Running Head: Patient-Reported Pulmonary Symptoms, Exacerbations
Funding Support: This study was funded by an unrestricted research grant from AlphaNet.
Date of Acceptance: September 7, 2022 | Published Online Date: September 14, 2022
Abbreviations: alpha-1 antitrypsin deficiency, AATD; chronic obstructive pulmonary disease, COPD; health-related quality of life, HRQoL; Step Forward Study, SFS; body mass index, BMI; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC
Citation: Choate R, Sandhaus RA, Holm KE, Mannino DM, Strange C. Patient-reported pulmonary symptoms, exacerbations, and management in a cohort of patients with alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis. 2022; 9(4): 549-561. doi: http://doi.org/10.15326/jcopdf.2022.0317
Online Supplemental Material: Read Online Supplemental Material (430KB)
Introduction
Exacerbations of chronic obstructive pulmonary disease (COPD) are episodes of acute worsening of pulmonary symptoms that often require a change or increase in regular medications.1,2 Exacerbations are common in individuals with COPD and have a high burden on health-related quality of life (HRQoL) and health care resource utilization.2-4 Exacerbations are the most frequent cause of physician visits, hospitalizations, and mortality in patients with COPD.5
Alpha-1 antitrypsin deficiency (AATD) is an autosomal codominant disorder characterized by an increased risk for early-onset COPD and pulmonary emphysema.4,6,7 Studies have shown that exacerbations are common in patients with AATD-related lung disease and often manifest as changes in their regular pulmonary symptoms like dyspnea, sputum volume, and color changes.8 Patients with PiZZ AATD, one of the severely deficient genotypes of AATD, tend to have frequent pulmonary exacerbations and often require management with antibiotics and/or steroids.8,9 Although studies have been published on this issue, it remains under evaluation whether exacerbations are more frequent in AATD-related COPD patients compared to non-AATD COPD.8,10,11 Some research has found that patients with AATD-related COPD have roughly twice the exacerbation frequency compared to non-AATD COPD individuals,8 while other research has found no differences between these groups with regard to exacerbation frequency.12 Prospective randomized studies of augmentation therapy with plasma-derived alpha-1 antitrypsin infusions prescribed to treat lung disease due to AATD have not shown that this therapy decreases the incidence and severity of exacerbations or lowers the odds of exacerbation-related hospitalizations.13,14 A single retrospective case series on AATD-associated COPD has shown a decrease in exacerbation severity and hospitalization risk after initiation of augmentation therapy.15
Considerable efforts have been made to reach an agreement and standardize the definition of exacerbations. There are 2 broad definitions of exacerbations that are based either on patients' symptoms or definitive events. Symptom-based definitions of exacerbations rely on patient-reported changes in usual respiratory symptoms that frequently prompt contact with health care professionals for a diagnostic assessment. Some of the challenges related to this definition are that patients can experience a wide range of pulmonary symptoms and have a heterogeneous clinical presentation that may not be recognized as an exacerbation.16 Hence, in some cases, the symptom-based definition may underestimate exacerbations due to under-reporting,17 whereas, in other cases, it may overestimate exacerbations due to symptoms related to coexisting conditions.18 Event-based definitions capture changes in treatment (antibiotics and/or steroids) or hospitalizations and aim to circumvent the challenges related to recognizing symptoms.16 However, event-based definitions of exacerbations may lack patients' perspectives in identifying events with the highest burden of symptoms.16
In this study, we aim to examine and describe patients’ perspectives in recognizing changes in usual respiratory symptoms as exacerbations and identify a potential gap in knowledge and barriers in reporting exacerbations in a cohort of patients with AATD.
Methods
The study population consisted of subscribers of AlphaNet, a not-for-profit health management organization for individuals with AATD in the United States.19 AlphaNet coordinators, individuals with AATD who have received specialized training, connect AlphaNet subscribers to various disease-related resources and provide regular support. Data collected by AlphaNet coordinators via regular monthly telephone calls to AlphaNet subscribers were used in this study.
