Running Head: Spirometry Underutilization in Tertiary Centers
Funding support: This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grants KL2TR002492 and UL1TR002494 (AKB). The views expressed in this article are those of the authors and do not reflect the views of the United States Government, the Department of Veterans Affairs, the National Institutes of Health, the National Institutes of Health’s National Center for Advancing Translational Sciences or any of the authors’ affiliated academic institutions.
Date of Acceptance: August 25, 2022│ Published Online Date: August 30, 2022
Abbreviations: chronic obstructive pulmonary disease, COPD; adjusted odds ratio, aOR; confidence interval, CI; Charlson Comorbidity Index, CCI; Veterans Health Administration, VA; VA Corporate Data Warehouse, CDW; International Classification of Diseases-Ninth Revision, ICD-9; ICD-Tenth Revision, ICD-10; Rural-Urban Community Area, RUCA; area deprivation index, ADI; Veterans Integrated Services Networks, VISN
Citation: Baldomero AK, Kunisaki KM, Bangerter A, et al. Beyond access: factors associated with spirometry underutilization among patients with a diagnosis of COPD in urban tertiary care centers. Chronic Obstr Pulm Dis. 2022; 9(4): 538-548. doi: http://doi.org/10.15326/jcopdf.2022.0303
Online Supplemental Material: Read Online Supplemental Material (371KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States and is a leading cause of disability and health care utilization.1-3 The physiologic hallmark of COPD is irreversible airflow obstruction, but clinical history and physical examination findings are not reliable indicators of the presence of airflow obstruction.4 To assess airflow obstruction, guidelines recommend spirometry to confirm a diagnosis of COPD in patients with respiratory symptoms.5-7 Spirometry is recommended among patients with respiratory symptoms and appropriate history and is required to diagnose COPD. Despite guideline recommendations, only a third of patients with a diagnosis of COPD receive confirmatory spirometry.8,9 Lack of spirometry results in both under- and overdiagnosis leading to suboptimal management.10-13 Underdiagnosis causes delayed initiation of appropriate therapies. Overdiagnosis leads to higher health care utilization and prescription of unnecessary therapies which could result in adverse side effects and health care costs.10-14
Prior studies cite poor access to spirometry as a partial explanation for underutilization of spirometry,15-19 but it is unclear how factors other than access contribute to underutilization. To address this, we aimed to identify individual (demographic, socioeconomic, health status, and health care utilization) and regional (geographic region of the tertiary care facility) factors that are associated with underutilization of spirometry among a cohort of patients who are less likely to face widely-recognized barriers to access (lack of insurance or high out-of-pocket costs, long travel to the service, and receiving care where the service is not available). To reduce the role of lack of insurance and out-of-pocket costs as barriers, we studied only patients enrolled in the Veterans Health Administration (VA). To reduce the impact of travel, we limited our analyses to urban dwellers. Finally, we limited our sample to patients who received their primary care in facilities that had spirometry available on site.
