Running Head: Elastin Degradation with Secondhand Smoke Exposure
Funding Support: This work was supported by: the Flight Attendant Medical Research Institute (FAMRI) (012500WG and CIA190001 to MA; CIA150034 to GMT; FAMRI Center of Excellence Award#012500 to RR); the California Tobacco-Related Disease Research Program (TRDRP) (T29IR0715 to MA); the Department of Veterans Affairs (CXV-00125 to MA); a National Library of Medicine Training Grant (NIH: T15LM007442 to SZ); Postdoctoral Training in Tobacco Control Research (NIH/NCI: T32 CA113710 to JMR); the Greenwall Foundation and Arnold Ventures (to RR); the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) (to RR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The statements and conclusions in this publication are those of the authors and not necessarily those of the funding agency. The mention of commercial products, their source, or their use in connection with the material reported herein is not to be construed as an actual or implied endorsement of such products.
Date of Acceptance: June 8, 2022 │ Published Online Date: June 13, 2022
Abbreviations: secondhand smoke, SHS; elastin degradation markers, EDM; parameter estimate, PE; confidence interval, CI; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; forced expiratory flow rate between 25% and 75%, FEF25%-75%; chronic obstructive pulmonary disease, COPD; dsemosine and isodesmosine, DI; Multicenter Ozone Study of oldER Subjects, MOSES; Flight Attendant Medical Research Institute, FAMRI; University of California San Francisco, UCSF; University of Rochester Medical Center, URMC; University of North Carolina, UNC; cellulose fiber, CF; pulmonary function test, PFTs; diffusing capacity, DCO; functional residual capacity, FRC; Global Lung Initiative, GLI; Global initiative for chronic Obstructive Lung Disease, GOLD; standard deviation, SD; body mass index, BMI; lower limit of normal, LLN
Citation: MustraRakic J, Zeng S, Rohdin-Bibby L, et al. Elastin degradation and lung function deterioration with remote secondhand tobacco smoke exposure in never-smokers. Chronic Obstr Pulm Dis. 2022; 9(3): 377-393. doi: http://doi.org/10.15326/jcopdf.2022.0289
Online Supplemental Material: Read Online Supplemental Material (425KB)
Background
Secondhand tobacco smoke (SHS), a heterogenous mixture of the side stream smoke from the burning end of the cigarette and the smoke exhaled by the smoker, is 4 to 12 times more toxic than the mainstream smoke inhaled by the smoker.1,2 Over the past several decades, a large body of scientific evidence has implicated long-term exposure to SHS as a risk factor for pulmonary diseases including chronic obstructive lung disease (COPD) in both smokers and never-smokers.3-6 Remarkably, even remote exposure to SHS has been shown to be associated with respiratory symptoms and lung function abnormalities consistent with obstructive lung disease.3,7-11
Desmosine and isodesmosine (DI) are 2 cross-linked pyridinoline amino acids specific to peptides that are generated from degradation of elastin, a key extracellular protein that provides resilience and elasticity to tissues and is primarily located in lungs, aorta, and skin.12 Lung elastin is a recognized target for injury in COPD, and systemic levels of DI, which are specific for elastin degradation, are elevated in COPD, as well as in those without COPD but with acute exposure to tobacco smoke, direct or secondhand.13-15 However, it is unclear whether past remote exposure to SHS in those without a diagnosis of COPD is also associated with continued lung injury, elastin degradation, and elevated levels of DI.
In this study, we aimed to investigate the relationship between remote prolonged exposure to SHS and molecular markers of elastin degradation (plasma DI) in never-smokers without a diagnosis of COPD, and how those levels are associated with lung function as a way to speculate about the source of elastin degradation products. Our research questions were whether plasma DI levels, which have been shown to increase with acute exposure to SHS,13 continue to be elevated years after the exposure has ceased, and whether plasma DI levels are associated with lower lung function. We hypothesized that there is a positive association between past SHS exposure and plasma DI, and that the level of plasma DI is inversely associated with lung function. Presence of such associations would then suggest that past exposure to SHS could result in ongoing lung injury and elastin degradation, contributing to obstructive lung disease.
Study Design and Methods
Study Design
To examine our hypothesis above, we took advantage of a “natural experiment” and examined the plasma levels of DI and lung function from a cohort of healthy flight attendants who worked for U. S. airlines before the smoking ban enactment, and had no known history of lung diseases, including no known COPD. These flight attendants were exposed to relatively heavy cabin occupational SHS during their employment for many years and for long periods of time each day.3 This set up allowed for a robust objective quantification of cabin SHS exposure using employment history and the years on which different airlines implemented the smoking bans on domestic and international flights, between 1988 to 1989 for U.S. domestic flights, and between 1994 to 1997 for international flights depending on the airline, respectively.3 Flight attendants who began working after the smoking ban enactment were also recruited as an “unexposed” reference group. Furthermore, blood samples and lung function data from the baseline visit of previous participants in the Multicenter Ozone Study of oldEr Subjects (MOSES) were also used as an additional “unexposed” non-flight attendant reference group.16
Study Population
Between June 2014 and October 2019, 241 flight attendants were enrolled as part of an ongoing clinical investigation of the health effects of exposure to cabin SHS in the Flight Attendant Medical Research Institute (FAMRI) Center of Excellence at the University of California San Francisco (UCSF). Participants with overt cardiopulmonary disease were not recruited into the study. Flight attendants were considered to be ever-smokers and excluded from the study if they had smoked more than 100 cigarettes in their lifetime. Accordingly, 32 of those flight attendants who were ever-smokers were excluded from the study. From the 209 remaining participants, 193 had begun their airline employment before the smoking ban enactment and had worked in a smoky cabin and were considered “exposed” and 16 were “unexposed.” All participants gave written informed consent, and the study was approved by the UCSF Institutional Review Board.
The MOSES cohort has been described previously.16 Briefly, between 2012 and 2015, 87 healthy nonsmoker adults between the ages of 55 to 70 years old were recruited to participate in a clinical trial investigating the cardiopulmonary health effects of exposure to ambient levels of ozone in controlled exposure experiments at 3 centers across the United States. The study consisted of an initial screening visit to determine the eligibility of participants during which blood samples and lung function measurements were collected and incorporated into a biorepository. Participants with clinically significant cardiopulmonary disease were excluded from the study. The data (including the lung function measurements) and blood samples collected at baseline visit, prior to any exposure, from MOSES participants were used in this study to provide a reference “unexposed” group.