The monthly survey data collected from a group of AlphaNet subscribers who participated in the Step Forward Study (SFS) were used in the present analyses. The SFS was a 5-year, prospective, double-blind, randomized interventional study designed to examine the effectiveness of an intensive exercise and dietary program to improve physical activity and body mass index (BMI) over time compared to standard care. Some of the inclusion criteria into SFS were: (1) males or females age at least 18 years at the time of entry, (2) diagnosis of AATD, (3) participant in AlphaNet program because of prescription for alpha-1 antitrypsin augmentation therapy, and (4) evidence of pulmonary disease with 1 or more of the following: (a) a forced expiratory volume in 1 second (FEV1) measure of less than 80% predicted and an FEV1 to forced vital capacity (FVC) ratio less than 0.70, (b) emphysema on a previous CT scan of the chest, and/or (c) receiving augmentation therapy for lung disease.20 The methodology and findings of the study are described elsewhere.20 Monthly survey data collected from the SFS participants during the first year of the study, between October 2009 and October 2010, were used in these analyses.
During the monthly telephone calls, AlphaNet coordinators asked the subscribers about changes in their usual respiratory symptoms (example: "Have you experienced any changes in your usual respiratory symptoms since the last time we talked?"). The 7 respiratory symptoms included in the survey were shortness of breath, cough, fever, sputum color and amount, and new or worsening wheezing. Participants who reported changes in 1 or more of their usual respiratory symptoms during 1 year were included in the study. For the purposes of these analyses, a positive response to changes in 1 or more respiratory symptoms was defined as a "symptom event." Each participant of this study contributed between 1 and 12 events of changes in respiratory symptoms in 1 year.
AlphaNet subscribers who reported changes in any respiratory symptoms during the monthly call were also asked whether they considered these changes a pulmonary exacerbation (example: "Have you had significant worsening of your lung problems [an exacerbation or flare]?). Participants who identified having an exacerbation were asked about its management, including increased or new inhaled medication, a burst of steroids (oral or injected), antibiotics, or increased oxygen.
An overlap between changes in 3 respiratory symptoms – increase in shortness of breath and change in sputum color and its amount – was examined considering the clinical significance of these symptoms in identifying exacerbations and guiding management by Anthonisen criteria.21 Sub-analyses of exacerbation management using steroids and antibiotics included participants who reported at least 1 exacerbation in which there was a change in these 3 symptoms.
The study was approved by the Western Institutional Review Board (approval #1107247) and the University of Kentucky Institutional Review Board (#43435).
Statistical Analysis
SAS 9.4 was used to perform all statistical analyses (SAS Institute, Cary, North Carolina). Data were evaluated using patient-level and event-level analyses. Patient-level descriptive statistics were computed for select demographic and clinical characteristics of the participants in the overall study sample and stratified by the presence and number of exacerbations in 1 year. All results were reported as frequencies and proportions for categorical variables and as means (±SD) for continuous variables.
Event-level analyses of changes in respiratory symptoms and management of lung disease were performed in the overall cohort and stratified by exacerbation status (yes, no, unknown). Management of the symptom events identified as exacerbations was summarized using descriptive statistics.
Proportional Venn diagrams were constructed to explore the overlap of changes in 3 respiratory symptoms: increase in shortness of breath and change in sputum color and amount. The association between the overlap of changes in the above 3 symptoms and events identified as exacerbations and their management was evaluated using additional Venn diagrams.
Results
Patient-level Analyses
During the 1-year follow-up, between October 2009 and 2010, 316 SFS participants reported changes in 1 or more usual pulmonary symptoms and were included in this study (Figure 1). The average age of the study participants was 58.2±9.7 years, 53% were female, 67% were married, and the majority (88%) had a PiZZ genotype. Participants of this study were equally distributed across the SFS randomization arms (standard care, 50.3% versus intervention group, 49.7%) (Table 1).

