Running Head: Antibody Response to SARS-CoV-2 Vaccination
Funding Support: Fondation de l’Institut universitaire de cardiologie et de pneumologie de Québec - fonds général et fonds Alphonse L’Espérance.
Date of Acceptance: July 29, 2022 │ Published Online Date: August 3, 2022
Abbreviations: severe acute respiratory coronavirus-2, SARS-CoV-2; chronic obstructive pulmonary disease, COPD; arbitrary unit, AU; forced expiratory volume in 1 second, FEV1; Global initiative for chronic Obstructive Lung Disease, GOLD; inhaled corticosteroids, ICSs; odds ratio, OR; confidence interval, CI; coronavirus disease 2019, COVID-19
Citation: Pelletier E, Desmeules P, Lacasse Y, et al. Antibody response to severe acute respiratory syndrome coronavirus-2 vaccination in COPD: a cohort study. Chronic Obstr Pulm Dis. 2022; 9(4): 591-595. doi: http://doi.org/10.15326/jcopdf.2022.0315
Introduction
The development of vaccines against the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) within a year after virus identification is a feat of modern medicine. Large cohort studies suggest adequate antibody response to SARS-CoV-2 vaccines1 and clinical effectiveness in the general population, including those with chronic diseases.2 In subanalyses, chronic obstructive pulmonary disease (COPD) did not predict poor response to SARS-CoV-2 vaccines1 or reduced clinical efficacy.2 However, detailed phenotyping of COPD was not provided in these studies, making hazardous any inference about diagnostic validity and disease severity. It is also likely that people with severe COPD were underrepresented due to multiple barriers to study participation.3 Early in 2021 in Quebec (Canada), people living in long-term health care facilities and health care workers were prioritized for SARS-CoV-2 vaccination, followed by elderly people, and individuals below the age of 60 with chronic disease. We took advantage of this vaccination campaign to document the antibody response to SARS-CoV-2 vaccines in patients with well-characterized COPD.
Patients of the COPD clinic of the Institut Universitaire de Cardiologie et de Pneumologie de Québec, a tertiary care hospital, were invited to participate (ethical approval number 2260). Out of 415 patients, 407 accepted to be vaccinated, 327 were enrolled in the SARS-CoV-2 vaccine study, and 191 agreed to repeated blood sampling. Sixteen healthy participants, all health care workers, served as controls. All study participants provided written consent. Immunization status against SARS-CoV-2 was assessed using a commercially available immunoassay (Siemens, Dimension Vista 1500; COV2T assay) that quantifies IgG and IgM against the spike (S) protein.4 To differentiate between natural and vaccine-related immunity, an immunoassay (Architect i2000; Abbott system; SARS-CoV-2 IgG assay) measuring IgG directed against nucleocapsid (N) protein was conducted. Blood was sampled on 3 occasions in patients with COPD, before the first dose (baseline), 28 to 35 days after the first dose, and 28 to 35 days after the second dose. Blood sampling was performed only after the second dose in healthy participants.
Three patients with COPD received only 1 dose of the vaccine, 2 tested positive for anti-N antibodies prior to the first vaccine, and 2 others became anti-N positive after the first vaccine. Therefore, 184 patients with COPD are the subject of the present report (Table 1). The first vaccine dose was administered between February 11 and May 24, 2021, with the second dose administered 81±18 days later (range 28 to 140 days).
Healthy participants included 4 males and 12 females, aged from 21 to 57 years. With the exception of 1 case, they were all non-smokers. They received the first vaccine dose between January 13 and May 1, 2021, with the second dose administered 99±23 days later (range 44 to 118 days). Their anti-N protein status was negative, excluding natural infection. Vaccine combinations were: 2 doses of BNT162b2 (n=12), 2 doses of mRNA-1273 (n=2), 2 doses of ChAdox nCoV-19 (n=1), and a combination of BNT162b2 and mRNA-1273 (n=1).