All MOSES participants gave written informed consent approved by the respective centers’ institutional review boards (the University of Rochester Medical Center [URMC], University of North Carolina [UNC], and UCSF).
Measurement of Cabin Secondhand Smoke Exposure
SHS exposure was characterized by a questionnaire developed by the UCSF FAMRI Center of Excellence,17 and modified to acquire information on airline-related occupational history, as described previously.3,10 Briefly, this included employer airline, duration of employment, and flight routes with quantification of “cabin SHS exposure” as the number of years during which the crewmembers were exposed to SHS in aircraft. As previously described, the smoking ban was put in effect between 1988 and 1989 for U.S. domestic flights, and between 1994 and 1997 for international flights depending on the airline.3 The duration of airline employment prior to those dates was used as the period during which the flight crew was exposed to cabin SHS. Other possible sources of SHS exposure were also explored by questioning participants about their non-cabin exposures in additional settings, as described previously.6 Consideration for cabin section was not made.
Plasma Collection and Measurements of Plasma Desmosine and Isodesmosine Levels
A non-fasting blood draw was obtained during the same visit as when the lung function measurements were performed. The blood samples were collected on ice and centrifuged at 4°C and 1200 x g for 10 minutes. Plasma was transferred in a new tube and stored at -80°C for further analyses.
Measurement of DI was done as previously described.18 Briefly, plasma samples were acid- hydrolyzed in concentrated hydrochloric acid at 100°C–110°C for 24 hours and were then applied to a cellulose fiber (CF1 or CF11) cartridge to purify. A synthetic desmosine-d4 served as the internal standard for processing plasma samples and measuring DI. High-performance liquid chromatography and tandem mass spectrometry methods were used for measuring DI levels.
Pulmonary Function Testing
Lung function testing for the flight attendants was performed between June 2014 and October 2019. Full pulmonary function testing was done for 82 of the eligible participating 209 flight attendants. The remaining 127 participating flight attendants underwent spirometry without plethysmography or diffusing capacity (DCO) measurement.
Full pulmonary function tests (PFTs) (N=82) were performed in the seated position using a model Vmax 229 CareFusion (CareFusion Corp., Yorba Linda, California) and nSpire body plethysmograph (nSpire Health Inc., Longmont, Colorado). This included measurement of the flow-volume curve and spirometry;19 lung volume by single breath dilution;20,21 and plethysmography;22 airway resistance during panting at functional residual capacity (FRC);23,24 and single breath carbon monoxide diffusing capacity.25 Spirometry without plethysmography or diffusing capacity measurement for the 127 flight attendants was done using a portable spirometer (EasyOne, NDD Medical Technologies) in the seated position. MOSES lung function testing procedures were performed between 2012 and 2015, and have been described previously.16 Briefly, spirometry was performed in a seated position: URMC used a KoKo PFT Spirometer (nSpire Health, Longmont, Colorado); UNC used VIASYS 10.2-L model 1022 (SensorMedics; Palm Springs, California); and UCSF employed an S&M Instrument, PDS Instrumentation (Louisville, California).
All pulmonary function studies were conducted according to the American Thoracic Society and European Respiratory Society guidelines.26-31 Participants did not undergo bronchodilator administration. The Global Lung Initiative (GLI) predicted formulas were used to compute the %predicted values as well as lower and upper limit of normal values for spirometry measures (FEV1/FVC, FEV1, FVC, FEF25%-75%).32 Crapo predicted formulas were used to compute the %predicted values as well as lower and upper limits of normal values for diffusing capacity.33,34 Spirometric COPD was defined using Global initiative for chronic Obstructive Lung Disease (GOLD) criteria unless otherwise specified.35
Statistical Analysis
Participants’ characteristics including demographics, years of SHS exposure, and lung function measures were examined and summarized within all participants and with respect to subgroups with or without cabin SHS exposure. The adjusted plasma DI levels were computed by calculating the residual values of the raw plasma DI levels and their predicted values from a linear regression model of the plasma DI levels over age, sex, height, and weight. A comparison of the distributions was performed using an unpaired t-test for each continuous variable or a Chi-squared test for each binary or categorical variable. The P-values and the descriptive statistics including the mean±standard deviation (SD), median (1st quartile, 3rd quantile) for continuous variables or N (%) for binary and categorical variables were presented.
The associations between plasma DI levels and lung function measures were examined, in the whole group of participants and the subgroup of those who had cabin SHS exposure, using linear regression modeling with adjustment for covariates including age, sex, height, and weight. The associations between having a history of cabin SHS exposure as well as years of cabin SHS exposure and lung function measures were examined using linear regression modeling with adjustment for the same covariates. For each individual model using one of the lung volume measures as the dependent variable, the total number of participants involved in the model and the parameter estimate with a 95% confidence interval and a P-value for plasma DI levels or years of SHS exposure were reported accordingly.
The associations between plasma DI levels and years of SHS exposure were examined using linear regression modeling with adjustments for the same covariates in the subgroup of those who had cabin SHS exposure. The difference in plasma DI levels between the subgroups with and without cabin SHS exposure was assessed using linear regression modeling with adjustments for the same covariates in the whole group of participants. For these models using plasma DI levels as the dependent variable, the total number of participants involved in the model and the parameter estimate with a 95% confidence interval and a P-value for years of SHS exposure or the binary indicator of having past SHS exposure were reported accordingly.
To assess whether associations between lung function and SHS exposure were potentially mediated through plasma DI, we performed mediation analyses with lung function measures (as dependent variable), SHS exposure (as independent variable; continuous or binary), and plasma DI (as mediator variable), with inclusion of covariates using the mediation package in R.36 Absolute proportion of mediated effects with corresponding P-values were reported.
Statistical analyses were conducted using the R (version 3.6) statistical software. A significance level of α<0.05 was used to determined statistical significance.