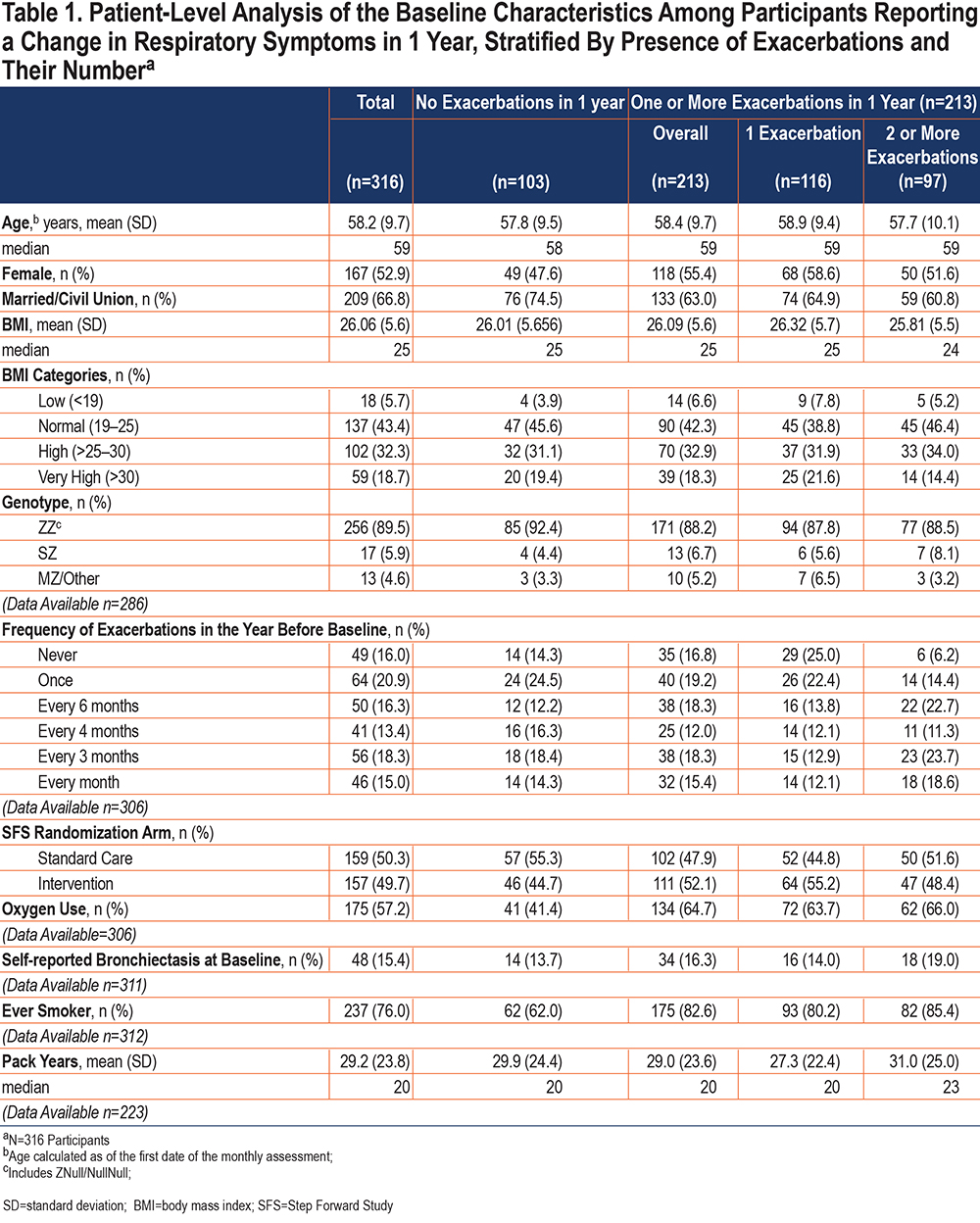
A third of the 316 participants (n=103, 33%) did not report having an exacerbation in 1 year. Of the remaining 213 individuals who recognized having 1 or more exacerbations, 55% (n=116) had 1 exacerbation, and 45% (n=97) had 2 or more exacerbations in 1 year. Table 1 shows the distribution of select baseline characteristics of the study participants by the number of exacerbations in 1 year. Supplemental Table 1 in the online supplement shows the distribution of select baseline characteristics of individuals who were included in analyses as compared to individuals who were excluded from analyses due to reporting no changes in pulmonary symptoms during the study period.
Event-level Analyses
Overall, 316 participants reported 797 events of changes in their usual respiratory symptoms during 1 year. The most prevalent were an increase in shortness of breath (75% of all events of symptom changes), an increase in cough (69%), and an increased sputum amount (53%) (Table 2). The average number of symptoms per event was 2.7±1.5 (range 1–7). Almost half (48%, n=380) of the reported 797 events of changes in the usual respiratory symptoms were identified by the participants as exacerbations. On average, the participants reported being sick for 12 ± 8.3 days during these exacerbations. Of the 380 events recognized as exacerbations, most of them (81%) were managed by taking antibiotics, over half (53%) were managed by taking steroids (Table 3). Both antibiotics and steroids were used in 41% of the exacerbations. Over 38% of the symptom events (n=308) were not recognized as exacerbations, and exacerbation status was missing for 14% of events (Table 2).