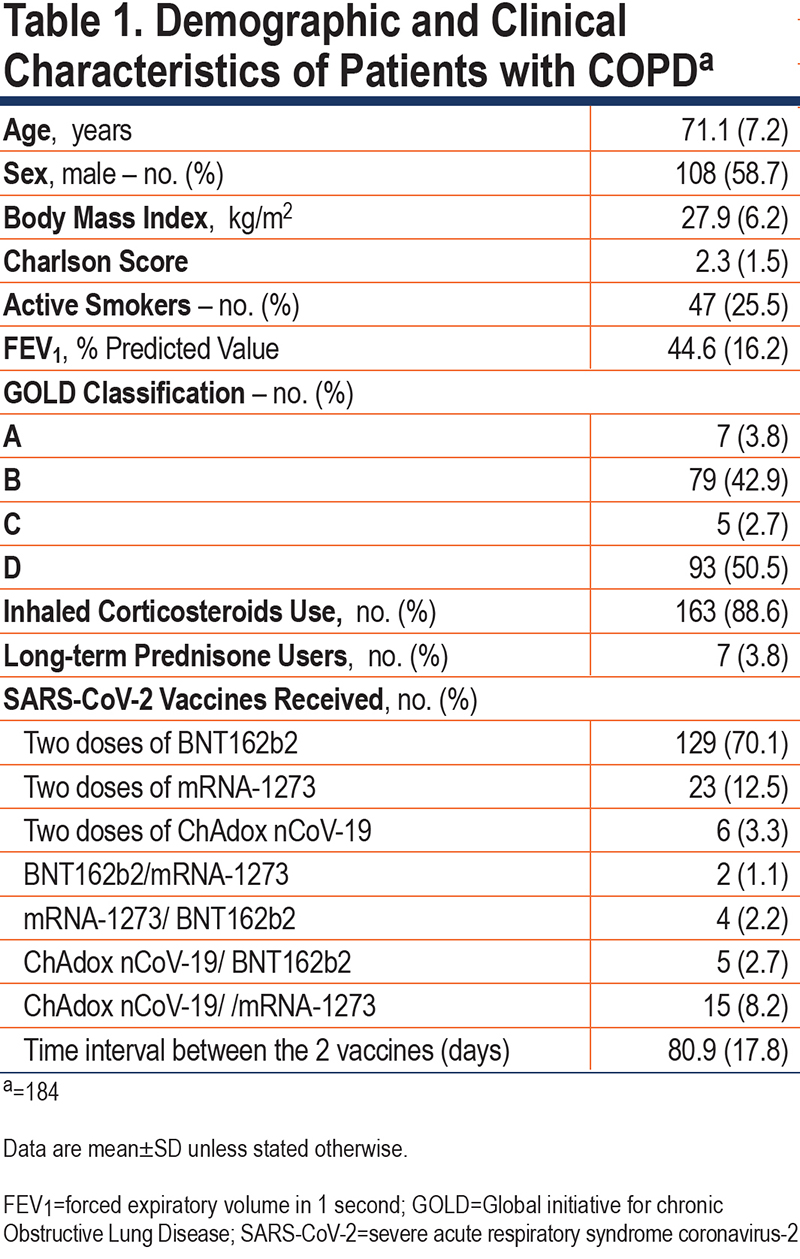
Circulating antibody levels against SARS-CoV-2 S protein are shown in Figure 1. A total of 53% of patients (97/184) were above the cutoff of 103 arbitrary unit (AU) proposed by the manufacturer5 after the first dose, and 98% (176/180) after the second dose. In comparison, the lowest value measured in healthy controls was above 104 AU, a threshold that was reached in 91% of patients (164/180) after the second vaccine. Patients with COPD tended to have a lower antibody response against S protein compared to healthy controls (p=0.0573).
Two approaches were used to study possible determinants of the anti-S antibody response in COPD; the first considered the antibody levels as a continuous variable and the second dichotomized the antibody responses according to the 104 AU threshold below which no healthy controls were seen. Age (< or ≥70), sex, comorbidities (Charlson index), severity of airflow limitation (forced expiratory volume in 1 second [FEV1] <30% or ≥30 % predicted value), Global initiative for chronic Obstructive Lung Disease (GOLD)6 classification (A or B versus C or D), use of inhaled corticosteroids (ICSs), long-term use of prednisone, smoking status (active versus former), and vaccine types (2 doses of mRNA vaccines versus any other combinations) were considered as potential determinants of the antibody response against S protein in univariate and multivariate models.