Results
Participants’ Characteristics
From the total of 241 flight attendants who were initially recruited into the study, 32 were excluded because they were not never-smokers. Among the remaining 209, 193 (92.3%) had been exposed to cabin SHS and 16 had not been exposed to cabin SHS. Additionally, 87 non-flight attendant healthy nonsmoking participants (from MOSES) were included in the SHS-unexposed group. Overall, 296 participants were included in the analyses (Figure 1) consisting of 193 (65.2%) exposed to cabin SHS and 103 (34.8%) unexposed.
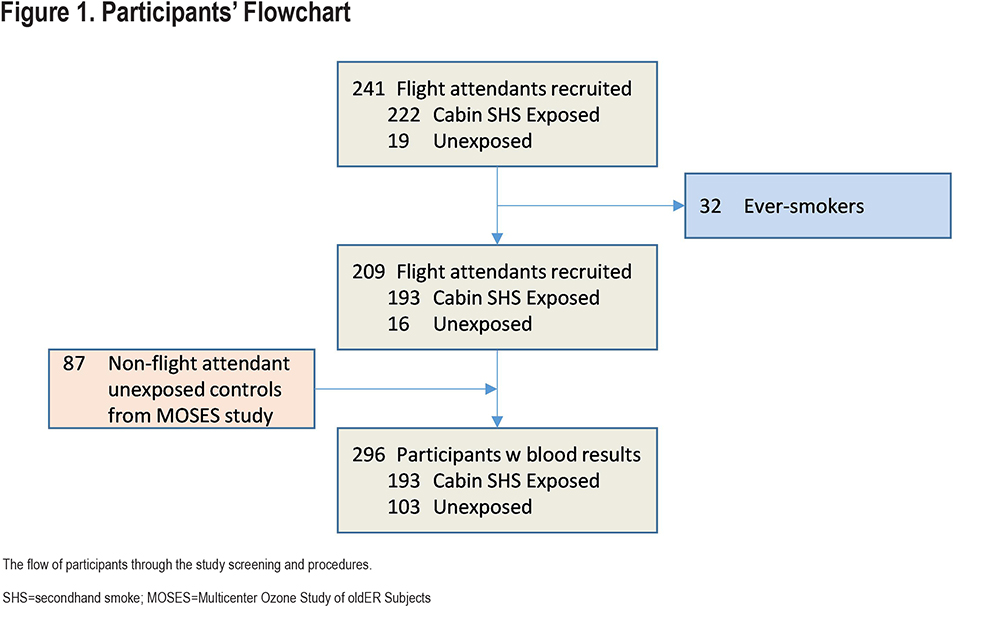
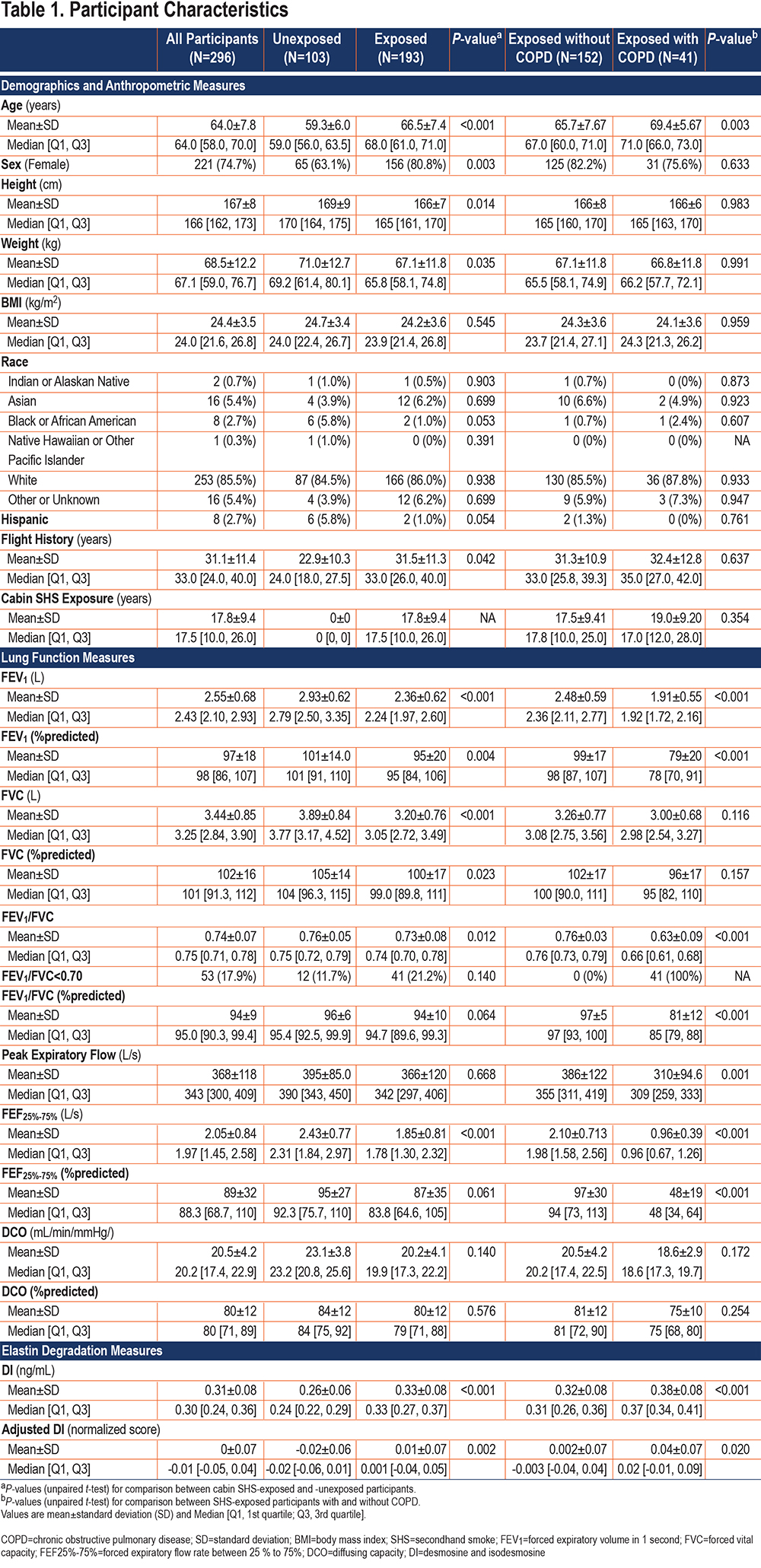
Participants’ characteristics are shown in Table 1. The average age of participants (N=296) was 64.0±7.8 years. The SHS-unexposed group was younger (59.3±6.0 years) than the SHS-exposed group (66.5±7.4 years) with the majority being women in both groups (63% and 81%, respectively). The cohort was primarily composed of people of White racial background (85.5%), but also included people who identified their racial background as Asian, African American, American Indian, Alaskan Native, and Native Hawaiian or other Pacific Islander. There was no significant race/ethnicity difference between SHS-exposed and -unexposed groups. The average body mass index (BMI) was 24.4±3.5Kg/m2 (21 participants were obese defined by BMI >30Kg/m2), with no significant difference in BMI between SHS-exposed and -unexposed groups. Among all flight attendant participants, the total years of airline employment was 31.1±11.4 years. Among the exposed flight attendants, the years of exposure to cabin SHS exposure was 17.8±9.4 years.