Methods
Patient Sample
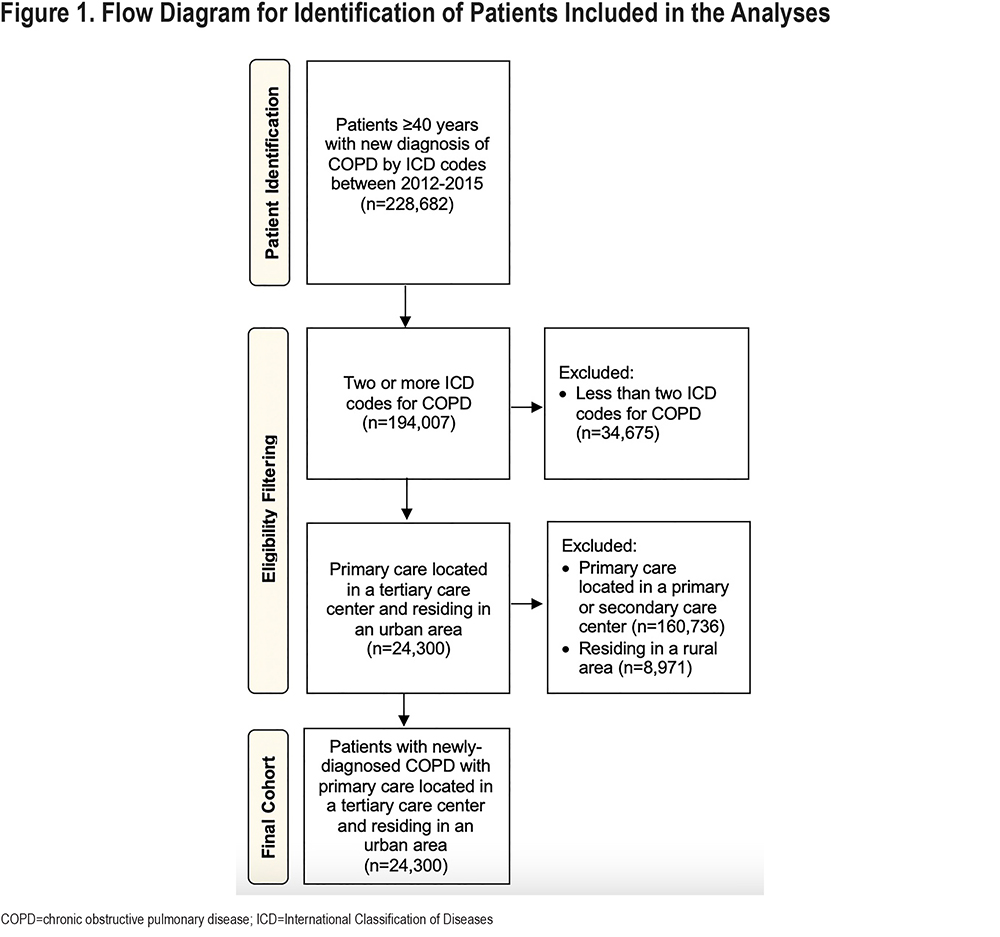
We conducted a retrospective cohort study of patients enrolled in the national VA with a new diagnosis of COPD between January 2012 and December 2015. We collected administrative data from the VA Corporate Data Warehouse (CDW). We included patients who were ≥40 years and had at least 2 visits with International Classification of Diseases-Ninth Revision (ICD-9) or Tenth Revision (ICD-10) codes (ICD-9: 490, 491.XX, 492.XX, 496 or ICD-10: J40, J41.X, J42, J43.X, J44.X) for COPD (Supplementary Table 1 in the online supplement).20 The date of the first COPD diagnosis code was assigned as the index date.
Among this COPD cohort, we then excluded patients whose primary care occurred anywhere other than a tertiary care center. Designation of tertiary level center is assigned to VA facilities with advanced specialized services which include, but are not limited to, cardiac surgery, neurosurgery, or organ transplant, with an academic affiliation for specialized training programs, and are capable of performing pulmonary function tests.21 Each patient’s geocoded residential address was designated as urban, rural, or highly rural by the VA Planning Systems Support Group using the Rural-Urban Commuting Area (RUCA) codes (version 2010) where urban is defined using RUCA codes 1.0 or 1.1.22-24 We excluded patients who were not designated as urban dwellers.
Dependent Variable
Spirometry was identified by Current Procedural Terminologycodes (94010, 94014, 94015, 94016, 94060, 94070, 94150, 94200, 94250, 94375, 95070, 94620, or 95071) or a pulmonary function test VA clinic stop code (stop code 104) within 2 years (before or after) of the index date. Clinic stop codes are 3- or 6-digit identifiers used by the VA to track outpatient visits to reflect the type of outpatient care and record workload. We used the VA outpatient files to identify spirometry received in the VA, while non-VA care program integrity tools and fee basis files were accessed to identify VA-reimbursed spirometry performed in non-VA settings.