When considering the antibody levels as a continuous variable, univariate analysis showed that age≥70 (p=0.0081) and having received 1 or 2 doses of non-mRNA vaccines predicted a lower antibody response against S-protein (p=0.0256). Females (p=0.0579) and ICS users (p=0.0643) tended to have a higher antibody response than males or ICS nonusers, respectively. The remaining independent variables, including smoking status, were not statistically associated with the anti S-protein level. According to the multivariate analysis, the probability of reaching the 104 AU threshold was lower in individuals ≥70 years (odds ratio [OR]: 0.104, 95% confidence interval [CI]: 0.021 to 0.520, p=0.0059) and in those who received any vaccine combination other than 2 doses of mRNA vaccines (OR: 0.135, 95% CI: 0.041 to 0.450, p=0.0011).
The present study provides an objective evaluation of the response to SARS-CoV-2 vaccines in patients with well-documented severe COPD. With 98% of vaccinated people, vaccination uptake was excellent. A typical patient followed at our clinic has severe airflow limitation, frequent exacerbations, and associated comorbid conditions predicting poor outcomes after coronavirus disease 2019 (COVID-19), including death.7,8 Therefore, we were pleased to note that 91% of our patients reached an anti S-protein level above 104 AU, the lowest value seen in healthy controls. Although adequate antibody response does not guarantee clinical protection, serum antibody levels measured with the Siemens’ immunoassay predicts serum viral neutralizing capacity9 which, in turn, predicts clinical protection10 against COVID-19. If the response observed in COPD is similar to that of healthy controls in whom vaccination is clearly associated with clinical protection, the same should be expected in patients with COPD.
We acknowledge that our study has potential limitations. Considering that older age is associated with lower antibody response to SARS-CoV-2 vaccination,1 and that patients with COPD were compared to younger, healthy controls, we provide a conservative estimate of the proportion of patients with COPD exhibiting an antibody response to SARS-CoV-2 vaccination in the normal range. Although, the antibody response assessed with the immunoassay used in the present study shows a good correlation with virus neutralization titers,9 we recognize that this was not specifically measured in our patients.
Age≥70 and non-mRNA vaccines were associated with a lower antibody response. These results are consistent with known risk factors for suboptimal vaccination response.1 As previously reported,1 males are also known to exhibit lower antibody responses than females; this did not emerge in multivariate analysis, possibly because of the relatively small sample size. The observed tendency for an increased antibody response in ICS users was unexpected. This finding should be interpreted cautiously given the small number of non-ICS users and the fact that other investigators did not report such a finding.11 It was not possible to make a firm conclusion about the impact of long-term use of prednisone given the small number of patients on this therapy. While the degree of efficacy of the SARS-CoV-2 vaccines in this clinical cohort is extremely encouraging, we propose that patients exhibiting poor antibody response after 2 vaccines, those ≥70 years and/or who have received 1 or 2 doses of non-mRNA vaccines during the first vaccination phase should be prioritized for booster shots.
Acknowledgements
Author contributions: All authors contributed to the design of the work and to data analysis. EP, YL, ST, JM, and FM contributed to data acquisition. PD supervised the laboratory aspects of the study including the antibody response measurement and interpretation. EP and FM drafted the manuscript. All authors were involved with data interpretation and critical review of the manuscript. All authors approved the final version of the manuscript. FM is accountable for all aspects of the work, on behalf of the co-authors.
The authors wish to acknowledge the contribution of Christine Racine and the whole team of the Biobank of the Institut de Cardiologie et de Pneumologie de Québec.
Declaration of Interest
EP, PD, YL, ST, JM, and MCM have no disclosures to report. FM reports grants from AstraZeneca and GlaxoSmithKline, Boehringer Ingelheim, Sanofi, and Novartis, and personal fees for serving on speaker bureaus and consultation panels from GlaxoSmithKline, Grifols, and Novartis. He is financially involved with Oxynov, a company which is developing an oxygen delivery system.