Lung Function Measurements
PFT measurements are shown in Table 1. For the flight attendant cohort, lung function measurements were carried out between June 2014 and October 2019, and for the MOSES cohort, lung function measurements were performed between 2012 and 2015. Although none of the participants had a clinical diagnosis of COPD, 53 (17.9%) had an FEV1/FVC ratio <0.70, consistent with spirometric COPD by GOLD,35 and 22 (7.4%) had an abnormal FEV1/FVC ratio by the lower limit of normal (LLN) criteria. Among the SHS-exposed group, 41 (21.2%) and 14 (7.3%) participants had an abnormal FEV1/FVC ratio consistent with spirometric COPD by GOLD and LLN criteria, respectively. Among the SHS-unexposed group, 12 (11.7%) participants had an FEV1/FVC <0.70, but only 8 (7.8%) had spirometric COPD by LLN criteria. The FEV1 was within the normal range for both, unexposed and exposed participants as a whole, but was reduced at 1.91±0.55 L (79±20 %predicted) in the SHS-exposed participants with spirometric COPD.
The diffusion capacity, which was measured only in 82 participants (all flight attendants), was (20.5±4.2mL/min/mmHg at 80±12 %predicted) and was below the LLN in 32 (39%) of the participants. Although not statistically significant, the diffusion capacity of the SHS-exposed group (N=73) was lower than that of the SHS-unexposed group (N=9) (20.2±4.1 versus 23.1±3.8mL/min/mmHg [80±12 versus 84±12 %predicted]). Similarly, the diffusing capacity of the SHS-exposed group with spirometric COPD was non-significantly lower than that of the SHS-exposed group without spirometric COPD (18.6±2.9 versus 20.5±4.2mL/min/mmHg [75±10 versus 81±12 %predicted]).
Plasma Levels of Elastin Degradation Products—Desmosine and Isodesmosine—Were Associated with Lung Function Measures
In linear regression models adjusted for age, sex, height, and weight, plasma levels of elastin degradation products (DI) were inversely associated with FEV1, FVC, FEV1/FVC, FEF25%-7%5, and DCO among all participants (FEV1: ß=-1.76L, P<0.001; FVC: ß=-1.43 L, P=0.002; FEV1/FVC: ß=-26%, P<0.001; FEF25%-75%: ß=-2.74L, P<0.001; and DCO: ß=-9.98mL/min/mmHg, P=0.037) (Table 2). Similar associations were found with the %predicted values (Figure 2).
