a mean score of 8.1 for the RC group and 9.4 for the PC group. The unadjusted linear random-effects model showed no significant difference in change in PSQI score between the 2 study groups in either week 6 (difference: −0.26; 95% CI: −0.93 to 0.42; P=0.46) or week 12 (difference: −0.07; 95% CI: −0.77 to 0.63; P=0.85). Removing interactions from the model, we found that the week 12 PSQI score was significantly lower than at baseline (difference: −0.59; 95% CI: −0.94 to −0.24; P=0.001) while controlling for the group, and the PC group had a significantly higher PSQI score compared with the RC group while controlling for the time point (difference: 1.19; 95% CI: 0.29 to 2.09; P=0.01).
- ESS. Baseline scores on the ESS were not significantly different between the 2 groups (P=0.16), with a mean score of 8.5 for the RC group and 9.5 for the PC group. The unadjusted linear random-effects model showed no significant difference in change in ESS score between the 2 study groups in either week 6 (difference: −0.06; 95% CI: −0.84 to 0.73; P=0.89) or week 12 (difference: −0.15; 95% CI: −0.96 to 0.66; P=0.72). Removing interaction from the model, we found that the week-12 ESS score was significantly lower than at baseline (difference: −0.66; 95% CI: −1.06 to −0.25; P=0.002) while controlling for the group, and the PC group had a marginally significantly higher ESS score compared with the RC group while controlling for the time point (difference: 0.92; 95% CI: −0.14 to 1.98; P=0.09).
- Comorbidities. The mean number of medical conditions using the FCI was 6.4±2.6 (range: 1-15). When the optional write-in medical conditions were included, the mean was 8.4±2.9 (range: 2-17). The top 5 endorsed medical comorbidities in this sample were: visual impairment (e.g., cataracts, glaucoma): 182 (54.8%); obesity (body mass index ≥ 30): 180 (54.2%); arthritis: 177 (53.3%); peripheral vascular disease: 168 (50.6%); and upper gastrointestinal disease: 148 (44.6%).
- Supplemental Oxygen Therapy Use. A total of 46% (n=136) of participants were using oxygen therapy to some degree while 54% (n=158) were not users of oxygen therapy.
- Smoking. A total of 210 participants (71.7%) reported being past smokers; 63 (21.5%) never smoked; 20 (6.8%) were current smokers; and 1 (0.3%) refused to answer. The past smokers reported smoking for 31.4±12.3 (range: 1–60) years and 9.7±5.4 (range: 1–30) packs/week. The current smokers reported smoking for 35.1±12.3 (range: 15–59) years and 5.6±4.5 (1–24) packs/week.
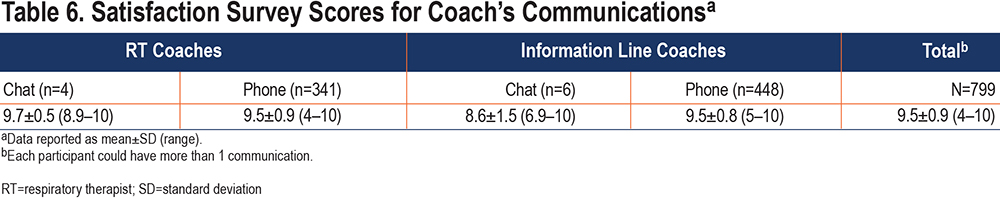
- Satisfaction. Participants in both groups were presented with an automated online satisfaction survey within the study portal after a communication with an RT and/or Information Line coach. Rating their communication was based on a scale 1 (dissatisfied) to 10 (satisfied). The overall satisfaction scores were high across both coach type and method of communication. Table 6 provides a summary of the satisfaction survey results.
Exploratory Analyses
CPAP Use and TSP: CPAP is prescribed for use during sleep period. Patients tend to wear CPAP for some portion of their sleep period and seldom use CPAP for longer than their sleep period. However, per anecdotal reports, some patients may use CPAP during non-sleep periods because they find that it helps with their breathing.
