Running Head: Varenicline for Smoking Cessation in COPD Individuals
Funding Support: This study was funded by a 2015 Global Research Award for Nicotine Dependence (GRAND), a competitive independent investigator-initiated research program supported by Pfizer, Inc. However, Pfizer had no further role in the study design, collection, analysis and interpretation of the data, writing of the report, or in decision to submit the paper for publication.Trial registration:ClinicalTrials.gov Identifier: NCT02894957
Date of Acceptance: July 14, 2022 | Published Online Date: July 22, 2022
Abbreviations: chronic obstructive pulmonary disease, COPD; target quit day, TQD; odds ratio, OR; confidence interval, CI; nicotine replacement therapy, NRT; randomized controlled trial, RCT; adverse events, AEs; Shaare Zedek Medical Center, SZMC; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; visual analog scale, VAS; carbon monoxide, CO; COPD Assessment Test, CAT; point-prevalence, PP; standard deviation, SD
Citation: Bohadana A, Rokach A, Wild P, et al. Varenicline for gradual versus abrupt smoking cessation in poorly motivated smokers with COPD: a prematurely terminated randomized controlled trial. Chronic Obstr Pulm Dis. 2022; 9(4): 486-499. doi: http://doi.org/10.15326/jcopdf.2022.0305
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by the triad of exposure to noxious agents, respiratory symptoms, and progressive airflow limitation that is not fully reversible by the inhalation of bronchodilators.1 Tobacco exposure from active smoking is the most common cause of COPD, affecting as much as 50% of smokers.1,2 While inhaled medication can be used to improve symptoms and reduce exacerbations,1 smoking cessation remains the only proven means of effectively slowing down the progression of COPD and preventing further decline in maximal expiratory flows.3,4
Despite the benefits of smoking cessation, a high proportion (35%-49%) of patients with COPD smoke,5 suggesting an increased resistance to smoking cessation in this population. For such patients, the usual “cold turkey” way to stop smoking i.e., abruptly on a given day, may be difficult to achieve, especially in those with low motivation to quit.6,7 An alternative strategy is the reduce to quit or gradual method of cessation. This may be because smoking reduction: (1) increases self-efficacy about quitting, (2) decreases the association between environmental cues and smoking, facilitating total cessation, (3) decreases nicotine dependence, and (4) provides an opportunity for the smoker to learn skills to tackle urges and relapse-inducing situations.8,9 Previous studies have shown that nicotine replacement therapy (NRT) products9 and varenicline10-12 can be used successfully as smoking reduction aids prior to cessation; however, these studies were conducted in smokers from the general population, not in patients with COPD. This is important, as it is crucial to ensure successful smoking cessation attempts in patients with COPD to avoid irreversible tobacco damage to their lungs.
Given the above considerations, we conducted a randomized controlled trial (RCT) to examine, in COPD patients receiving regular 12-week varenicline therapy, whether using varenicline for a 6-week gradual cessation before the target quit day (TQD), rather than starting 1 week before the TQD as currently labeled, would improve quit outcomes 30 weeks after randomization. We chose varenicline over NRT because varenicline is arguably the most effective treatment for smoking cessation13 and, second, because varenicline has been found to increase motivation to quit in smokers who are not ready to make a serious attempt.14 This effect justified our secondary hypothesis that the use of varenicline for gradual smoking cessation might increase motivation to quit in smokers with COPD. In addition, we investigated the impact of this treatment on pulmonary parameters, as well as on adverse events (AEs) and safety.
Methods
Study Design and Setting
This randomized, single-center, open-label study was conducted at the Shaare Zedek Medical Center (SZMC) Lung Institute in Jerusalem, Israel, between January 2019 and October 2020. The study was conducted in 3 phases:
- Pre-quit phase, i.e., the 6-week period from baseline (week 1) to TQD (week 6);
- Treatment phase, i.e., the 12-week abstinence period from TQD to week 18, during which regular varenicline treatment was administered;
- Follow-up phase, i.e., the 12-week treatment-free period from the end of week 18 to the end of week 30.
The trial was in accordance with the recommendations adopted by the 18th World Medical Assembly, Helsinki, Finland 1964 and its subsequent revisions. The Helsinki Committee of the SZMC approved all study procedures and all participants provided written informed consent before any procedure. The trial was registered on ClinicalTrials.gov (ID: NCT02894957).
Study Population
Participants were men and women aged ≥35 years who received a clinical diagnosis of COPD and:
- Smoked ≥15 pack years;
- Had a COPD diagnosis confirmed by a forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio <70% after bronchodilation;
- Were poorly motivated to quit smoking (see below for definition);
- Were willing to sign an informed consent statement;
- Were committed to trying to quit smoking by a TQD; and
- Were willing and able to comply with study procedures.
We excluded patients with a history of:
- Systemic corticosteroid treatment or hospitalization for a COPD exacerbation within 4 weeks before enrollment;
- A severe psychiatric disorder precluding rational use of medication;
- Myocardial infarction within the past 3 months;
- Unstable angina;
- Severe cardiac arrhythmia;
- Use of any form of smokeless tobacco or nicotine replacement or completion of a withdrawal program within the past 3 months; or
- Alcohol or other drug abuse.