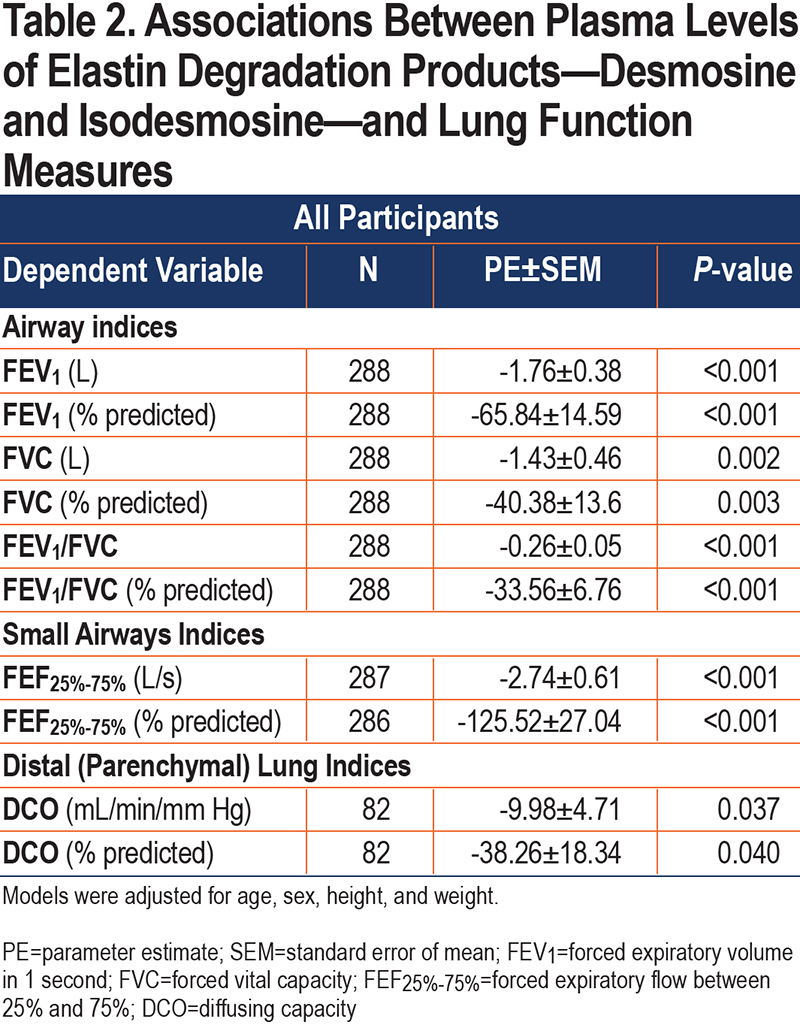
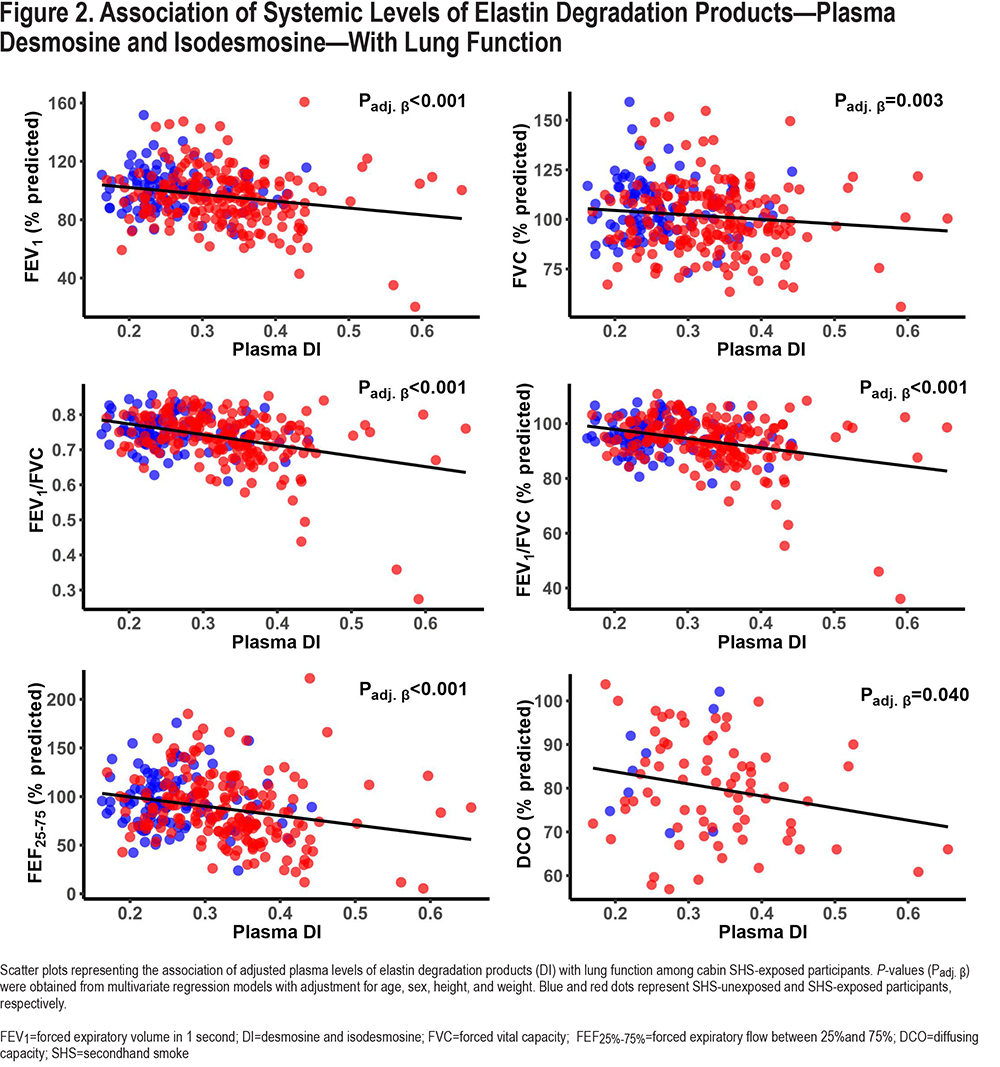
Among the subgroup with cabin SHS exposure, we found a similar inverse association between plasma levels of DI and FEV1, FEV1/FVC, FEF25%-75%, and DCO in adjusted models (FEV1: ß=-1.47L, P=0.001; FEV1/FVC: ß=-31%, P<0.001; FEF25%-75%: ß=-2.55L, P<0.001; and DCO: ß=-10.92mL/min/mm Hg, P=0.025; respectively) (Table S1 in the online supplement). Similar associations were found with the %predicted values(Figure 2). When the SHS-exposed group was divided into the subgroups with and without spirometric COPD, similar directions of the associations were observed among SHS-exposed without COPD, although they did not reach statistical significance. However, among the SHS-exposed group with COPD we observed significant inverse association between plasma levels of DI and FEV1/FVC (FEV1/FVC: ß=-0.57%, P<0.001), and border significance for FEV1 and FEF25%-75% (ß=-1.88L, P=0.076, ß=-1.63L, P=0.054) (Table S1 in the online supplement).
Secondhand Smoke Exposure Was Associated with Plasma Levels of Elastin Degradation Products
Among all participants, the plasma levels of DI were significantly elevated in the SHS-exposed group compared to the unexposed group (unadjusted levels: 0.33±0.08 versus 0.26±0.06ng/mL, P<0.001 (Table 1); age, sex, height, and weight-adjusted levels in Figure 3; Padj<0.001). Within the SHS-exposed group, those with spirometric COPD had significantly elevated levels of plasma DI compared to those without spirometric COPD (unadjusted levels: 0.38±0.08 versus 0.32±0.08ng/mL, P<0.001 [Table 1]; adjusted levels in [Figure 2]; Padj<0.001). Of note, the 12 unexposed participants who met GOLD COPD criteria had a slight but non-significant higher level of plasma DI than those unexposed participants without COPD (Figure 2).
