Running Head: Air Cleaner Adherence Among Adults with COPD
Funding support: The Clinical Trial of Air Cleaners to Improve Indoor Air Quality and COPD Health study (CLEAN AIR) was supported by the National Institute of Environmental Health Sciences (NIEHS) R01ES022607. AF was supported by the National Heart, Lung, and Blood Institute T32HL007534. The funders of the study (NIEHS) had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. Air cleaners used in this trial were provided by Austin Air Cleaners, however, the company did not have any input on study design, analysis, or manuscript preparation. ClinicalTrials.gov Identifier/Registration: NCT02236858.
Date of Acceptance: June 17, 2022 │ Published Online Date: June 22, 2022
Abbreviations: chronic obstructive pulmonary disease, COPD; odds ratio, OR; confidence interval, CI; particulate matter, PM; emergency department, ED; high efficiency particulate air, HEPA; Clinical Trial of Air Cleaners to Improve Indoor Air Quality and COPD Health, CLEAN AIR study; St George’s Respiratory Questionnaire, SGRQ; nitrogen dioxide, NO2; exhaled carbon monoxide, eCO; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; socioeconomic status, SES; Hospital Anxiety and Depression Scale, HADS; quality of life; QoL; modified Medical Research Council, mMRC; 6-minute walk distance, 6MWD; standard deviation, SD; high school, HS; inhaled corticosteroid, ICS; long-acting beta2-agonist, LABA; long-acting muscarinic antagonist, LAMA; air cleaner, AC
Citation: Lorizio W, Woo H, McCormack MC, et al. Patterns and predictors of air cleaner adherence among adults with COPD. Chronic Obstr Pulm Dis. 2022; 9(3): 366-376. doi: http://doi.org/10.15326/jcopdf.2022.0309
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease that causes obstructed airflow from the lungs, and it is typically caused by long-term exposure to irritating gases or particulate matter. COPD creates a significant health care burden, which includes hospitalizations, emergency department (ED) visits, and economic costs.1 The quality of indoor environments where people spend a large part of their life is an essential determinant of healthy life and people's well-being.2
Indoor particulate matter (PM) concentrations have been associated with respiratory morbidity including worse respiratory symptoms, worse quality of life, and increased respiratory exacerbations in individuals with chronic airways obstruction, such as asthma and COPD.3 Further, most individuals, and in particular, the elderly with chronic respiratory conditions, spend the majority of their time indoors.4 Unlike outdoor air, the indoor air environment may be modified at the individual level.5 Portable air cleaner (high efficiency particulate air [HEPA] filters) intervention strategies are practical and easily implemented by individuals at the household level, and may improve respiratory symptoms.6-9
While adherence to medications has been studied and assessing adherence is currently recommended as part of guideline-based care, very few studies have examined adherence to environmental interventions, despite evidence suggesting that environmental interventions may improve respiratory health. The Clinical Trial of Air Cleaners to Improve Indoor Air Quality and COPD Health (CLEAN AIR study) (ClinicalTrials.gov Identifier: NCT02236858) was a 6-month, randomized controlled trial that showed potential health benefits of HEPA and carbon filter air cleaners among former smokers with COPD, which was more pronounced among individuals with higher adherence to air cleaners use.10 Briefly, inintention-to-treat analysis, former smokers with COPD residing in homes with active air cleaners experienced clinically meaningful benefits, including significantly lower rates of moderate exacerbations, fewer respiratory symptoms, and less frequent rescue medication use at 6 months. A priori per protocol analysis, limited to participants who used at least 1 air cleaner greater than 80% of the study period, met the primary endpoint of treatment difference in St George’s Respiratory Questionnaire (SGRQ) and suggested increased treatment response among those adherent to air cleaner use. Thus, understanding factors that are associated with air cleaner adherence are important to maximize benefit and to implement future environmental interventions.
Adherence is a complex concept with which a multitude of factors have been associated. For example, previous studies on medication adherence in asthma and COPD have suggested that individual factors such as age, gender, socioeconomic status, and smoking behaviors have been associated with adherence.11-13 An association between perception of one’s health, psychological well-being (depression and anxiety), and adherence has also been documented.14,15 In addition, worse disease control, including lower daily functioning and frequent and longer hospitalizations, was also a significant predictor of adherence.16,17 On the other hand, there may be factors that are unique to adherence in air cleaner use, as suggested by a recent study of a mostly African-American cohort of children with asthma,18 in which the authors found adherence to air cleaner use to be the lowest during winter, suggesting the role of draft in air cleaners and its impact on air cleaner use during cold temperatures.