Prior to inclusion in the trial, each participant received adequate verbal and written information regarding the purpose and procedures of the trial and the potential risks and were informed of their right to withdraw from the trial at any time.
Interventions
Randomization and Group Assignment: Two statisticians (YF-D; PB) working in a separate institution from SZMC used the Graph Pad random number calculator to randomize patients at the baseline visit to receive varenciline either for gradual cessation or abrupt cessation and informed the research team of the allocation by telephone. Participants and investigators were masked as to group assignment but could not be masked as to treatment assignment.
Treatment: To lessen the risk of adverse events, especially nausea, patients in the gradual cessation group received 1 week, low-dose titration of varenicline (0.5mg 1×day for 3 days, 0.5mg 2×day for 4 days) followed by 1mg 2×day from week 2 to week 6. All patients were recommended to reduce their baseline smoking rate by 25% by week 2 and by 50% by week 4, with further reduction to 75% by week 6 with the goal of quitting by the end of week 6. To help achieve reduction, 3 methods were suggested, according to Hughes and colleagues9 namely:
- Gradually increasing the time interval between cigarettes;
- Eliminating the easiest cigarette first, thus, giving the smoker an initial success and the confidence to tackle more difficult cigarettes; or,
- Eliminating the hardest cigarette first in order to build confidence, increase the likelihood of success, and reduce the likelihood of slip (i.e., occasionally smoking cigarettes after cessation).
Patients in the abrupt cessation group were instructed to smoke ad libitum for 5 weeks, after which they received a 1-week titration of varenicline as above, with the goal to quit at the end of week 6. They were not encouraged to cut down during the first 5 weeks prior to the TQD. However, to avoid disengagement, they were instructed to use these 5 weeks to analyze their smoking patterns and prepare for cessation. After the TQD, patients in both groups received regular varenicline treatment (1mg 2×day) for 12 weeks. For patients with tolerance problems, the maintenance dose could be temporarily or permanently reduced to 1mg daily.
Smoking Cessation Counseling: After the TQD, patients in both groups received smoking cessation counseling consisting of a short (approximately 10 min.) explanation about: (1) how cigarette smoking causes COPD and affects spirometry; (2) the fact that smoking cessation is the only treatment likely to slow the progression of COPD, and (3) how varenicline works, and the importance of taking the drug as prescribed.
Clinical Visits: Participants in the 2 groups attended 10 clinical visits, at week 1 (visit 1), week 2 (visit 2), week 4 (visit 3), week 6 (visit 4; TQD), week 7 (visit 5), week 9 (visit 6), week 12 (visit 7), week 15 (visit 8), week 18 (visit 9; end of treatment), and week 30 (visit 10; end of study).
Measures
Baseline Assessment: Baseline assessment included demographics, ethnicity, marital status, employment status, education, general health and comorbidities, current medical treatment, and alcohol use. Patients were weighed at all visits.
Smoking History: Smoking history was assessed by questionnaire. Cigarette dependence was assessed using the Fagerstrom Test for Cigarette Dependence (FTCD: 0-10 pts).15 Motivation to quit smoking was assessed at all visits using a 10-point visual analog scale (VAS) (How much do you want to quit smoking? not at all=0; extremely=10). A value <5 points was considered low.
Smoking Status: This was evaluated by measuring exhaled carbon monoxide (CO) levels at each face-to-face contact with a Bedfont monitor. Smoking abstinence was defined as self-reported abstinence biochemically validated by exhaled CO measurements ≤5ppm.16 Effective smoking reduction was defined as a self-reported decrease of 50% or more in the number of cigarettes smoked per day.
COPD Symptom Burden in Daily Life: This was measured using the COPD Assessment Test (CAT) (range: 0-40 points).17 Spirometry was performed before and after bronchodilator inhalation according to American Thoracic Society/European Respiratory Society recommended standards.18 AEs and new medications were assessed at all contacts. AEs, including serious AEs, were documented on case report forms and followed through to resolution.
Outcomes
Endpoints were selected according to the recommendations of the Society for Research on Nicotine and Tobacco working group.19 Primary outcome was the biochemically verified continuous abstinence rate (CAR) during weeks 6 (TQD) through 30 (24-week CAR).
Secondary Outcomes: The secondary outcomes included the:
- 7-day point prevalence (PP) abstinence at all time points from week 4 to 30, defined as the rate of biochemically-validated abstinence from smoking during the 7-day time window immediately preceding follow-up;
- Effective (≥50%) smoking reduction in the pre-quit phase;
- Motivation to quit smoking; and
- Number of cigarettes smoked by day.
Additional outcomes included the changes in:
- Respiratory symptoms and
- Lung function between baseline and week 30 (end of study).
Adverse Events
These were monitored by counting participants for each type of AE each time an event occurred. An AE was considered serious if it was life-threatening, resulted in death or hospitalization, prolonged an existing hospitalization, caused persistent or significant disability, or significantly disrupted the patient's ability to perform normal life functions.