Covariates
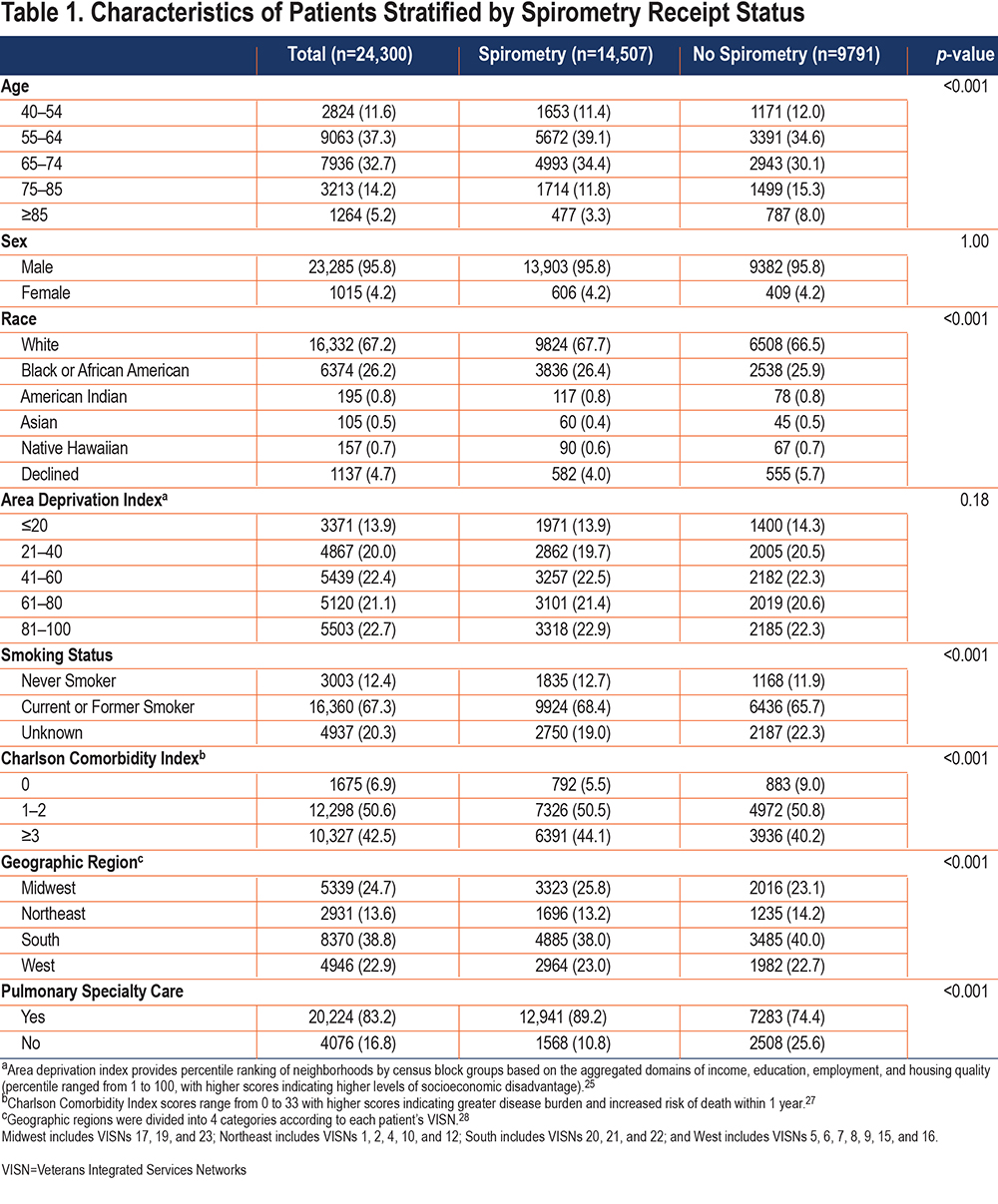
To account for the potential associations between demographic characteristics and spirometry utilization, we included age (40–54, 55–64, 65–74, 75–84, and ≥85 years), sex (male versus female), and self-reported race (White, Black or African American, American Indian, Asian, or Native Hawaiian) as covariates. As a proxy for socioeconomic disadvantage, we used the area deprivation index (ADI), which provides percentile ranking of neighborhoods by census block groups based on the aggregated domains of income, education, employment, and housing quality (percentile ranged from 1 to 100, with higher scores indicating higher levels of socioeconomic disadvantage).25 The ADI was categorized into ≤20, 21–40, 41–60, 61–80, 81–100 for analysis.