Based on these studies and others, we aimed to explore whether characteristics of the individual, COPD disease severity, and the home environment characteristics, in addition to time in study and season, are associated with adherence to air cleaner use in the CLEAN AIR study.
Methods
The CLEAN AIR study was a double-blind randomized controlled trial where participants were randomly assigned to receive (provided by the study) either 2 portable air cleaners (Austin HealthMate HM400) with HEPA and carbon filters for the reduction of PM and nitrogen dioxide (NO2) or 2 sham (placebo) air cleaners without internal HEPA and carbon filters but with similar noise, airflow, and overall appearance compared to active air cleaners. All air cleaners (including placebo) featured 3 different speed settings for filtration (high, medium, or low). Participants were instructed to run the air cleaners continually during the course of the study (6 months). The air cleaners were placed in the bedroom and in the room the participant reported spending most waking time (usually a living room or family room). The intervention lasted 6 months, with outcome assessment at pre-randomization and at 1 week, 3-and 6-months post intervention. Participants received $60 compensation at 3 and 6 months for participation in the study and to cover the costs of running the air cleaner.
The study population was recruited from April 2014 to January 2019 and included former smokers (self-reported and exhaled carbon monoxide [eCO] ≤6ppm),19 age ≥40 years with physician-diagnosed COPD (forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio ≤70%, FEV1 <80 % predicted)20 and a ≥ 10 pack-year cumulative smoking history. During the clinic visit, participants provided demographic data and health-related and COPD/respiratory information. Of the 116 who were eligible and randomized (58 into each treatment group), only 109 participants had valid air cleaner monitoring data and were included in the final sample for adherence analysis. The Johns Hopkins Medical Institutional Review Board approved the study (NA_00085617), and all participants provided written informed consent before beginning the study.
Adherence to Air Cleaner Use
To provide an objective record of adherence and total time of air cleaner use, current sensors (electronic adherence monitors) with data logging capabilities (Split-core AC current sensor model CTV-A connected to HOBO analog data logger model UX120-006M) were used. Air cleaner usage was monitored continuously and reported as the percentage of time that each air cleaner was turned on during each 24-hour period. In our analysis, “air cleaner adherence” was defined a priori as turning on either one of the air cleaners for more than 80% of the time during the full course of intervention (6 months), in either of the 2 locations at any filtration speed setting, as used in the per-protocol analysis of the primary study.
Potential Predictors of Adherence
Baseline predictors of adherence (Figure 1) considered to potentially be related to air cleaner use included: (1) Individual factors: demographics (age, gender and race), socioeconomic status (SES) measured by individual household income and education, psychological well-being measured by anxiety and depressive symptoms as measured by the Hospital Anxiety and Depression Scale (HADS),21 and smoking history, including time since quitting smoking, time living with a smoker, and pack years; (2) COPD disease severity and; (3) housing characteristics.
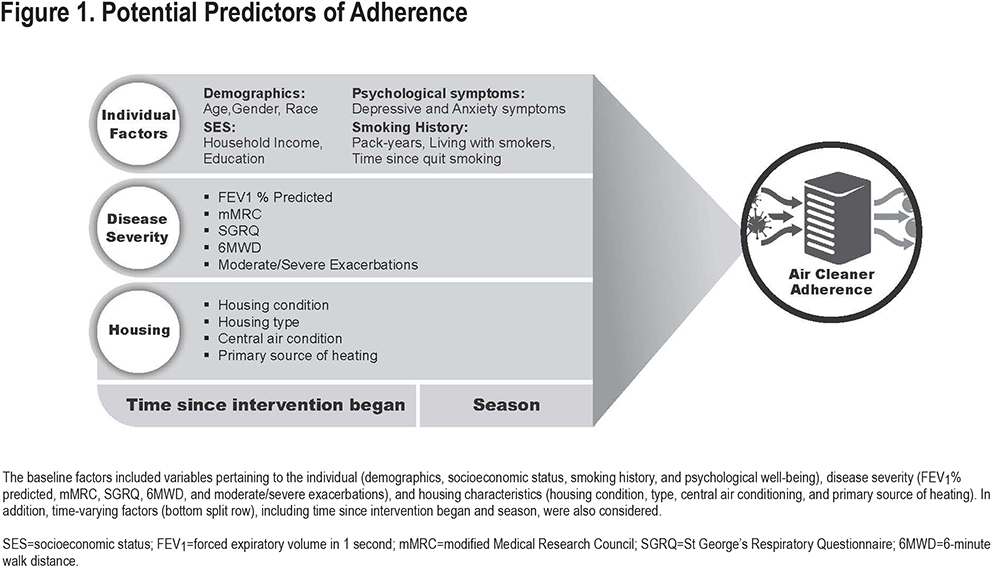
Baseline COPD disease severity was assessed by patient-reported outcomes, exacerbation history, and lung function. Respiratory-specific quality of life (QoL) was measured using the SGRQ;22 respiratory status was assessed by the modified Medical Research Council Dyspnea Scale (mMRC). Information on retrospective 12-month moderate and severe exacerbations was collected and defined as those requiring use of either systemic steroids and/or antibiotics or an urgent health care visit for a moderate exacerbation or those requiring an ED visit or hospitalization for a severe exacerbation. Functional capacity was determined by the 6-minute walk distance (6MWD).23 Baseline pulmonary function was measured as FEV1% 24 according to American Thoracic Society guidelines.25
Baseline housing characteristics, including housing type (detached, duplex, semi-detached, row, townhouse, or apartment), presence of air conditioner, type of heating, and general condition of home were determined by a detailed visual home assessment and conducted by a trained home inspector.