Sample Size and Power Calculations
As no data are available in the literature on the efficacy of varenicline for gradual cessation in patients with COPD who are poorly motivated to quit, our sample size calculation was based on the cessation rates observed for abrupt cessation in patients with COPD motivated to quit smoking receiving a combination of varenicline and smoking cessation counseling.20 In this population, continuous abstinence rates of 42.3% at 3 months were reported. Given the low motivation and the low level of cessation support, lower rates are expected in our population. For a projected 6-month cessation rate of 15% in the abrupt cessation group versus 30% in the gradual cessation group, 121 patients per group were needed.However, the study was stopped prematurely because the study sponsor, Pfizer Inc., USA, voluntarily recalled all batches of varenicline due to elevated nitrosamine levels.
Statistical Analysis
Statistical analysis was performed using the Stata (version 16) statistical software (Stata Corp, Texas) The primary analyses were carried out on an intention-to-treat basis where participants lost to follow-up are presumed to be smoking (i.e., non-abstainers).
Descriptive Statistics: Baseline demographic and clinical characteristics of the participants were described according to the study groups: the test group (gradual cessation) and the control group (abrupt cessation). In order to test the main hypothesis that in smokers with COPD with low motivation to quit treated with the standard 12-week varenicline treatment gradual cessation produced higher quit rates at 6 months than abrupt cessation if varenicline is used to assist pre-treatment reduction, continuous abstinence was cross tabulated with the study group and tested using a 1-sided Fisher exact test. Mixed models, i.e., models with both fixed and random independent variables, were fitted to analyze the different outcomes, with the patient ID as a random effect. Such models take into account the within participant correlations in the analysis of longitudinal data and are less sensitive to the dropouts than analyses carried out at each visit. Being an abstainer was analyzed using a mixed logistic model with visit and study group fitted in interaction. With respect to secondary outcomes, cessation motivation, and daily number of cigarettes, these outcomes were analyzed using a linear mixed model with study group fitted in interaction with the visit. Similarly, the test of the study group on the changes of the indices of lung function and respiratory symptoms was done using linear mixed models with visit and study group in interaction. P values <0.05 are considered significant.
Results
Participants Disposition
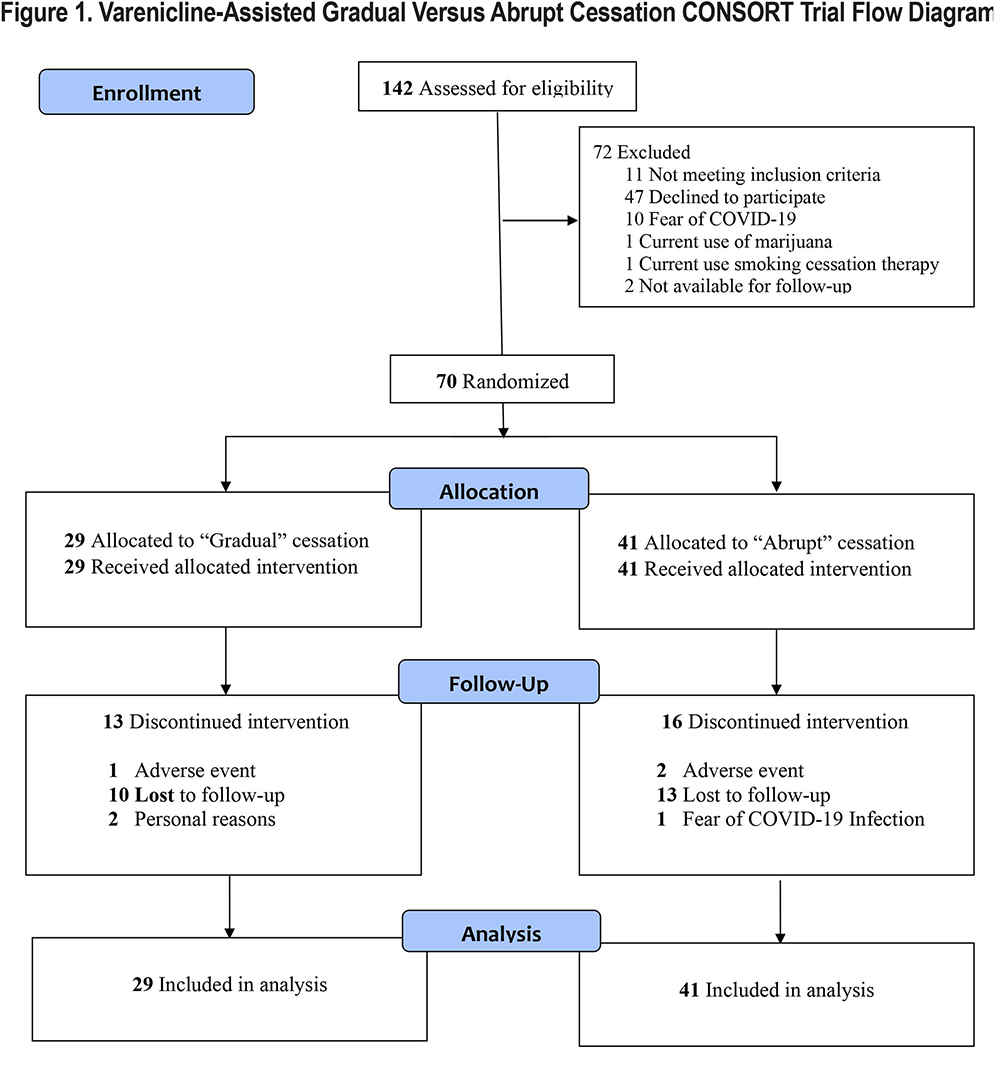
The flow of participants through the study is shown in the CONSORT diagram depicted in Figure 1. At the time of premature termination, 70 of 142 (49.3%) patients who responded to our invitation had been randomized either to gradual (n=29) or abrupt (n=41) cessation. The imbalance in the number of participants in the 2 groups is explained by the fact that the random assignment assumed a sample of 242, not 70, participants.