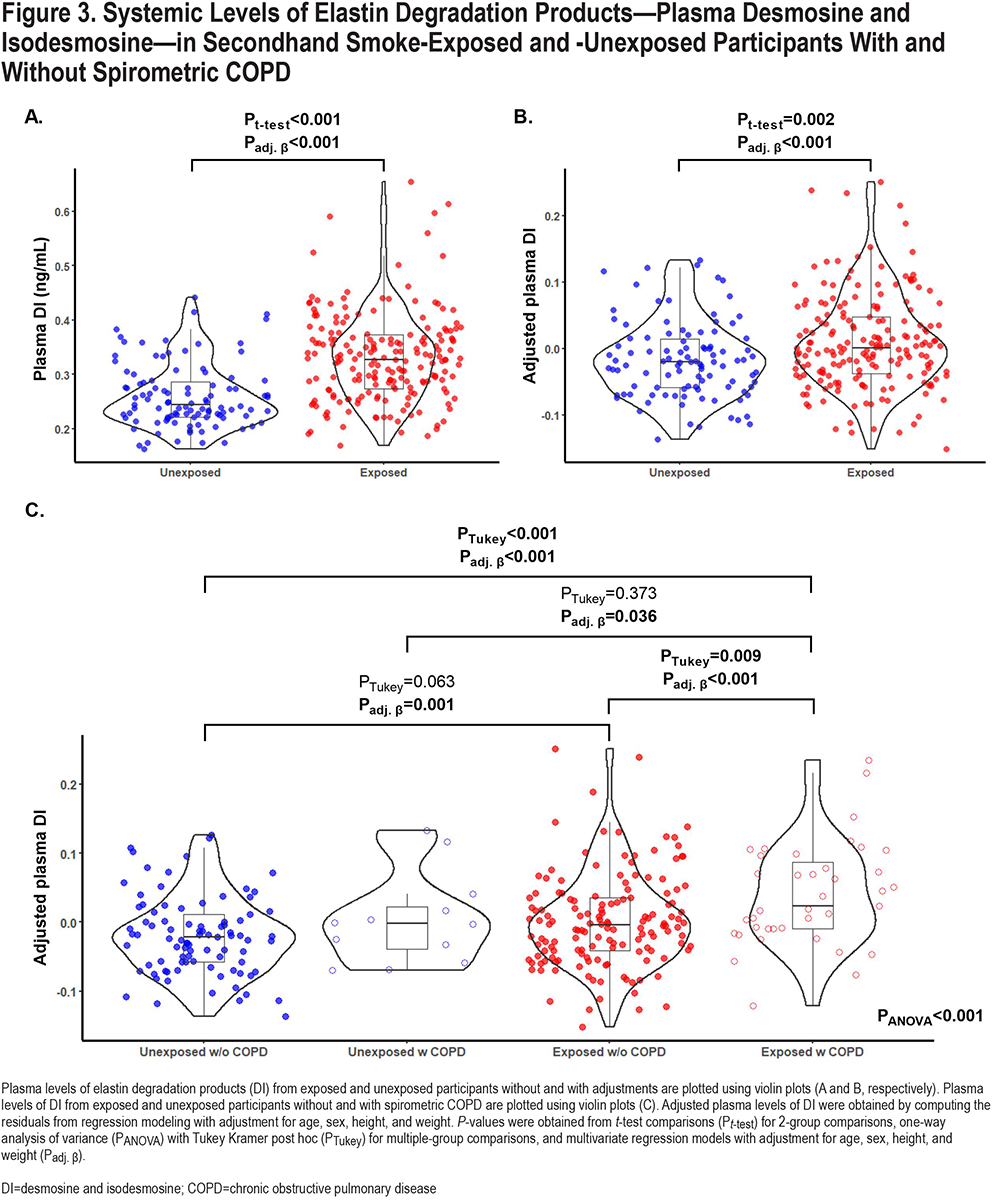
In univariate unadjusted models, plasma levels of DI were positively associated with years of SHS exposure, and with age, but not with total length of airline employment (P<0.001, P<0.001, and P=0.43, respectively) (Table S2 in the online supplement). However, in multivariate models adjusted for age, sex, height, and weight, years of cabin SHS exposure and total years of flight employment were not associated with plasma DI levels (Table S2 in the online supplement).
Secondhand Smoke Exposure Was Associated with Lung Function Measures
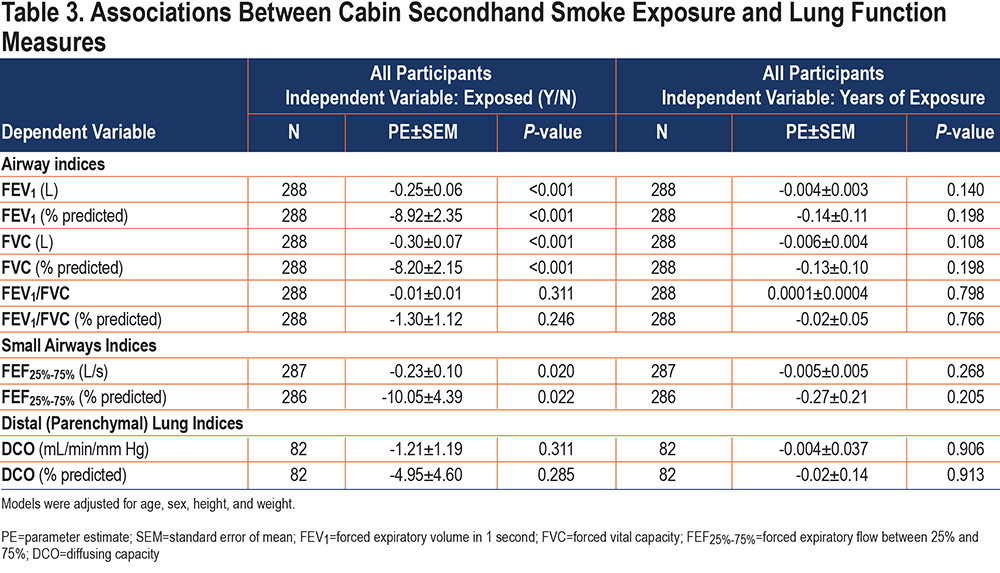
Among all participants, history of exposure to cabin SHS was inversely associated with FEV1, FVC, and FEF25%-75% in models adjusted for age, sex, height, and weight (FEV1: ß=-0.25L, P<0.001; FVC: ß=-0.30L, P<0.001; and FEF25%-75%: ß=-0.23L, P=0.020) (Table 3). As an example, those exposed to cabin SHS smoke had an FEV1 that was 247mL lower compared to those who were not exposed to cabin SHS. The associations between exposure to SHS and FEV1/FVC or DCO were in the hypothesized directions but did not reach statistical significance.