In addition to the baseline factors, 2 time-varying factors were considered: time since intervention began and season, which were each measured at monthly intervals for the 6-month intervention period.
Statistical Analysis
Descriptive analysis was run to assess sample characteristics at baseline. To assess cross-sectional bivariate associations between adherence (turning on any of the air cleaners for more than 80% of the time) and its potential predictors, t-tests or Chi-squared tests were run comparing the candidate predictors at baseline between those who adhered to air cleaner use and those who did not. In cross-sectional adjusted analysis, stepwise logistic regression (backward elimination with threshold p=0.1) of adherence was performed among those predictors that suggested bivariate correlation with adherence in the previous step (p<0.2). Additionally, to assess whether participants’ adherence diminished over time or was influenced by season, a longitudinal fixed effect analysis was performed, regressing the within-person change in adherence against change in time and season. For the latter, adherence was modeled continuously, with its within-person standard deviation (SD) and intraclass correlation assessed. The regression analysis was performed with time and season included as mutual covariates. Statistical significance was defined as 2-sided P-values less than 0.05. Analyses were performed with Stata statistical software, version 15.1 (Stata Corp, College Station, Texas).
Results
Participant Characteristics
Of the 109 participants who were randomized and had valid adherence data, 83 (76.1%) were adherent—or turned on at least 1 air cleaner for more than 80% of the time they were in the study (Table 1). On average, participants turned on at least 1 air cleaner 86.7% (SD=23.2) of the