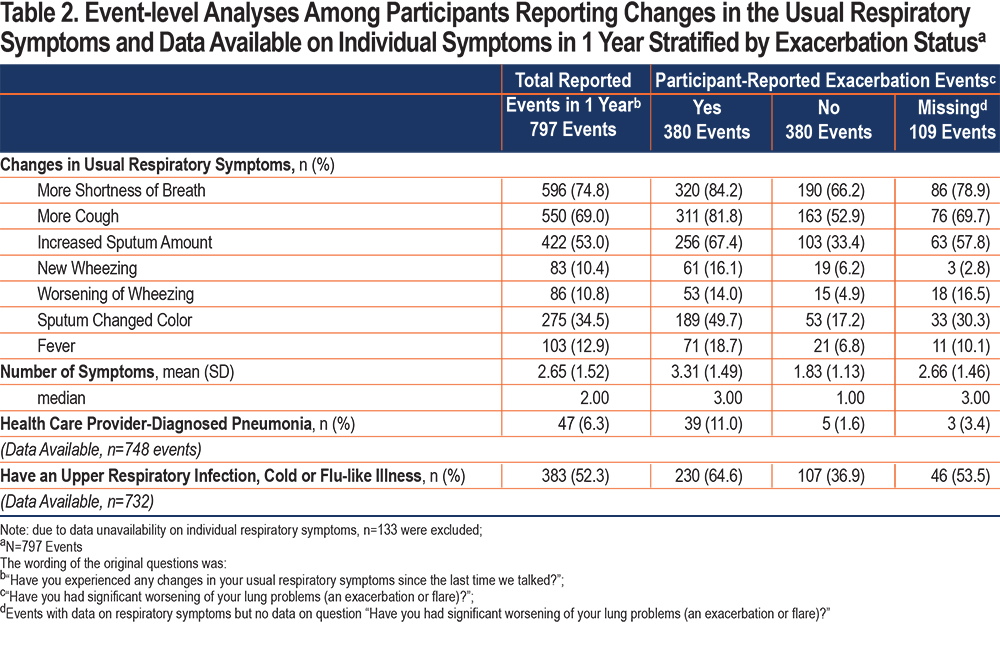
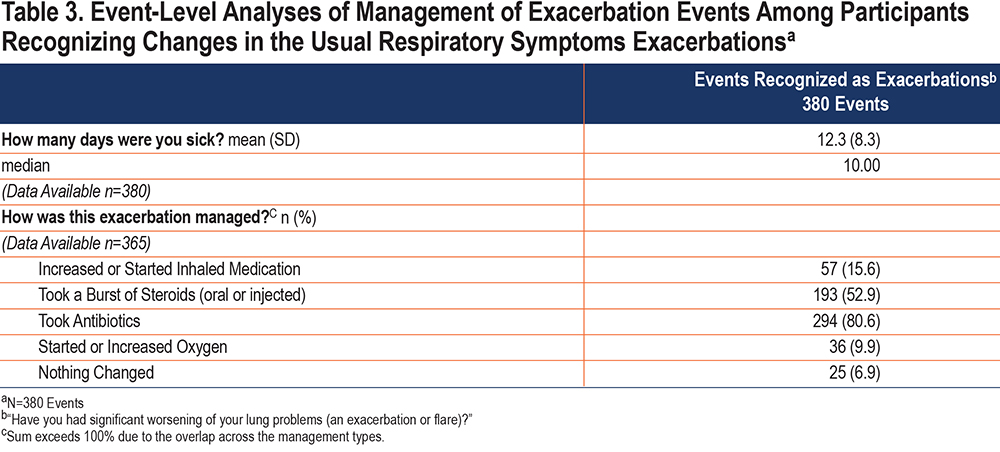
The symptom events identified by the participants as exacerbations had, on average, a higher number of respiratory symptoms compared to those not identified as exacerbations (3.3±1.5 versus 1.8±1.1). The symptom events identified as exacerbations were mainly managed by consulting with a pulmonary specialist (39% of the events) or going to a doctor's office (37%) (Table 4). Participants mainly "treated the problem themselves" (56%) during the symptom events not identified as exacerbations (Table 4). In 17.6% of the symptom events recognized as exacerbations, participants went to an emergency department or urgent care center compared to 4.2% of the events not identified as exacerbations.