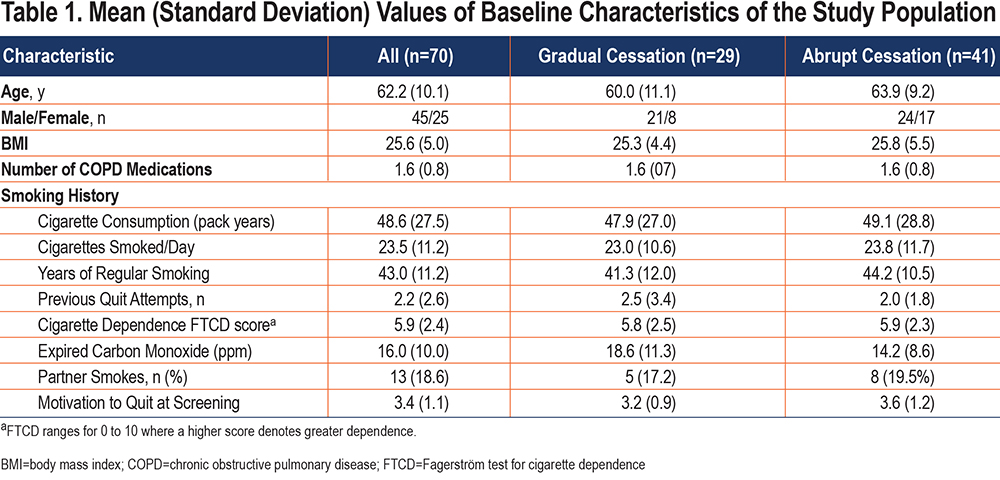
Baseline Characteristics
As shown in Table 1, participants assigned to study groups were similar at baseline. The premature interruption probably explains the slight imbalance in the proportion of females (41.5% versus 28%) in favor of the abrupt cessation group. No differences were observed between the 2 groups in the other variables, including smoking variables. Incidentally, mean cigarette consumption was high, almost 50 pack years, while mean motivation to quit smoking at the pre-enrollment screening interview was low (<4 pts) in the 2 groups.
Primary Outcome: Continuous Abstinence
Table 2 shows 24-week continuous abstinence rates for both groups. Intent-to-treat analysis, which assumes that missing participants are active smokers, shows that better outcomes for the gradual cessation group were significant at week 30 (p=0.048).
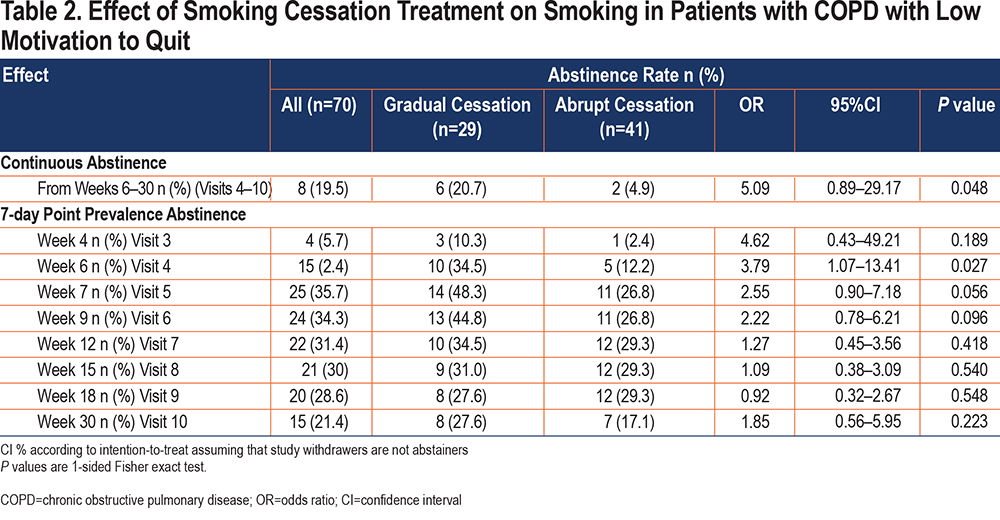
Secondary Outcomes
7-Day Point-Prevalence Abstinence: 7-day PP rates are shown in Table 2. Overall, there was a trend toward better cessation rates for the gradual cessation group at all visits except visit 9 (week 18- end of treatment). The between-group differences were significant at visit 4 (week 6) (p=0.027) and of borderline significance at week 7 (visit 5) (p=0.056) and week 9 (visit 6) [p=0.096].