time, with half of the sample turning it on more than 98.9% of the time. Looking at air cleaner use by location, 69% and 70% of the participants had the air cleaner in the bedroom and the room they spent the most waking time in, respectively, turned on for more than 80% of the time. A little less than two-thirds of the participants (62%) turned on air cleaners in both locations more than 80% of the time.
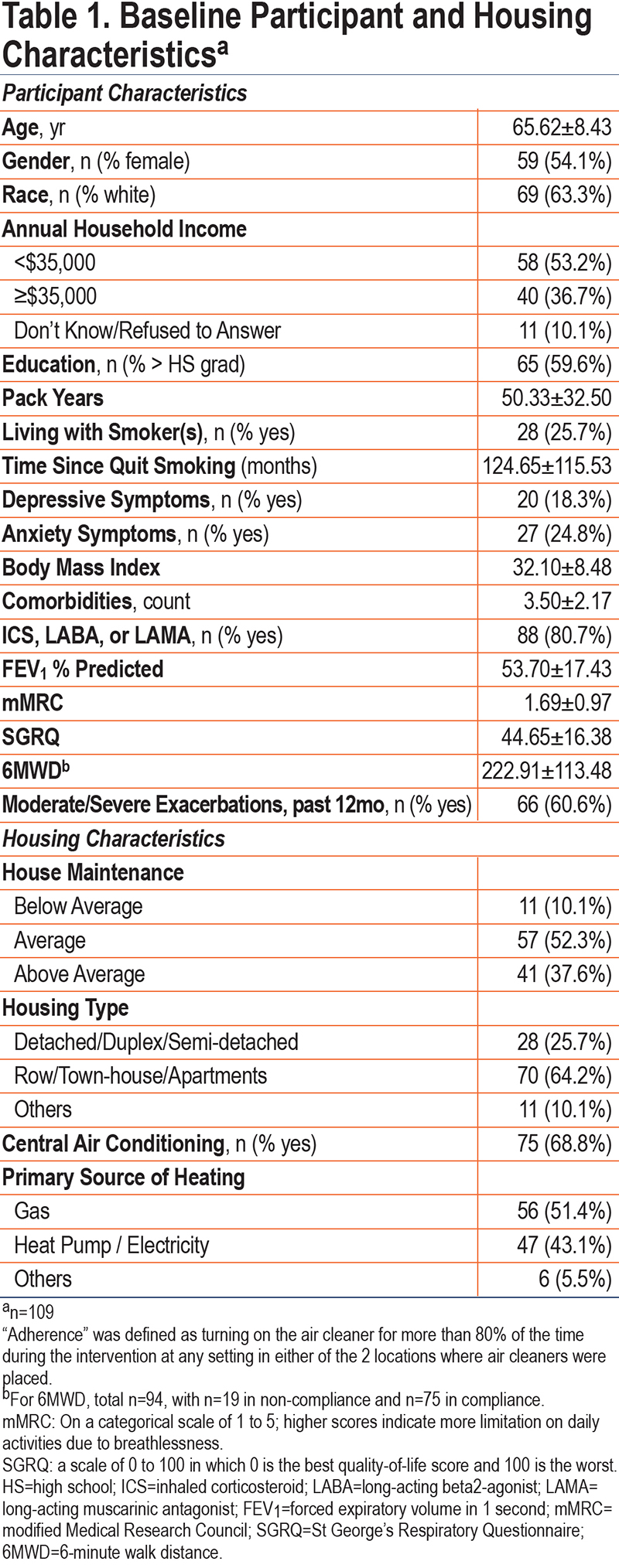
Participants’ mean age was 65.7 years (SD=8.4), about half females (54%), and 63% Whites. Less than half of the sample (40%) had a high school or less education and about half (53%) reported an annual household income below $35,000. Participants were recruited evenly across seasons. Participants’ mean (SD) smoking pack years was 50.3 (32.5), and most participants (81%) were on some respiratory medication and had 3 or more comorbidities (mean [SD] 3.5[2.2]), with a mean (SD) FEV1 % predicted of 53.7 (17.4) at baseline. A total of 61% of the participants reported having moderate and severe exacerbations in the 12 months prior to the beginning of the study. Participants were distributed evenly (51%) across active (n=56) and placebo (n=53) groups.
Bivariate Associations
In the unadjusted analysis (Table 2), participants who adhered to air cleaner use reported better QoL scores (SGRQ: 42.1 versus 52.9, p=0.003) and a lower rate of exacerbations (moderate and severe exacerbations in the prior 12 months: 55% versus 77%, p=0.05) than did participants who did not adhere at baseline. Income was also associated with adherence (p=0.02), with the adherent participants (versus non-adherent participants) more likely to report an annual household income above $35,000 (43% versus 15%).
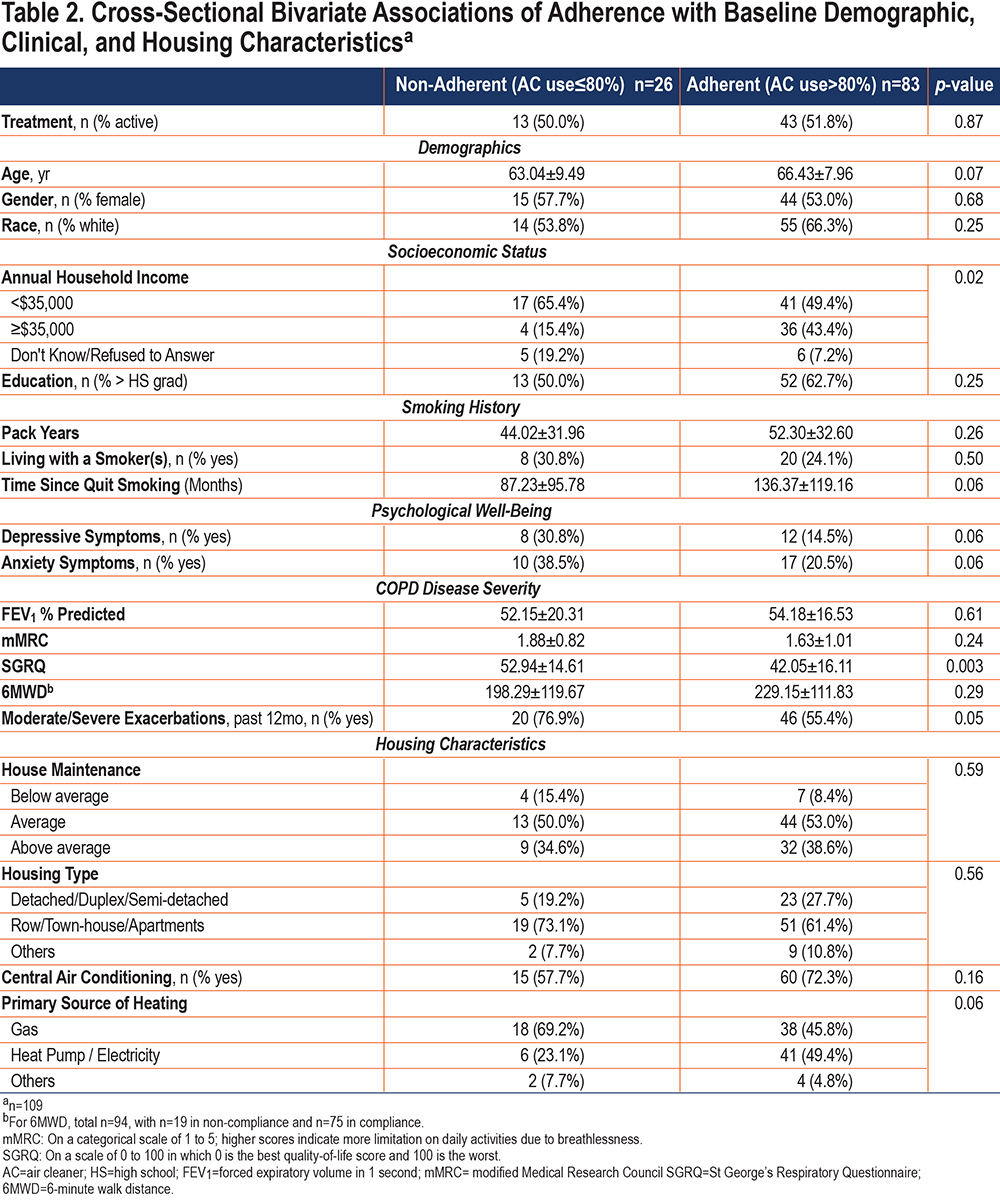