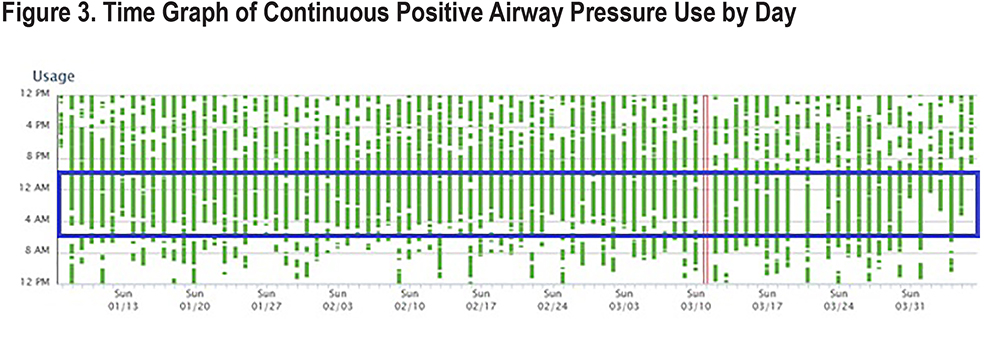
TSP was calculated as uptime minus bedtime, and its units are in hours. The source of uptime and bedtime data was the PSQI. Table 7 provides the TSP by group and time point. Note that the average TSP for the entire group at each time point was quite high at 8.1 hours/night, and that it ranged quite substantially from 2 hours on the low side to 14 hours on the high side.
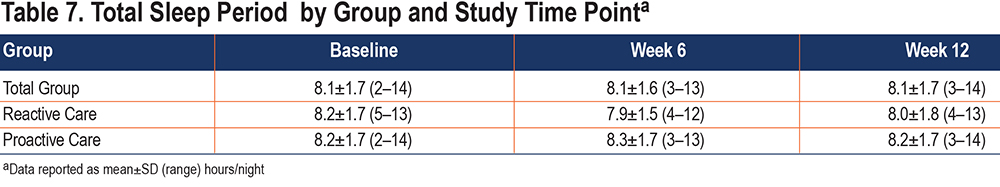
Figure 3 shows a time graph of CPAP use over the course of an approximately 90-day period for 1 non-identified participant to graphically demonstrate when CPAP is used during sleep. The green bars indicate the times when CPAP was used during each 24-hour period. Breaks in the green bar indicate when the CPAP mask was removed. The single red bar on March 11 indicates a day when CPAP was not used. The blue box indicates when a normal, approximately 8-hour TSP typically occurs (i.e., 10PM to 6AM). The green bars outside of the blue box show those times when CPAP was used outside of the normal sleep period. The y-axis represents a 24-hour period (12PM to 12PM), and the x-axis shows the number of days. To be clear, the CPAP data in Figure 3 is from a non-identified study participant and the blue box indicating the sleep period is hypothetical, with the goal to demonstrate when CPAP is used beyond TSP.
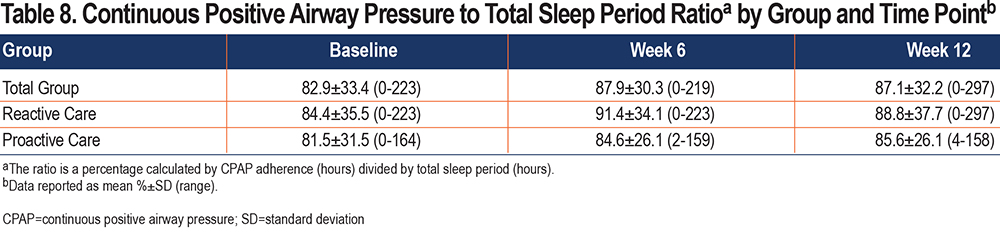
The percentage of CPAP use during TSP was calculated as CPAP use (hours) divided by TSP (hours), which we hereafter refer to as “CPAP/TSP ratio.” A CPAP/TSP ratio of 1.0 means that CPAP use and TSP are equal and 100% CPAP use (e.g., a CPAP user who slept 6 hours used CPAP for the full 6 hours). The mean CPAP/TSP ratio at baseline was 83%±33% (range: 0%–223%) and at 12 weeks was 87%±32% (range: 0%–297%). A total of 30% of the sample had a CPAP/TSP ratio ≥1.0. Table 8 provides the CPAP/TSP ratio by group and time point.