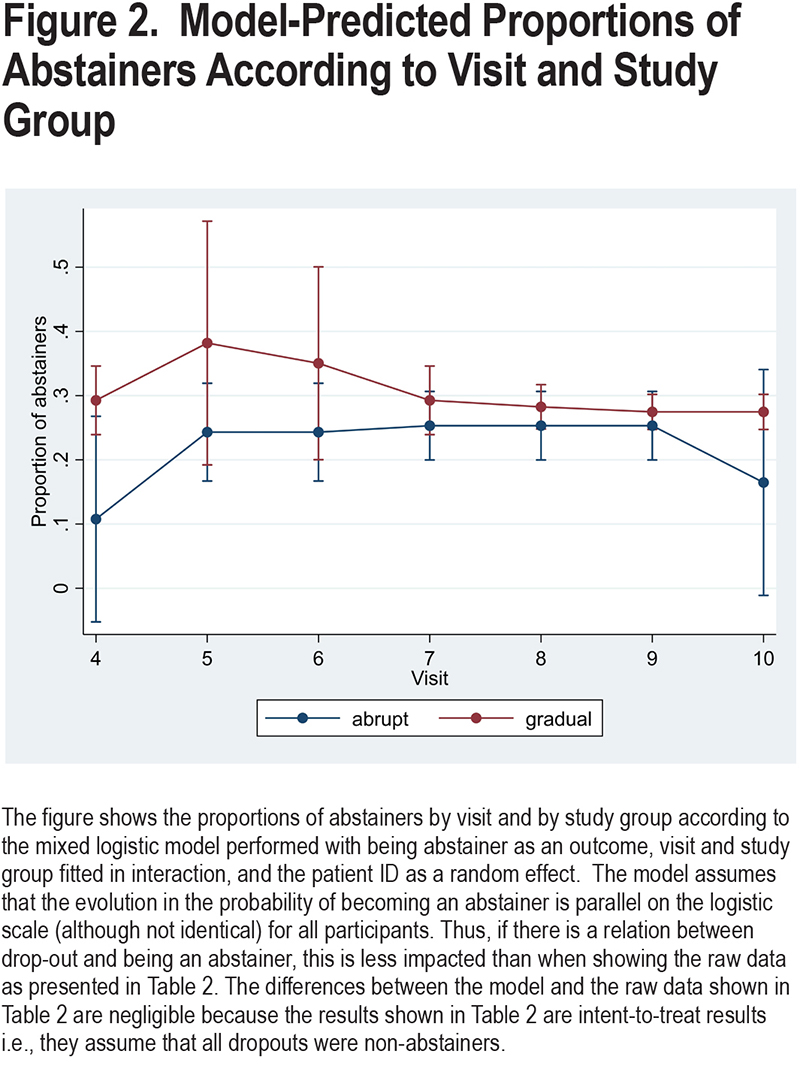
Figure 2 shows the proportions of abstainers by visit and by group, predicted by the mixed logistic model performed with being abstainer as an outcome, visit and study group fitted in interaction, and the patient ID as a random effect. As can be seen, the probability of becoming an abstainer is greater for smokers included in the gradual cessation group than in the abrupt cessation group.
Effective, Pre-Quit Smoking Reduction: In total, 30%-35% of patients achieved efficient smoking reduction in the pre-quit phase with no significant differences being noted between the 2 groups. At week 2 (visit 2) there were 22 effective reducers, 11 in each group. At week 4 (visit 3): there were 25 effective reducers, 12 in the gradual cessation group, and 13 in the abrupt cessation group. Finally, at week 6 (visit 4 TQD) there were 24 effective reducers, 8 in the gradual cessation group, and 16 in the abrupt cessation group. The between-group differences were not significant.
Motivation to Quit: Figure 3 shows the evolution of motivation to quit smoking across the trial for the 2 groups.There was a steady increase in average values in both groups from baseline onwards, with higher values in the abrupt cessation group at all visits except visit 6 (TQD). While the differences in the evolution between the 2 groups were not significant, for each group the increase across the visits was significant (p=0.002).

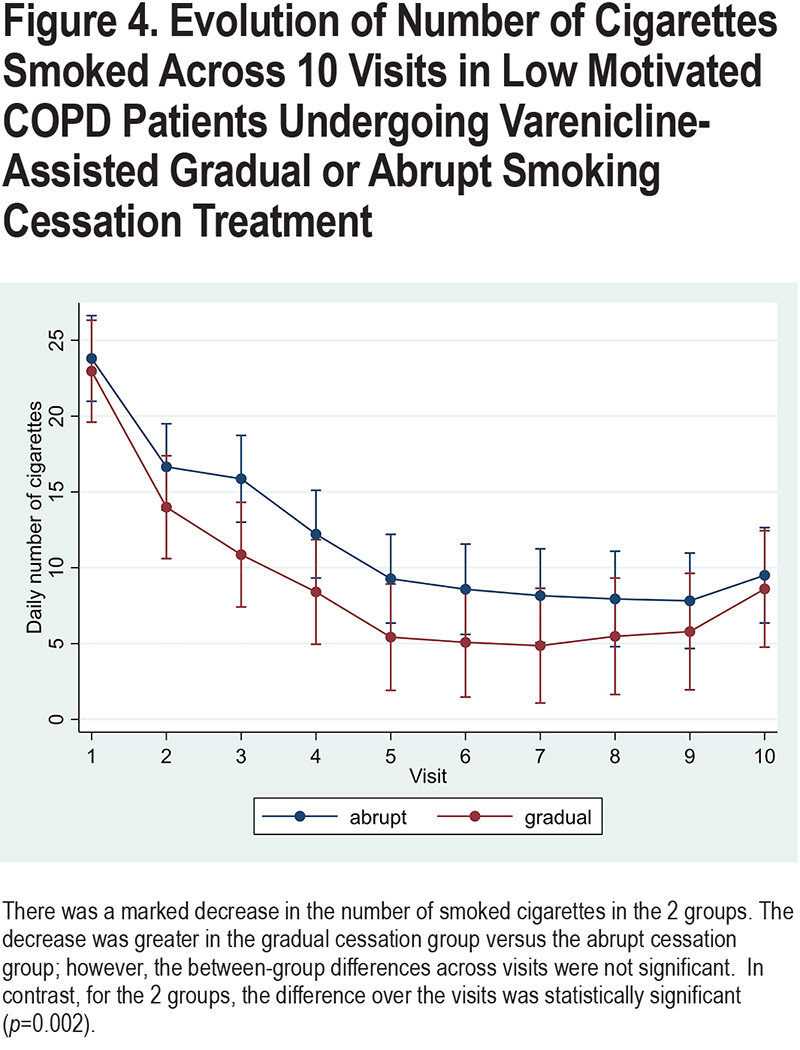
Number of Daily Cigarettes: Figure 4 shows the evolution, across the visits, of the number of daily cigarettes smoked by each group. There was a marked decrease in the number of daily cigarettes in the 2 groups, mainly in the gradual cessation group. While no significant differences between the 2 groups were noted; for each group, the decrease in the number of cigarettes across the study was significant (p=0.002).