Several other factors showed directional trend with adherence (p<0.2), though not reaching statistical significance. The participants who adhered, in comparison to those who did not, tended to be older, be leaner, report fewer anxiety and depressive symptoms at baseline, have abstained from smoking longer, have central air conditioning in their home, and be more likely to have heat pump/electricity than gas as the primary source of heating in home (Table 2).
Treatment assignment was not associated with adherence, with similar percentage of adherent participants between active and placebo groups (76.8% versus 75.5%, p=0.9). Gender, race, smoking pack years, and individuals living with a smoker, as well as lung function, dyspnea, and 6-minute walk distance did not significantly differ between the adherent and non-adherent participants. Similarly, housing type (e.g., detached, apartment, etc.,) and the level of house maintenance did not differ across adherence (Table 2).
Multivariable Associations
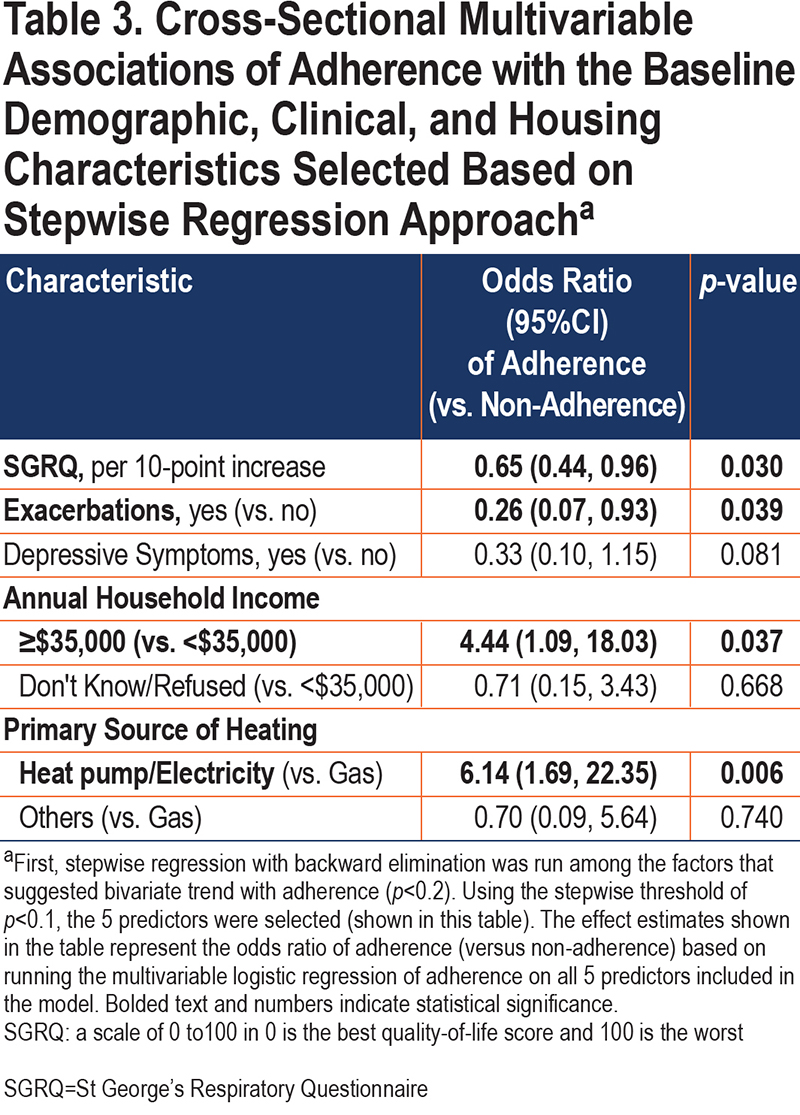
Stepwise regression identified 5 variables that independently predict adherence. Higher adherence was associated with an annual household income above $35,000 (versus below $35,000) (OR=4.4, 95%CI: 1.1–18.0), lower baseline SGRQ (OR=0.65 per 10-point increase in SGRQ, 95%CI: 0.44–0.96), reporting no exacerbations in the 12 months prior to baseline (versus 1 or more exacerbations) (OR=0.26, 95%CI: 0.1–0.9), lack of depressive symptoms (versus some depressive symptoms) (OR=0.33, 95%CI: 0.1–1.2), and using heat pump or electricity (versus gas) as the primary source of heating in the home (OR=6.1, 95%CI: 1.7–22.4) (Table 3).
Longitudinal Associations with Time and Season
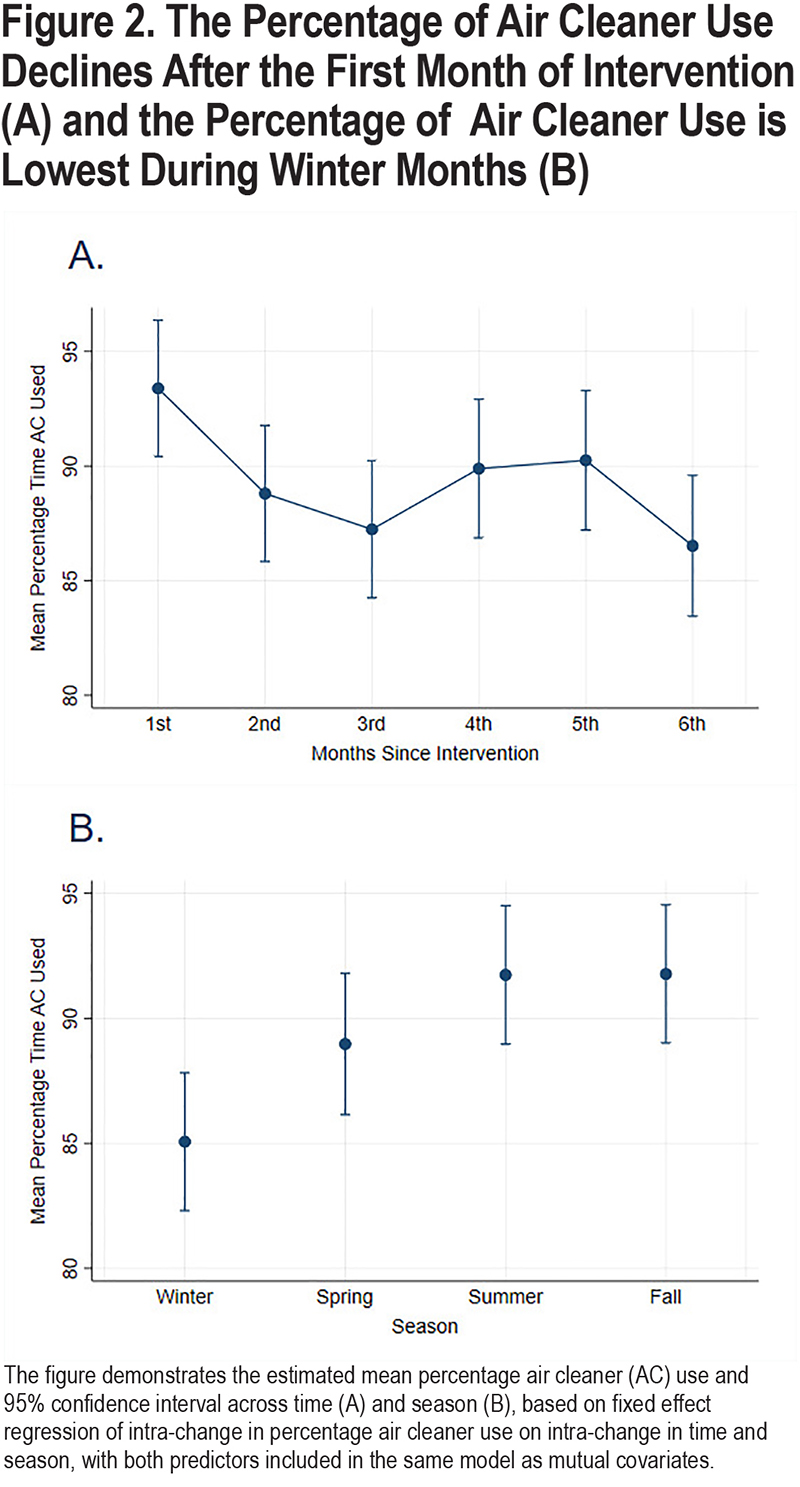