We used smoking status and the Charlson Comorbidity Index (CCI) as covariates26 to assess the associations between health status and spirometry utilization. Smoking status, dichotomized into never smoker or current or former smoker status, was determined using health factors, a data field in the VA electronic health record that is used in routine clinical care.27 CCI was used to quantify health status.26 CCI scores range from 0 to 33 with higher scores indicating greater disease burden and increased risk of death within 1 year.26 CCI was categorized into 0, 1–2, and ≥3 for analysis. We also included specific comorbidities (congestive heart failure, coronary artery disease, chronic kidney disease, diabetes mellitus, and obesity) in sensitivity analyses.
To account for the potential regional variability in clinical practice patterns, we also included geographic regions for the location of the tertiary care facility as a covariate. Geographic regions were divided into 4 categories (Midwest, Northeast, South, and West) according to each patient’s Veterans Integrated Services Network (VISN). VISNs are regional systems of care working together to meet local health care needs and provide access to care.28 The Midwest includes VISNs 17, 19, and 23; the Northeast includes VISNs 1, 2, 4, 10, and 12; the South includes VISNs 20, 21, and 22; and the West includes VISNs 5, 6, 7, 8, 9, 15, 16.
We also included pulmonary specialty care encounter to evaluate the association between involvement of a pulmonary subspecialist and health care utilization with spirometry utilization. We identified patients with at least 1 pulmonary specialty care encounter before or after the index date of their COPD diagnosis. Pulmonary specialty care VA clinic encounters, including in-person, phone, and video visits, were identified (stop codes 312 and 324).
Statistical Analysis
We calculated the percentage of patients who had spirometry (binary outcome). We compared the characteristics of patients who did and did not receive spirometry. We used Pearson’s chi-square tests to identify differences in categorical demographic characteristics between patients with and without spirometry. We used univariable and multivariable logistic regression models to analyze the associations between patient characteristics and receiving spirometry. Using a directed acyclic graph to guide the modeling strategy, we identified the minimum sufficient adjustment variables as age, ADI, and CCI (Supplementary Figure 1 in the online supplement). We used a multivariable logistic regression model that adjusted for these potential confounders and another model that adjusted for all covariates. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute).
Study Oversight
This study was approved and the requirement for informed consent was waived by institutional review boards at the Minneapolis VA Health Care System (VAM-20-00583) and the University of Minnesota (STUDY00011069).
Results
Among 24,300 patients with newly diagnosed COPD who receive primary care in a tertiary care facility and reside in an urban area (Figure 1), 59.7% received confirmatory spirometry within 2 years of the initial COPD diagnosis.
Patients ≥75 years old had lower rates of receiving spirometry than patients <75 years (48.9% versus 62.1%, p<0.001). Compared to patients with a CCI score of ≥3 (61.9%), patients with a CCI score of 0 (47.3%) or a CCI score of 1–2 (59.6%) had lower rates of spirometry (p<0.001, overall test). Additionally, patients without a pulmonary specialty care visit had lower rates of undergoing spirometry compared to those who had a pulmonary specialty care visit (38.5% versus 64.0%, p<0.001) (Table 1). By VISN regions, the rate of spirometry was: Midwest 62.2%, Northeast 57.9%, South 58.4%, and West 59.9% (p<0.001, overall test).