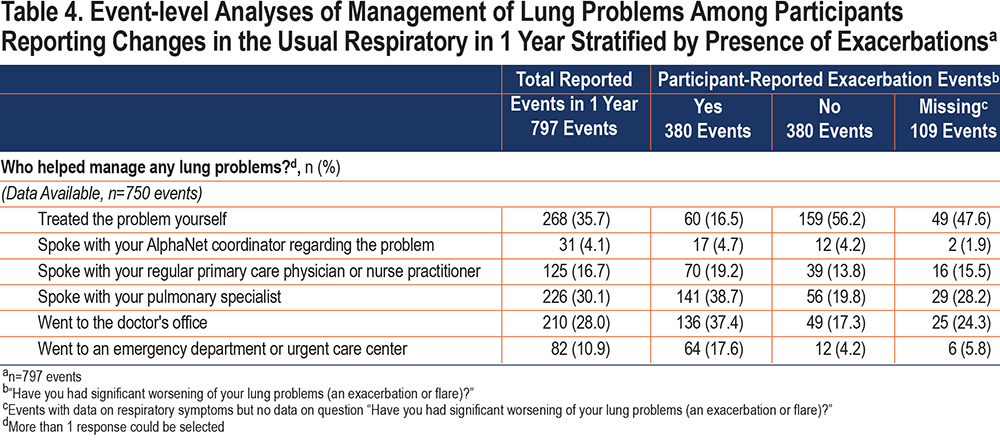
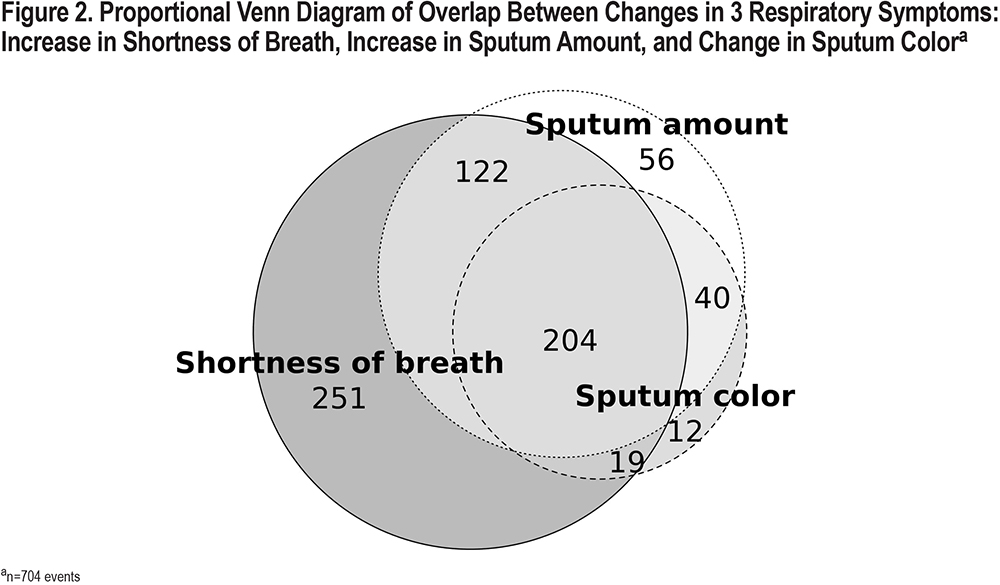
Of the 797 events of changes in any respiratory symptoms, 88% (n=704) of events indicated changes in 1 or more of the following 3 symptoms: increase in shortness of breath, increase in sputum amount, and change in sputum color. The proportional Venn diagram examining the overlap between the above symptoms showed that 204 (29%) had all 3 symptoms concurrently (Figure 2). About a third of these 3-symptom events (36%, n=251) involved shortness of breath only, 8% increased sputum amount only, and 2% change in sputum color only.