Given anecdotal reports of CPAP use during the day to aid breathing, a relationship between severity of COPD and CPAP use was explored. The study team examined the relationship between COPD impact on a participant’s life as measured by the CAT score and CPAP adherence. For the entire group, there was not a significant relationship between the CAT score and CPAP adherence at the 12-week time point. However, when the subgroup of high CPAP users (defined as CPAP/TSP ratio >1.0) was analyzed separately, the correlation coefficient was 0.250 (P=0.04).
Discussion
In the O2VERLAP study, a difference in CPAP adherence between the 2 intervention groups (PC and RC) was not found at either of the follow-up time points. No differences in secondary outcomes (daytime functioning, sleep quality, and daytime sleepiness) were found between the groups at follow-up. The baseline CPAP adherence level for the entire sample was 6.7 hours/night. While most studies of CPAP adherence are focused on new users, this study was focused on existing users because it is well known that most CPAP users do not use CPAP as prescribed, which is whenever asleep.49 The baseline CPAP adherence level in this study was based on the immediate prior 30 days to study start. The study sample overall were quite experienced users of CPAP therapy, with 55% of the sample having been diagnosed with OSA ≥6 years prior to enrollment and only 19% having been diagnosed <2 years prior to enrollment.
It has been shown in a larger review of the literature (82 studies with sample sizes ranging from 13 to 356) that the overall average CPAP use level is 4.6 hours per night.50 This study’s baseline CPAP adherence level of 6.7 hours/night was approximately 45% higher than the typical level of CPAP use described in the literature. The high baseline adherence rate appears to have resulted in a ceiling effect, meaning that there was little room for improvement of any interventional effort. To our knowledge, this high level of CPAP adherence in patients diagnosed with COPD and OSA is a finding that has not been previously reported in the medical literature. It is, therefore, considered an unexpected and novel finding of this study.
In addition, the significant baseline difference between the 2 groups (RC: 7.3 hours/night; PC: 6.1 hours/night) was also a surprising finding. Most interventional studies show an effect of increasing CPAP use levels by 0.7 to 1.5 hours/night.29 The groups differed at baseline in this study by 1.3 hours/night, which is on the high end of that range. While most CPAP adherence interventional studies are focused on new users, this study focused on existing users. However, it does not appear that this factor alone would account for the observed difference between the groups at baseline. Typically, study randomization helps to ensure that groups are equivalent at baseline. In this study, randomization was carried out as planned.
Two large systematic reviews provide us with information on normative total sleep time values across the age span.48,51 Both are in relative agreement that individuals who are approximately 65 years average approximately 375 minutes (6.25 hours) of sleep per night. The current study used self-reported TSP from the PSQI, based on a simple difference between bedtime and uptime, finding a mean of 486 minutes (8.1 hours). Adding in the mean wake after sleep onset (WASO) of 70 minutes results in an estimated total sleep time of 416 minutes in our sample, which is about 40 minutes higher than normative values. There are few studies in the literature that provide sleep parameters for OVS patients. One study from Japan found that total sleep time in OVS patients (5.8 hours/night) did not differ significantly from healthy participants (6.1 hours/night).52
The study team decided to use TSP as the primary sleep metric to compare CPAP use because patient report of bedtime and uptime are more reliable than the amount of WASO. TSP is by definition greater than or equal to total sleep time and therefore, for this analysis, was considered a more conservative value. Our study found that on average across the entire group, that CPAP was used for 83% of TSP at baseline and 87% of TSP at the 12-week follow-up. The literature shows that the average CPAP user wears CPAP for about 4.6 hours over a 7.5-hour sleep period for a ratio of about 60%. What appears to be driving the high CPAP use in our sample are the approximately 35% of participants who used CPAP outside of their sleep period. This means that approximately 1 out of 3 participants in our study appear to have used CPAP during non-sleep time. While we have heard of anecdotal use of CPAP during non-sleep periods, this study would seem to be one of the first that has found a sizable group who are users of CPAP while awake.