In multivariable logistic regression analyses adjusted for all covariates, older age, lower comorbidity burden, and not having had a pulmonary specialty care visit were associated with lower odds of undergoing spirometry (Table 2). Patients aged 75–84 years had an adjusted odds ratio (aOR) of 0.80 (95% confidence interval [CI]: 0.72–0.92) while patients ≥85 years had an aOR of 0.47 (95% CI: 0.40–0.54) of receiving spirometry compared to patients <55 years. Compared to patients with a CCI of ≥3, patients with a CCI of 0 had an aOR of 0.60 (95% CI: 0.54–0.67) and patients with a CCI of 1–2 had an aOR of 0.95 (95% CI: 0.90–1.01). Sensitivity analysis also showed higher odds of spirometry utilization in patients with comorbid conditions such as obesity (aOR 1.14 [95% CI: 1.06–1.22]) and congestive heart failure (aOR 1.08 [95% CI: 1.00–1.16]) (Supplementary Table 2 in the online supplement). Patients without a pulmonary specialty care visit also had lower odds of receiving spirometry (aOR 0.38 [95%CI: 0.35–0.41]). Compared to patients in the Midwest VISN regions, patients in the Northeast (aOR 0.79 [95% CI: 0.72–0.87]) and Southern (aOR 0.90 [95% CI: 0.84–0.97]) VISN regions had slightly lower odds of receiving spirometry. Although race and smoking status were not associated with receipt of spirometry, patients who had declined to identify their race or had an “unknown” smoking status, had a slightly lower likelihood of not undergoing spirometry than White individuals or those with “never smoker” status, respectively.
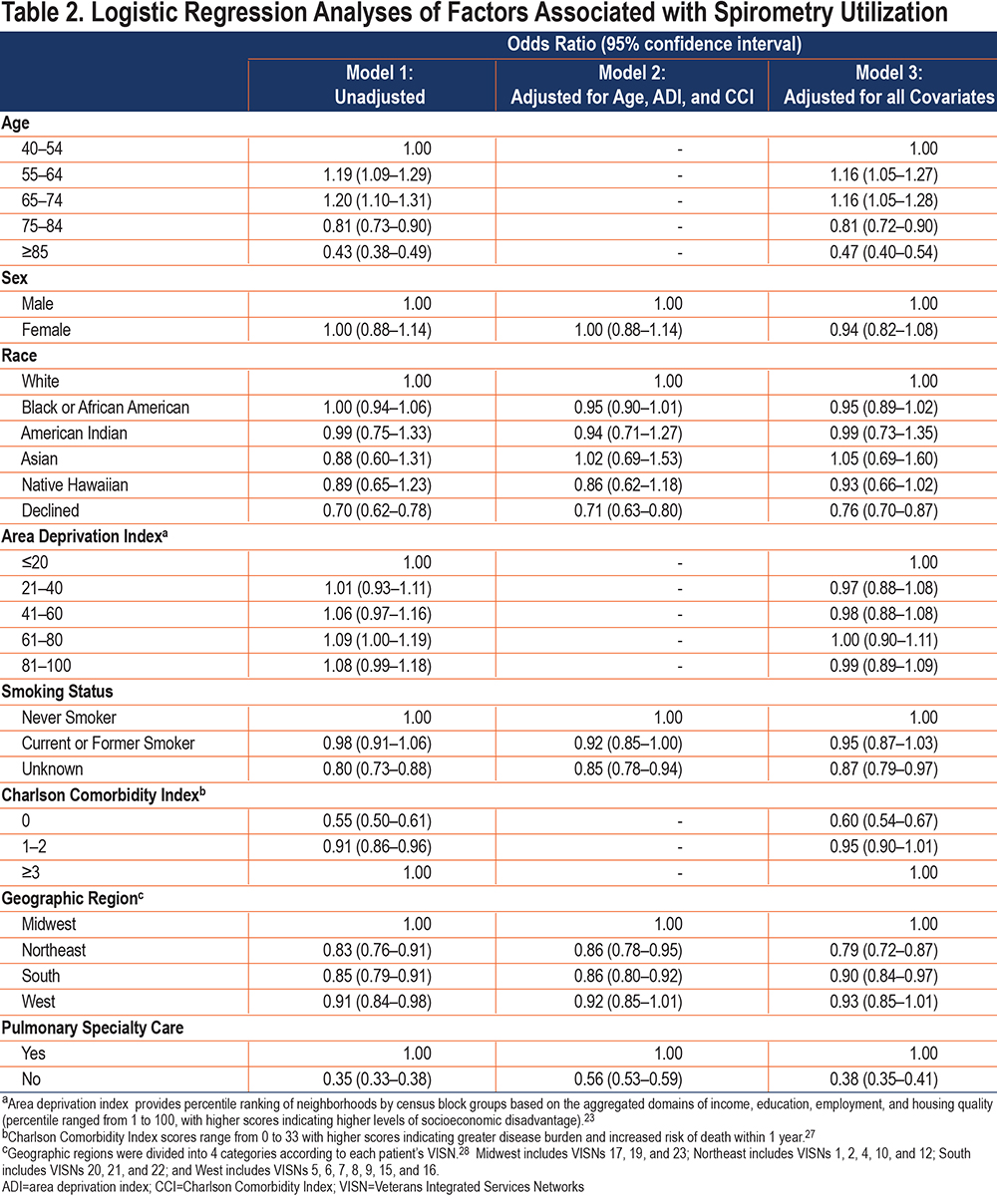
Similarly, in models that adjusted for age, ADI, and CCI, patients without a pulmonary specialty care visit also had lower odds of receiving spirometry (aOR 0.56 [95%CI: 0.53–0.59]), while sex and race were not associated with receiving spirometry.
Discussion
Spirometry underutilization persists among patients suspected of having COPD, including for those who seem to have minimal typical barriers to accessing spirometry. We find spirometry underutilization is associated with older age, not having had a pulmonary specialty encounter, and lower comorbidity burden. The percentage of patients undergoing spirometry, although higher than prior reports, remains low, suggesting that other factors will also need to be addressed to optimize spirometry utilization.