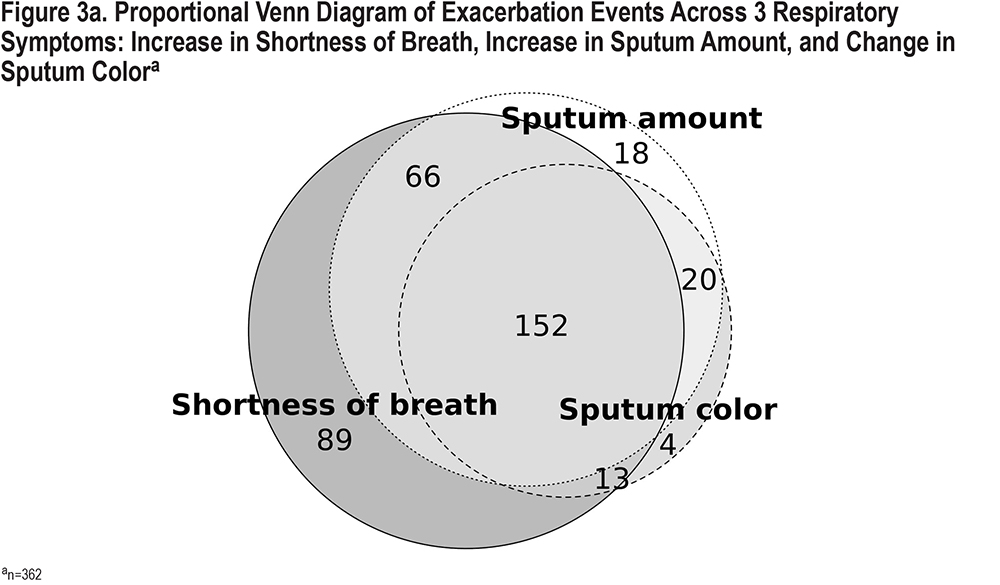
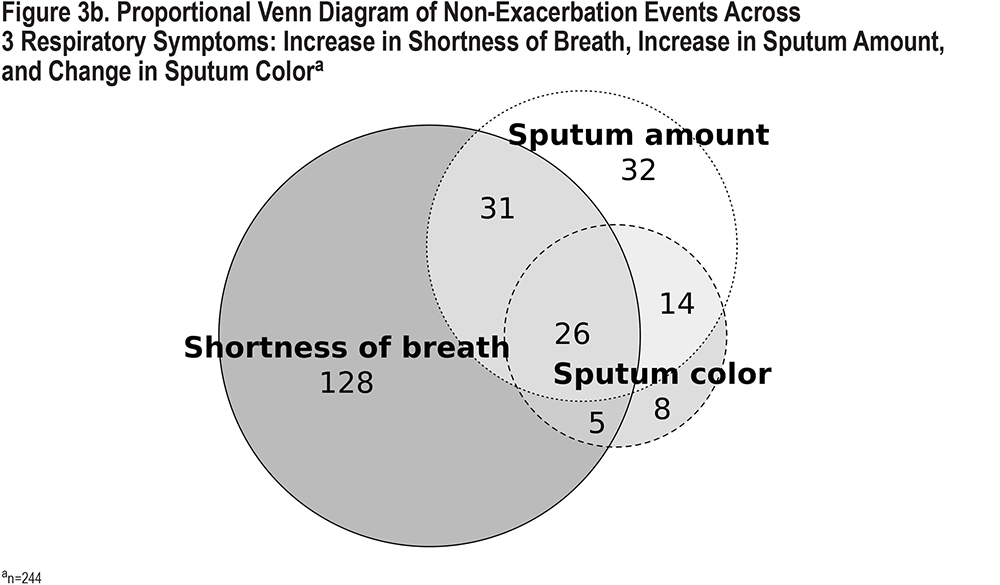
Over half of the 704 events with any of the 3 symptoms (51%, n=362) were identified as exacerbations. Three-quarters (75%, n=152) of the 204 events with all 3 concurrent symptoms were recognized as exacerbations. Of the remaining 25% of the events with all 3 concurrent symptoms, approximately half were not identified as exacerbations, and half had unknown or missing responses (Figures 3a and 3b).
More than half (55%) of the events with 2 of the 3 Anthonisen symptoms and about a third (35%) of the events with 1 of the 3 symptoms were identified by the participants as exacerbations. Eighty-seven percent of the exacerbation events (n=314) were managed with either antibiotics or corticosteroids. Of those, 41% were managed with antibiotics only, 11% with corticosteroids only, and 48% with both (Supplemental Figures 1 and 2 in the online supplement).
Discussion
Exacerbations of COPD are extremely common in AATD.6,8 A high background exacerbation frequency allows studies like the current one to inform differences in patient-reported and health care utilization-reported COPD exacerbations. Using monthly questionnaires, we found that approximately half of all clinical worsening events are missed when assessing exacerbation frequency by antibiotic and/or corticosteroid use. The results of this study have potentially important implications on better understanding the symptom-based definition of pulmonary exacerbations from the patients' perspective.
Often the term exacerbation is not clearly understood by the patients; and some of these acute events are not timely reported and appropriately managed. A multinational study in Europe identified that a large proportion of patients with COPD were not familiar with the word exacerbation and could not recognize these acute worsenings of their symptoms.22 The data used in our study were collected by the AlphaNet study coordinators during regular monthly calls with subscribers. The trained coordinators regularly communicate with the participants and are able to clarify the meaning of the survey questions asking about "worsening of their lung problems,” "flares," or "exacerbations." Patient-perceived recognition of changes in their usual respiratory symptoms as "worsening" or "flare" is critical in identifying pulmonary exacerbations and reporting them to the coordinators.