There was a weak/moderate variability in air cleaner use over time within participants, with intraclass correlation of 0.61 and within-person SD of 16 for the continuous percentage use of air cleaners. Participants also experienced change in season during the course of the study. In the longitudinal analysis, change in time and season were each independently associated with change in adherence. (Figures 2A and 2B) For example, adjusting for season, air cleaner usage significantly declined after the first month for each participant, with statistically significant lower percentage use in the 2nd, 3rd, and 6th months in comparison to the first month (all p<0.05); the percentage decline ranged from -4.6% to -6.9%. Beyond the first month, there was no statistically significant reduction over time (Figure 2A). Adjusting for time trend, season was associated with adherence, with participants turning on air cleaners the least during winter than in any other season (Figure 2B). For example, participants turned on air cleaners 7% more during summer and fall in comparison to winter (βsummer=6.67, 95%CI: 2.3–11.0, βfall=6.71, 95%CI: 2.8–10.6).
Discussion
In this air cleaner intervention trial of participants with moderate to severe COPD, using a novel method of monitoring and collecting continuous adherence data over 6 months, we found that more than three-quarters of participants were adherent to the air cleaner intervention. However, non-adherence was independently associated with lower income, cold weather season, and use of gas for home heating. Poor quality of life and worse disease control were also associated with decreased adherence. These factors should be considered when designing future environmental clinical trials and larger-scale implementation studies.