Previous studies that have evaluated barriers to utilization of spirometry found multiple patient-, provider-, and health system-related factors.12-13 Inadequate access to spirometry has been cited as a barrier.15-19 To investigate factors while minimizing the potential contribution of access barriers, we evaluated patients who receive care from the VA, live in urban areas, and were assigned to primary care in a center that offers spirometry. In this cohort, the proportion of patients with a diagnosis of COPD who had spirometry is higher than prior reports (59.7% versus 30% across different health plans in the United States).8,9 Nonetheless, spirometry is still underutilized in our cohort, suggesting that expanding access alone is not sufficient to address underutilization. Other investigators have suggested that potential contributors to underutilization may include lack of training in COPD management and lack of knowledge about guideline recommendations, insufficient time, and competing priorities during primary care visits.29,30 COPD care quality initiatives may need to address these other factors, in addition to spirometry access, to improve spirometry utilization.
Among individual-level characteristics, we found that older age was associated with lower spirometry utilization. Compared to younger patients, COPD patients who were ≥75 years have lower odds of receiving spirometry, consistent with findings from Lee et al9 and Han et al.8 Not performing spirometric confirmation of COPD among older patients may lead to a higher risk of misdiagnosis than among younger patients, since older patients are more likely to have other potential explanations for chronic dyspnea than younger patients. Older patients also are at higher risk of medication side effects, so the risks to empiric therapeutic trials without first confirming a COPD diagnosis are also higher. Prescription of unnecessary therapies will result in harm, including adverse events and higher health care costs. There is also no evidence to suggest that the quality of spirometry declines with increasing age.31,32
We also found that lower comorbidity burden is associated with underutilization of spirometry. Prior studies have reported mixed results.9,33 In a large national sample of veterans, Lee et al9 showed slightly higher odds of spirometry for patients with underlying hypertension, heart disease, cancer, depression, and arthritis, whereas Joo et al, 33 who evaluated patients from a single center with smaller sample size, found no association between comorbidities and spirometry utilization. The reason for higher rates of spirometry among patients with underlying comorbidities is unclear but could be related to more health care engagement compared to patients with lower comorbidities.