Additional Outcomes
Respiratory Symptoms: Table 3 shows that there was a decrease in mean (standard deviation [SD]) CAT score from 18.8 (8.7) points, observed at baseline, to 14.7 (10.3) observed at week 30. Analysis by group showed that this decrease was similar in the 2 groups although the mean score values remained higher in the abrupt cessation group versus the gradual cessation group.
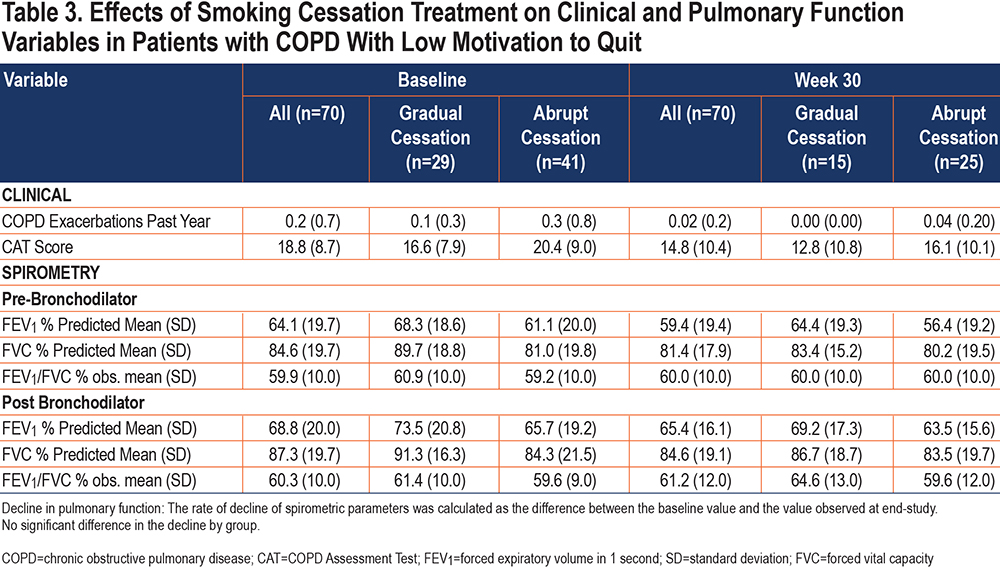
Spirometry: Table 3 shows that the severity of airway obstruction remained constant in the population as a whole between baseline (mean [SD] FEV1/FVC=59.9% [10.0]) and week 30 (FEV1/FVC [%]=60.0% [10.0%]). Analysis by group showed a similar trend, with no between-group differences. Comparison restricted to abstainers (not shown) showed no significant difference between the values of FEV1, FVC, or FEV1/FVC observed at enrollment versus those observed at week 30.