Several reasons may account for the uniquely high CPAP adherence levels found in this study. The mean age group in this study was of retirement age, so the participants likely had more free time during the day to clean, prepare, and use CPAP. It appeared that more use, especially for daytime CPAP users, was associated with worse COPD impact on health status. It may be that other published studies did not have samples with the degree of COPD impact on health status that this sample had. It could also be that the combination of COPD with OSA could produce a more noticeable health improvement from using CPAP. Finally, this study was unique in its national, electronic recruitment method conducted via the COPD and OSA patient communities. It may also be the case that patients who are actively involved in monitoring social media channels and who are willing to respond to research opportunities were in some ways different than those who are not.
In addition to the other limitations of the study, it is acknowledged that the study was designed to be conducted online and remotely, and therefore, the study team did not have the opportunity to conduct medical record reviews or access each participant’s electronic medical record. For these reasons, we had to rely entirely on self-reported health information for this study, including for the diagnoses of COPD and OSA.
The study was designed to help answer the question, “should a clinic or patient advocacy organization be more or less proactive in setting up online and personnel support for their communities?” In the end, because we found patients who were already using CPAP at a high level, the findings of the present study cannot help to answer this contextual question. In fact, because there was a downward trend in the PC group at 12 weeks (after an initial slight increase), it may be that providing structured support to active, consistent users has a slightly negative effect. A recommendation from the study team to a clinic or organization that is considering the decision to staff up or provide minimal resources would be to do the latter and build up only if the demand can be quantified and/or if the outcomes warrant it. This is not to say that support should not be provided, but it is recommended that it be on an as-needed basis based on documentation of poor adherence. Good care for chronic illness is providing the right support at the right time to the right person.53
Acknowledgements
Author contributions: JS, CP, BC, EM, LL, and CS were involved in the study design. SM, JS, CP, BC, EM, and CS were involved in execution of the project. SM, SD, LL, and CS had full access to the data and ensures the accuracy of the data analysis. SM, EM, LL, and CS contributed to the drafting of the manuscript. SM, JS, CP, BC, EM, SD, LL, and CS were involved in the review and editing of the manuscript. All authors approved this version of the manuscript for submission.
The study team would like to acknowledge: (1) the contributions to the project by David Mannino, MD, who was the original study principal investigator and was involved with the study for the first year; (2) the support from Linda Walsh, BS, Brandon Holmes, and the COPD Information line Associates with carrying out the study’s peer interventions; (3) the American Association of Respiratory Care and the respiratory therapists who carried out the coaching portion of the intervention; (4) the help and assistance of the American Sleep Apnea Association for their efforts in helping to recruit participants and develop the intervention; (5) the many additional groups that helped with study promotion including: a PCORI Clinical Data Research Network; (pSCANNER, University of California San Diego); the PCORI Patient-Powered Research Network community (COPD PPRN, PRIDENet, PI Connect, Health eHeart Alliance, and the ABOUT Network); the Alpha-1 Registry; the American College of Chest Physicians; the American Thoracic Society and the American Association of Sleep Technologists.
Declaration of Interest
The authors have no conflicts of interest to report. The authors disclose in-kind contributions from ResMed and Phillips for donating CPAP data integration services to the study.