Symptom-based definitions of exacerbations rely primarily on patient-reported changes in usual symptoms. Previous research suggested that patients perceive events of changes in respiratory symptoms differently when not recognizing them as exacerbations.23 These events usually were found to be shorter in length and with less frequently reported dyspnea.23 Our study examined symptoms reported by the participants during the events recognized as exacerbations and not recognized as such. We found a higher proportion of all respiratory symptoms during exacerbation events than those not identified as exacerbations, with a mean reported duration of 12.3±8.3 days. Other studies in COPD exacerbations reported similar or longer durations.1
The classic exacerbation definition by Anthonisen et al is characterized in terms of patient symptoms and defines an episode of increased shortness of breath, sputum volume, and purulence as a "type-1 exacerbation."21 In our study, changes in 1 or more of these 3 symptoms were reported in 88% of the events, and of those, 29% had all 3 symptoms concurrently. This rate is almost identical to the findings by Vijayasaratha et al of type-1 exacerbations (worsening of all 3 symptoms), accounting for 34% of total exacerbations in a cohort of patients with AATD COPD.23,24 In our study, approximately 75% of the symptom events with all 3 of the above respiratory symptoms were identified by the participants as exacerbations, and 13% of these symptom events were not recognized as exacerbations (the status of the remaining events was undetermined). Research shows that not all symptom-based exacerbations are reported due to factors like not understanding the disease or the importance of seeking treatment.25
Patients' awareness of changes in their usual respiratory symptoms and reporting the onset of exacerbations is critical, so that diagnostic assessment takes place and optimal treatment is started where indicated. Reducing the impact of the current exacerbation and decreasing the occurrence of future exacerbations are the main goals of timely management of pulmonary exacerbations.26 Widely-used Anthonisen criteria guide the use of antibiotics in the management of pulmonary exacerbations.21,27 Research shows that incomplete resolution or persistence of symptoms was identified in 33% of type-1 COPD exacerbations not treated with antibiotics.28 In our study, 77% of the exacerbation events with any of the 3 Anthonisen symptoms and 88% of the events with all 3 symptoms were treated with antibiotics. Short bursts of steroids are often indicated in the management of COPD exacerbations and are shown to reduce the recovery time and lessen the risk of treatment failure.26,29,30 In our study, approximately half (54%) of exacerbation events with any of the 3 respiratory symptoms treated with antibiotics also received a burst of steroids. This is similar to Anthonisen's findings of 42% of exacerbations treated with both antibiotics and steroids.21
The major difference in patient-reported symptoms between events that were reported as exacerbations and those that were not, was the prominence of sputum volume and color change in events called exacerbations. Symptom events not called exacerbations were predominantly those in which worse dyspnea prevailed. Whether this is unique to AATD, a COPD endotype characterized by more emphysema than usual COPD, is unknown. Since patients take bronchodilator inhalers for worsening dyspnea frequently, the designation of exacerbation based on this symptom alone may always be problematic.
Research shows that patients with pulmonary exacerbations have better health outcomes when they recognize exacerbations, seek medical care, and receive appropriate treatment in a timely fashion.31 In our study, the majority of the events of changes in usual respiratory symptoms not perceived by the participants as exacerbations were self-treated at home. And, during the symptom events recognized as exacerbations, most individuals sought medical care. Exacerbations that are not recognized or treated in a timely fashion drive the rates of hospitalizations and health care utilization costs.32
Studies show that exacerbations frequently occur in individuals with AATD-related COPD and are often associated with poor outcomes.6 Augmentation therapy, infusion of plasma-derived alpha-1 antitrypsin, is the only licensed disease-specific treatment aimed to restore its physiological levels.33 Our study participants are subscribers of AlphaNet, a disease management organization, and the vast majority of them receive augmentation therapy. One retrospective study demonstrated that chronic augmentation therapy effectively lowers the incidence of severe exacerbations, which leads to a decrease in hospital admissions and additional concurrent medications.15 Other studies showed no change in exacerbation frequency but suggested a reduction in exacerbation severity with augmentation therapy.34 In short, no study has been adequately powered to study the effect of augmentation therapy on exacerbation frequency.