Plasma Levels of Elastin Degradation Products —Desmosine and Isodesmosine— Mediated Association of Secondhand Smoke with Lung Function
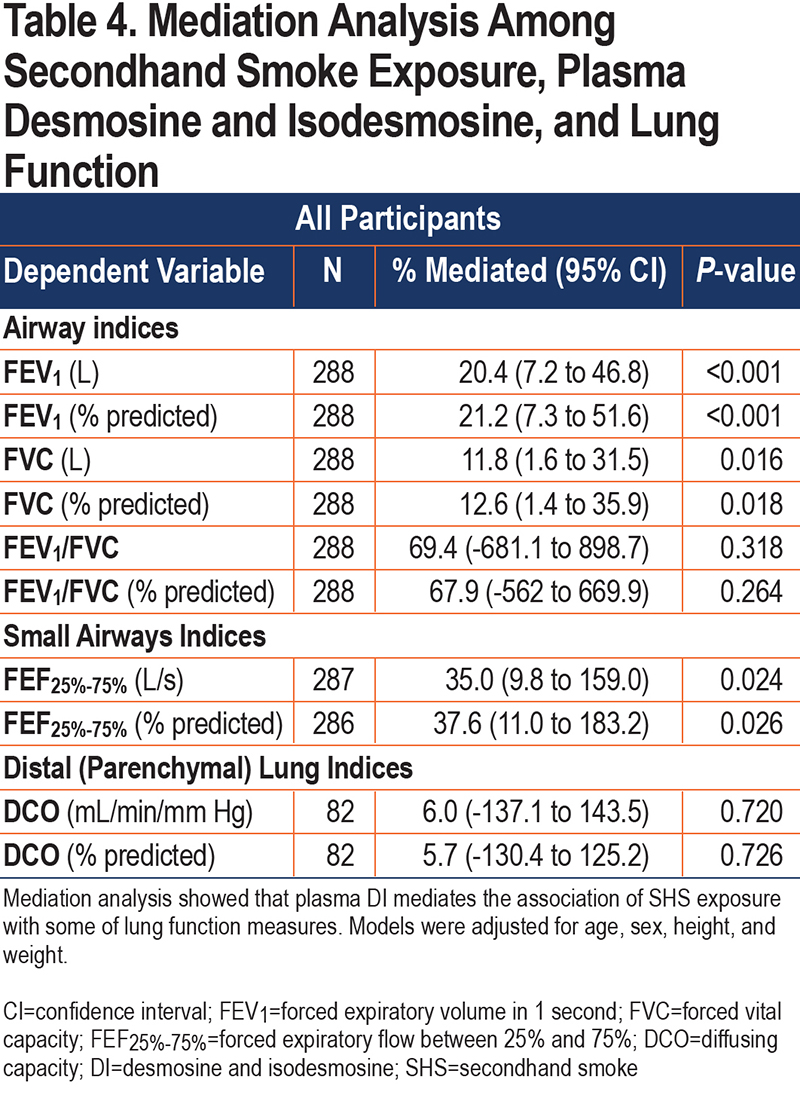
To determine whether the associations of lung function measures with SHS exposure were mediated through plasma DI, we performed a mediation analysis with adjustment for covariates. When SHS exposure was used as a continuous variable, the mediation model was not significant. However, using SHS exposure as a binary variable, mediation analysis showed that plasma DI significantly mediated the association of lung function measures with SHS exposure (for example, plasma DI accounted for 20% of the reduction in FEV1 due to past cabin SHS exposure; P<0.001) (Table 4).
Discussion
In this study, we found that never-smoking flight attendants who had a remote history of occupational exposure to SHS had significantly higher systemic (plasma) levels of elastin degradation products (DI) compared to nonsmoking individuals with no such history of occupational SHS exposure. Furthermore, we found that among never-smoking, SHS-exposed flight attendants, those with spirometric COPD had higher systemic levels of DI compared to those without spirometric COPD. This is remarkable because it implies that even 25 years after the occupational exposure to cabin SHS (smoking was banned on all domestic and international flights after 1995), there is ongoing differential elastin damage in these never-smokers who were exposed to SHS in the cabin, with the damage being even greater for the COPD-susceptible people. While the source of the higher systemic elastin degradation markers in those exposed to cabin SHS (and also those with spirometric COPD) that we observed in this study is unknown, the inverse association of lung function with plasma DI does suggest that pulmonary elastin degradation and lung tissue destruction at least in part contribute to this process. Corroborating this conclusion is the finding that a fraction of lung function measure associations with SHS exposure was mediated through plasma DI when using SHS exposure as a binary variable. Although there have been previous reports about increased systemic levels of DI in the setting of acute exposure to SHS,13 to our knowledge, this is the first report of describing evidence of lung damage with such remote exposure to SHS.