This is similar to a study of children with asthma18 where investigators instructed participants to use an air cleaner on high or turbo settings continuously during the 12-week study. This study reported that on average participants turned on/used the air cleaner approximately 80% of the time, and participants demonstrated adherence to high or turbo settings for 60% of the time. These findings, along with the results of our study, where approximately three-quarters were adherent—or turned on any air cleaner for more than 80% of the time they were in the study, and on average, participants had an air cleaner turned on 86.7% of the time, with half of the sample turning it on more than 98.9% of the time—suggest that participants will use air cleaner devices for the majority of the time.
It is not surprising that adherence was greatest during the first month of the study, with a lower percentage use, but still a > 85% use time, in subsequent months; and it is reassuring that the adherence remained similar throughout the 6-month study duration. Air cleaner use was similar between the active and sham (placebo) arms of the study suggesting appropriate blinding. Air cleaner adherence was lower in the winter months, compared with summer or fall. This is similar to results of Kaviany et al.18 It was postulated that the lower adherence during winter months is due to the cold draft potentially generated by the equipment. Though it is unclear why participants living in homes with gas heating had lower adherence compared to homes with electric heating, we suspect that differences in cost or home heating efficiency between homes with electric or gas heating may play a role. Indeed, participants with lower annual household income were similarly less likely to be adherent to air cleaner use, suggesting that cost of air cleaner use or heating during winter seasons may be important considerations to take into account when addressing strategies to improve adherence.