Adverse Events
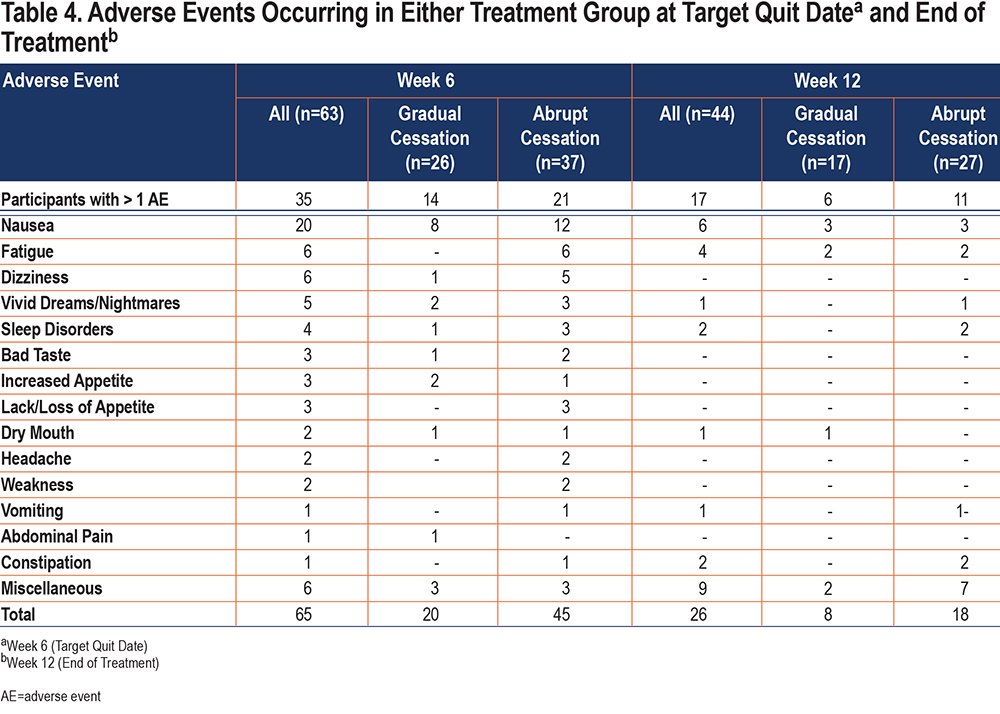
As shown in Table 4, patients in the gradual cessation group reported AEs on 28 occasions: 20 at week 6 (TQD) and 8 at week 12 (end of treatment) while patients in the abrupt cessation group reported AEs 63 times: 45 at week 6 (TQD) and 18 at week 12 (end of treatment) (p=NS). Overall, there were 35 patients with more than 1 AE at week 6 (TQD) and 17 at week 12 (end-of-treatment) (p=NS). Nausea was the most frequent adverse event in both groups (gradual cessation=30.1%; abrupt cessation=32.4%). There were 6 serious AEs: 5 in the gradual cessation group and 1 in the abrupt cessation group. In theory, 2 of these serious AEs could have been related to the drug study, but this was unlikely. In the first case, participant 2, a 62-year-old man with 62 pack years of tobacco consumption, presented with a clinical picture of coronary artery disease treated by percutaneous coronary intervention with stent implantation. The second, a 72-year-old man with 120 pack years of smoking, presented with a possible stroke. All participants with serious AEs recovered uneventfully.
Discussion
In this study, varenicline-assisted gradual cessation for 6 weeks prior to TQD, as opposed to ad libitum smoking for 5 weeks followed by varenicline titration for 1 week prior to TQD, significantly increased continuous abstinence from week 6 to week 30, and point-prevalence abstinence at week 6 in patients with COPD with initially low motivation to quit smoking. In both study arms, motivation to quit smoking increased significantly from baseline to week 30, with no significant difference between the 2 groups. Consistent with the study design, patients in the gradual cessation group significantly reduced the number of cigarettes smoked per day from baseline to week 30. However, although they were not instructed to do so, participants in the abrupt cessation group also reduced the number of cigarettes smoked per day. Although it was less marked than in the gradual cessation group, this reduction was high enough to make the difference between the groups nonsignificant. Overall, the results suggest that varenicline-assisted cessation is beneficial in patients with COPD with poor initial motivation to quit smoking.
To our knowledge, there is no research similar to this study for comparison purposes. In fact, there are very few studies of pharmacotherapies for smoking cessation in patients with COPD, and even fewer studies examining the effectiveness of varenicline in this population. As of 2016, only 16 studies had been published on this topic, of which only 1 used varenicline.20 Subsequently, a few more studies on the use of varenicline in patients with COPD have been published21-24 none of which examined the efficacy of varenicline-assisted gradual cessation. The paucity of smoking cessation RCTs in patients with COPD can be explained by several factors, including higher tobacco consumption, higher prevalence of psychiatric disorders, lower self-efficacy to abstain from smoking,25,26 higher cigarette dependence and lower motivation to quit smoking,27 and tendency to smoke when requested to stop7; however, a detailed analysis of such factors is outside the scope of this study. Incidentally, studies of varenicline for gradual cessation prior to smoking cessation in the general population have shown improved quit rates versus placebo.10,11,17,28
The better smoking cessation results in the gradual cessation group could be due to the increase in post-baseline motivation to quit. Indeed, while motivation has been classically associated with attempted cessation but not with maintenance of abstinence, later studies have shown that motivation can predict abstinence.29 The rapid increase in motivation in the gradual cessation group is consistent with the concept that motivation is a fluctuating dimension that can vary even over a short period of time. In this context, it could be postulated that because of its partial agonist effect –whereby it activates the a4b2 cholinergic nicotine receptor while blocking the effects of smoking on this receptor—varenicline might have produced fewer withdrawal symptoms and less pleasure in the gradual cessation group, resulting in increased confidence and motivation. (This mechanism was suggested to us by Karl Fagerström, PhD, Sweden.)Alternatively, varenicline may have produced a reduction in pre-cessation rewards and cravings that persisted into the post-cessation period, in a pattern consistent with a reinforcement-reduction mechanism similar to that described recently in smokers from the general population.30 However, in the present study, we did not assess the cravings and reinforcing effects of smoking, so we can only speculate on this issue. Although appealing, the hypothesis of a varenicline-mediated increase in motivation is contradicted by the fact that in the gradual cessation group, motivation increased early, even before varenicline was administered, and that a similar increase – albeit less marked—was also observed in the abrupt cessation group, although this group did not receive varenicline until week 6 of the study. A final possibility is that the significant difference in quit rates between the 2 groups is simply the result of the low continuous abstinence rates observed in the abrupt cessation group. Indeed, the long 5-week pre-titration before TQD could have produced a disengagement effect in this group, leading to dropouts, relapse, and uncontrolled smoking. However, disengagement is usually caused by a loss of motivation and, as mentioned earlier, motivation increased throughout the study in the abrupt cessation group as well.