Prior studies have shown that pulmonary specialty care is associated with having spirometry.9,33 In our study, patients who had a pulmonary encounter were more likely to receive spirometry than those who did not have a pulmonary encounter. This may be because pulmonologists are more familiar with COPD guidelines34-36 or have fewer competing, non-respiratory priorities during visits.
Joo et al37 found a 3-fold difference in spirometry utilization among individual VISNs within the VA. We also found variations in spirometry use among geographic regions, though not as large. This may have been because we only included patients receiving primary care in tertiary care facilities, likely resulting in less variability in facility characteristics.
Our study suggests that expanding access to spirometry alone will not be sufficient to address spirometry underutilization. Quality improvement initiatives addressing guideline adherence, particularly among older patients, patients with lower comorbidity burden, and patients who are not receiving pulmonary specialty care may be necessary.
Our data cannot explain why spirometry is underutilized for certain patient groups. Researchers could adopt mixed methods, using both quantitative and qualitative assessments, to explore why spirometry is underutilized among certain patient groups and focus on addressing these responses. Additionally, urban location does not guarantee that there are no travel issues. Future research could address this, for example, by directly asking patients about the time they must allocate to travel to the clinical site.
Our study has important limitations. First, we did not have access to spirometry from Medicare data, but a prior study has reported only 4% of patients were misclassified as not having spirometry based on VA data alone.9 Therefore, misclassification of spirometry status from Medicare is unlikely to significantly impact the results of our study. However, we also did not have data from private insurance. By using pulmonary function testing clinic stop codes, it is possible that we misclassified patients who received arterial blood draws and 6-minute walk tests as having undergone spirometry, which would result in even lower spirometry rates. Patients also could have undergone spirometry outside our ascertainment period (that is, more than 2 years before or after the index diagnosis date), but our objective was to evaluate spirometry utilization around the time of a new diagnosis of COPD. Additionally, by using ICD codes without confirmation of airflow obstruction, it is possible that some patients were mis- or over-diagnosed with COPD. Our analysis does not include rural patients; our findings may not be generalizable to other undeserved patient populations.Finally, we used the VA definition of urban which only includes census tracts with a metropolitan area core. However, using the more restrictive VA definition of urban should have led us to include patients whose residential addresses were closer to their clinic sites. Thus, using the VA’s urban definition was most consistent with our goal of studying spirometry underutilization among patients with fewer barriers to accessing care.
Conclusion
Spirometry underutilization persists among COPD patients with minimal access barriers to receiving confirmatory spirometry and is associated with older age, absence of pulmonary specialty care, and lower comorbidity burden. COPD care quality initiatives will need to address other factors, in addition to spirometry access, to improve spirometry utilization.
Acknowledgments
Author contributions: AKB, KMK, and RAD conceived the current analysis. AKB, KMK, RAD, and DBN designed the analysis. AKB obtained funding. AB acquired the data. DBN provided statistical expertise. AKB and DBN performed the data analysis. AKB and RAD drafted the manuscript. All authors provided critical input and revised the manuscript for important intellectual content and approved the final manuscript. All authors take responsibility for the integrity of the data and the accuracy of the data analysis.
This material is the result of work supported with resources and the use of facilities at the Minneapolis VA Health Care System.
Declaration of Interest
KMK reports personal fees from Nuvaira (data safety and monitoring board) and Allergan (consulting). All other authors declare that they have no conflict of interests related to this study.