The air cleaners were provided by the study and the participant received compensation at 3 and 6 months for participation in the study to cover the costs of running the air cleaner. Thus, it is possible that in a real-life setting the impact of cost on air cleaner adherence may be even greater including the need to replace the air cleaner filters. It is important to note that the cost of a commercial air cleaner is between $100 to $800 and the electricity to run the air cleaners is estimated to be 17.1 kWh – 646 kWh (dependent on model and setting used). Extrapolating to 30 days with the current average national residential electricity rates, 2 air cleaners per home would cost between $9 to $30 of electricity per month for continuous use, which is generally lower than the cost of medications for the treatment of COPD. Similarly, economic difficulties have also been implicated in affecting adherence to medications.13,17
Participants with worse respiratory-specific QoL and/or a history of exacerbations were also less likely to be adherent to air cleaner use. This is similar to findings in asthma where Kaviany et al reported that poor asthma control was associated with poor air cleaner adherence.18 It has also been shown that poor quality of life and disease control are associated with poor medication adherence not only in asthma26,27 but also in COPD.28,29 This may be because those who are less likely to be adherent to the air cleaner are less adherent to other medications or disease management strategies linked to poor disease control. It is also possible that those with worse disease control face other challenges which hinder better adherence. In particular, depression was associated with poor air cleaner adherence in bivariate analyses with continued trends in multivariate models. Depression has been associated with low medication adherence in COPD as well as other chronic diseases.30,31
This study has several limitations, including inherent limitations of a clinical trial setting, where the responsibility of the initiation and continuation of the intervention was not placed on the participant. The air cleaners were provided by the study and participants received reimbursement for participation in the study. Therefore, these findings of high air cleaner adherence were in the context of a clinical trial and may not represent real world settings. There may also be lack of generalizability due to geographic location and study trial completed at a single city site/center. Additionally, other barriers to adherence such as draft, noise, or reported trouble with the air cleaners, were not assessed. Further, “air cleaner adherence” was defined a priori as turning on either one of the air cleaners for more than 80% of the time during the 6-month study period as used in the per-protocol analysis of the primary study.10 Though there is no accepted threshold of adherence in environmental studies, this cut-off has been used in other air cleaner studies 32 and was shown to be associated with improved treatment effect in per protocol analysis of the primary study.10
In conclusion, results from the HEPA air cleaner intervention study suggest that in-home air cleaner use is a potential therapeutic strategy to improve COPD outcomes and that adherence is critical to achieve maximal benefit.10 Our findings suggest that adherence, as measured by novel method of monitoring electronic current within air cleaners, is well maintained over a 6-month period. Winter season, annual household income, and home heating characteristics may impact adherence. These results suggest that coverage of air cleaners by third-party payers may help address financial burden in low-income populations, and additional guidance during cold seasons may help improve adherence. Further, adherence may be lower in patients with worse quality of life and disease control, therefore, patients most likely to benefit from an air cleaner intervention may need the most support to address barriers to air cleaner adherence. For future studies, it would be valuable to consider incorporation of qualitative assessments to further evaluate potential etiologies for lower adherence and factors associated with air cleaner adherence should be taken into consideration when developing multimodal implementation strategies or designing future studies in an effort to reduce respiratory morbidity.
Acknowledgements
Authors Contributions: NNH contributed to the conception and design of the study. NNH, WL, and HW drafted the manuscript with input from all coauthors. HW analyzed the data. HW, WL, and CL contributed to the data cleaning. NNH, WL, HW, MW, and TG contributed to the acquisition of the data. KK, MW, and TG managed the environmental assessment team, air cleaner deployment, and compliance data collection and NNH, WL, and HW contributed to the data interpretation. All authors discussed the results and reviewed, edited, and approved the final manuscript for submission and publication. All authors had full access to the study data and accept responsibility to submit for publication. The corresponding author had final responsibility for the decision to submit for publication.
The authors thank the CLEAN AIR trial participants and staff for making this research possible. More information about the CLEAN AIR trial is available at ClinicalTrials.gov NCT02236858.
Data Sharing: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, and figures), beginning 9 months and ending 5 years following article publication will be available to researchers who provide a methodologically sound proposal. Study protocol and statistical analysis plan will also be available upon request. Proposals should be directed to nhansel@jhmi.edu.To gain access, data requestors will need to sign a data access agreement.
Declaration of Interest
NNH reports grants from the National Institutes of Health (NIH) , grants from the COPD Foundation, grants and personal fees from AstraZeneca, grants and personal fees from GlaxoSmithKline, grants from Boehringer Ingelheim, and personal fees from Mylan during the conduct of the study. NP reports a grant from the NIH K award, during the conduct of the study. MCM reports grants from the NIH and the Environmental Protection Agency during the conduct of the study, and personal fees from Aridis, GlaxoSmithKline, and Celgene outside the submitted work. KK reports grants from the NIH during the conduct of the study. WL, HW, PK, CL, MW, TG, DB, AF, SC, and MNE have nothing to disclose.