Consistent with previous studies,31 we found a clinically meaningful decrease in CAT scores (mean 4 points) in the 2 groups, while the number of exacerbations fell to zero in the gradual cessation group and decreased by a factor of 7.5 in the abrupt cessation group. Since this improvement was disproportional to the continuous abstinence rates of the 2 groups (Table 2), we speculate it was underpinned by the reduction in cigarette consumption. Finally, also in agreement with previous studies,31 no significant changes were found in spirometry at week 30 in either group.
In this study, varenicline was safe and well accepted for gradual cessation in patients with COPD, with a safety profile similar to that of previous studies.13,30 Nausea was the most common AE in both groups, while the reported serious AEs were unlikely to be treatment-related and all patients recovered without problems.
Strengths of this study include the rigorous execution according to a pre-specified published protocol, as well as the randomized, controlled design. In addition, we were able to recruit 50% of potential participants despite the fact that the start of the study coincided with the onset of the coronavirus disease 2019 (COVID-19) pandemic, which presented an additional barrier to recruitment. Finally, our use of a 5-ppm CO threshold for abstinence was important because reducers may have CO values between this value and the usual 10-ppm threshold and may be incorrectly counted as abstainers if the 10ppm threshold is adopted.30
This study has limitations. The small sample size, due to the unexpected drug recall by the study sponsor, reduced the ability to demonstrate differences between the 2 groups. Thus, the power of the study is lower than originally expected. This means that, if the results had been identical for the participants who were to be included as the results among the included participants , some of the non-significant observed trends would have become statistically significant. On the other hand, we must emphasize that the results that were found to be statistically significant in this smaller-than-expected study are not affected by the size of the study because the statistical analyses are based on the actual sample and, therefore, take into account the number of participants. By consequence, the small sample size does not invalidate our findings. A second limitation was the use of a single measure of motivation. Indeed, motivation is a broad, multidimensional, and fluctuating dimension that cannot be captured by a single instrument or scale and is best examined using a variety of tools.29 However, such an assessment was beyond the scope of this study. In fact, we chose the VAS because it is practical and useful if used to assess willingness to make a quit attempt. Finally, it could be argued that this study is limited by the lack of a placebo condition. However, it would have been unethical to assign patients with a serious disease to a placebo condition. In addition, we did not study the effect of varenicline per se, but rather the effect of a method of drug administration. Therefore, as previously recommended,32 we tested the new treatment (gradual cessation) as an add-on to the existing proven treatment with varenicline (abrupt cessation), so that even in the control condition, smokers were receiving proven treatment. Incidentally, this strategy tends to decrease the sensitivity of gradual cessation, an effect that reinforces the significance of the observed differences.
This is not the first smoking cessation trial to be stopped prematurely. Although they have mobilized far greater resources than ours (300 community pharmacies in the United Kingdom,33 11 hospitals in France34 and 17 centers in Canada35) in the last 3 years alone, 3 RCTs have been stopped prematurely, having recruited only 3.5%, 30%, and 77% of their respective target population. Nevertheless, all 3 studies provided useful clinical information that justified their publication. Incidentally, it is important to publish prematurely completed RCTs to avoid publication bias, provide useful data for meta-analyses, and, especially, recognize the valuable contribution of participants (and research staff) who may have volunteered their time in the hope of contributing to the advancement of medicine and improved medical care for others.
In conclusion, although stopped prematurely, this clinical trial showed that the use of varenicline as an aid to gradual smoking reduction before cessation was potentially useful in increasing abstinence rates in smokers with COPD with low initial motivation to quit. Although further studies are needed to corroborate our results, our study is important because there is an urgent need for new smoking cessation strategies for COPD patients, as smoking cessation is the only treatment capable of halting disease progression and increasing survival.
Acknowledgments
Author contributions: AB, PW, AR, BP, YF-D, PB, and GI had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis. Study conception and design was provided by AB. Randomization monitoring was provided by PB and YF-D. Collection of follow-up data was provided by AB, BP, AR, GI, and NA. Data analysis and interpretation was provided by AB and PW. Statistical analysis was provided by PW. AB drafted the manuscript with help (PW). All authors revised the manuscript for important intellectual content.
The authors are grateful to Karl Fagerström, PhD, for his helpful comments and suggestions, and for the critical revision of the manuscript. They also thank the patients for their participation.
Data sharing: Data are available from the corresponding author upon reasonable request after authorization from the Shaare Zedek Helsinki Ethics Committee.
Declaration of Interests
The authors have no interests to declare.