Studies investigating the source of plasma DI in people who smoke tobacco or those with COPD have been inconsistent. Many studies have reported DI levels to be an indicator of lung damage associated with decreased lung function.14,37-41 The initial studies that supported the association between destruction of lung elastin with occurrence of airspace enlargement characteristic for emphysema and increased urinary DI were done in animal models.38,39,41 Later, human studies confirmed similar findings.15,40,41 Stone et al extensively explored levels of DI in relation to presence of COPD, or with smoking status.42 They found higher excretion of DI in urine of COPD patients, and in smokers without presence of COPD compared to healthy lifetime nonsmokers, suggesting smoking and the presence of COPD to be independently associated with elevated urinary DI levels. Other studies have reported increased DI levels in smokers and COPD patients, even before clinical symptoms of COPD occur.14 Slowik et al demonstrated that not only smokers, but also non-smokers exposed to SHS, have higher levels of DI compared to a control without SHS exposure.13
On the other hand, other studies of COPD patients have indicated an association between plasma DI and elastin degradation in skin or vascular tissue,43,44 suggesting other tissues as a source of elastin breakdown in COPD. Rabinovich et al44 reported plasma DI to be related to cardiovascular comorbidities and aortic stiffness as well as age in patients with COPD. Although in univariate analysis, they observed weak correlations of plasma DI with airflow obstruction and dyspnea at the baseline, in multivariate analysis they did not find any significant associations of plasma DI with baseline airflow obstruction (FEV1) or emphysema, or with FEV1 decline or emphysema progression over time. In our study of people with a history of past SHS exposure, we found plasma DI levels to be inversely associated with lung function measures, including those representing airflow obstruction (FEV1) and lung tissue destruction (DCO). One possible explanation for these differences in findings could be the variable contribution of different organ systems to the amount of plasma DI in the cohorts studied. The population studied by Rabinovich et al had on average >40 pack years of direct smoking and relatively severe COPD with an average FEV1 of <60% of predicted value, 25% of whom had known significant cardiovascular disease. Our cohort was selected based on a history of SHS exposure, of whom only 18% had a diagnosis of mild to moderate spirometric COPD, and none with a known history of cardiovascular disease. It is possible that inclusion of a large number of patients with cardiovascular disease may have resulted in a plasma DI pool with greater contribution from vascular tissue, which could have then masked any existing associations of lung function decline or emphysema with plasma DI. Furthermore, though Rabinovich et al44 did not find association between plasma DI and FEV1 decline, when patients were divided into quartiles according to their DI levels, those in high quartile of DI levels had significantly lower FEV1 compared to patients in other quartiles, which suggests lung tissue as a possible source of plasma DI. Altogether, the reported associations between measures of lung function (including lung function indices suggestive of small airways and distal lung damage) and plasma levels of DI support the notion that systemic DI could at least in part originate from the lung elastin damage.
Our finding that cabin SHS exposure from many years ago is associated with a current elevation of systemic levels of elastin degradation products is quite remarkable, but its biological plausibility is supported by the available literature. Excessive and persistent inflammation is a driving force in lung injury and development of COPD, and several studies have shown that airway inflammation persists long after cessation of smoke exposure, including SHS.45-50 These findings suggest exposure to SHS could initiate self-perpetuating inflammatory processes, which in turn can cause persistent injury and damage to the lung, as evident from the elevated levels of elastin degradation products many years later despite removal of the original insult. Furthermore, given that we observed a significant or border significant inverse association between plasma DI levels and several lung function measurements among those SHS exposed with COPD, but not among the larger group of those SHS exposed without COPD, suggests greater ongoing effect of SHS exposure on lung deterioration in susceptible people years after cessation of the exposure.
Although no longer being exposed to the high intensity SHS that they experienced in aircraft cabins, flight attendants experience increased rates of respiratory illnesses, compared to the general population.3,7-9 McNelly et al9 reported a roughly 3-fold increase in chronic bronchitis prevalence among flight attendants, when compared to the age-matched general U.S. population in the National Health and Nutrition Examination Survey, despite the flight attendants having lower prevalence of smoking. Furthermore, the odds of being diagnosed with chronic bronchitis increased with the tenure of flight attendants.9 In addition, Beatty et al found an increased prevalence of chronic bronchitis, emphysema/COPD, and sinus problems among flight attendants compared to the U.S. general population.7 Arjomandi et al reported never-smoking flight attendants who were exposed to past cabin SHS had decreased diffusing capacity, with more than half of them having diffusing capacity below the lower limit of the 95% prediction interval for their age, sex, and height.3,10 Further, they also had decreased maximal airflow at mid- and low-lung volumes together with pulmonary function evidence of air trapping suggesting airflow obstruction.3
Our study has several limitations. First, exposure to cabin SHS was estimated based on self-reported data regarding airline employment years. Yet, airline employment history could provide a relatively accurate measure of cabin SHS exposure. Moreover, any error in the flight attendants’ recall of employment history is not expected to be related to plasma DI or lung measurements. Second, given the cross-sectional nature of this study, the analysis could only provide estimation, as opposed to direct proof, of any causal relationships. However, this study does provide indirect evidence about the long-lasting effects of remote SHS exposure on elastin breakdown and lung function decline years after cessation of the exposure. Third, while we observed a significant association between history of cabin SHS exposure and higher plasma DI levels in the univariate linear regression modeling, in the multivariate modeling, years of cabin SHS exposure association with plasma DI levels did not reach statistical significance after adjusting for age, sex, height, and weight. Nevertheless, in the current study, in the categorical comparisons of exposed and unexposed participants, plasma DI levels were significantly higher in those exposed to SHS as a group compared to the unexposed after adjusting for covariates (age, sex, height, and weight). Finally, beyond the history of cabin SHS exposure in the past, flight attendants face other environmental exposures, such as low atmospheric pressure and radiation exposure as well as other sources of SHS exposure such as childhood, home adulthood, or non-airline occupation, which could contribute to lung damage measured by DI levels. While the effects of those exposures could be significant, the significant association between years of cabin SHS exposure, and not length of airline employment, and plasma DI that we observed implicates the role of past airline related occupational SHS exposures on elastin degradation and lung function decline.
Collectively, our study documents the long-term adverse effects of exposure to SHS on pulmonary structure and function. It provides evidence that past exposure to SHS, even when remote, is associated with higher systemic elastin degradation markers, which in turn is indicative of continued lung damage and declining lung function despite the cessation of culprit exposure. Our study furthermore implicates plasma DI as a sensitive biomarker of lung damage in at risk populations with a history of exposure to secondhand tobacco smoke.
Acknowledgements
Author Contributions: Designed the current manuscript study: JMR, SZ, RR, GMT, EOS, MA. Developed study protocols: XL, SM, RR, GMT, EOS, MA. Collected samples: LR, RR, EOS, MA. Analyzed samples: XL, SM, GMT. Collected, analyzed, and interpreted data: JMR, SZ, LR, ELVB, MA. Prepared the manuscript: JMR, SZ, MA. Edited the manuscript: JMR, SZ, ELVB, LR, XL, SM, JK, RR, GMT, EOS, MA. Obtained funding: JMR, SZ, RR, GMT, EOS, MA. All authors approved the final version of the manuscript.
Declaration of Interest
Authors report no conflict of interest related to this work