Reducing the frequency, duration, and severity of exacerbations is essential as they often have a negative effect on disease progression and a patient's HRQoL.8,35 However, the awareness of exacerbations and recognition of these events by patients are not always straightforward. While clinicians strive to establish a standardized definition of exacerbations and measure their effect on health outcomes, patients' perspectives and experiences in recognizing these events might be overlooked.21 Unfortunately, many studies of COPD exacerbations have given up on this important aspect of the COPD experience using patient-reported outcomes and instead use health care utilization methodology to define the benefits of therapies. Our reminder that these studies tell only half of the story is important.
Strengths and Limitations
This study has several strengths. The data collected by AlphaNet provides access to a large population of lung-affected individuals with AATD participating in a disease management program. Critical patient-reported data collected by a unique network of AlphaNet coordinators using monthly questionnaires provided access to a geographically diverse study population.
The findings of this study should be interpreted in light of limitations. First, our study participants are subscribers of AlphaNet, a highly engaged and motivated group of individuals with personalized support through a network of study coordinators who also have AATD. AlphaNet provides education to their subscribers regarding self-monitoring of symptoms, recognition of exacerbations, and exacerbation management. In addition, our study participants may be especially engaged and motivated due to the fact that they were enrolled in the SFS study, an intervention designed to increase physical activity and improve BMI. Therefore, the findings may not be generalizable to other patients with AATD-related lung disease. The study period for the present analyses included the first year of the SFS study and utilized the data collected from the cohort of participants of this intensive intervention trial. Hence, selection bias cannot be excluded. It is important to highlight that the data used in the current analyses were not collected as a part of the SFS and represent regular monthly data collection using standardized questionnaires utilized for all AlphaNet subscribers. As such, we have been careful to report where missing data is present. In addition, recall bias and reporting bias cannot be excluded due to the self-reported nature of the data used in this study. Although, studies utilizing monthly surveys are superior to other studies with less frequent follow-up. In addition, some study participants consulted a medical professional regarding their symptoms prior to participating in their monthly survey, which may have influenced whether the participant defined the increase in symptoms as an exacerbation.
Second, data on treatment with antibiotics and steroids were collected only in those participants who identified changes in their respiratory symptoms to be exacerbations. Thus, we could not evaluate and compare the management of the events not recognized by the participants as exacerbations. The purpose of this study was to provide descriptive, real-world data based on patient-perceived day-to-day changes in their usual pulmonary symptoms and recognition of these symptom changes as significant to the patients’ exacerbations.
Conclusions
Worsenings of usual pulmonary symptoms are recognized as exacerbations in approximately half of an informed AATD population. Worse dyspnea is commonly not called an exacerbation while worsening of sputum volume and color is more commonly designated as an exacerbation event. An improved definition of COPD exacerbations would incorporate both symptom-based and management definitions. Some AATD-related lung disease exacerbations are managed with conventional COPD treatments, including antibiotics and/or corticosteroids. Future research is needed to investigate the effect of AATD-specific management on exacerbation prevention and treatment.
Acknowledgements
Author Contributions: CS and RAS contributed to study conception and design, and data interpretation. KEH and DMM contributed to data interpretation. RC contributed to data analysis and drafted the manuscript, and all authors revised it critically. All authors approved the final version.
This work was funded by AlphaNet, Inc., United States. We are extremely grateful for the superb work of all the AlphaNet Coordinators. We also thank the AlphaNet subscribers who participated in this study.
Declaration of Interest
DMM is a former employee and current shareholder of GlaxoSmithKline, a consultant to AstraZeneca, the COPD Foundation, and Schlesinger Law Firm, and receives royalties from Up-to-Date. RAS has grants to National Jewish Health from the Alpha-1 Foundation, Grifols, Vertex, and the National Institutes of Health /National Center for the Advancing Translational Sciences. He is a consultant/scientific advisor to Dicerna, Grifols, CSL Behring, Takeda, and Vertex and a Medical Director with AlphaNet. KEH has received consulting income from AlphaNet. RC has received research support from AlphaNet. CS has grants in alpha-1 antitrypsin deficiency or COPD paid to the Medical University of South Carolina from Adverum, Arrowhead, AstraZeneca, CSA Medical, Grifols, Nuvaira, Takeda. and Vertex. He is a medical director at AlphaNet. CS has consulted for Bronchus, Dicerna, GlaxoSmithKline, Pulmanage, and Vertex for alpha-1 and/or COPD.