Running Head: Constant Work Rate Endurance: a COPD Database
Funding support: Funding for the COPD Biomarkers Qualification Consortium Constant Work Rate Exercise project was provided by Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, and Chiesi.
Date of Acceptance: August 27, 2022 | Published Online Date: September 1, 2022
Abbreviations: COPD Biomarkers Qualification Consotium, CBQC; chronic obstructive pulmonary disease, COPD; constant work rate cycle ergometry, CWRCE; U.S. Food and Drug Administration, FDA; drug development tools, DDTs; clinical outcome assessments, COAs; endurance time, ET; COnsensus-based Standards for the selection of health Measurement INstruments, COSMIN; body mass index, BMI; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; St George’s Respiratory Questionnaire, SGRQ; 6-Minute Walk Test, 6MWT; oxygen uptake, V̇O2; heart rate, HR; inspiratory capacity, IC; pulmonary ventilation, V̇E; carbon dioxide uptake, V̇CO2; tidal volume, VT; respiratory rate, RR; blood oxygen saturation, SpO2; long-acting muscarinic antagonist, LAMA; long-acting beta2-agonist, LABA; inhaled corticosteroid, ICS; standard deviation, SD; European Respiratory Society, ERS
Citation: Casaburi R, Merrill D, Dolmage T, et al. Endurance time during constant work rate cycle ergometry in COPD: development of an integrated database from interventional studies. Chronic Obstr Pulm Dis. 2022; 9(4): 520-537. doi: http://doi.org/10.15326/jcopdf.2022.0331
Online Supplemental Material: Read Online Supplemental Material (783KB)
Introduction
In 2010, the United States Food and Drug Administration (FDA) issued a draft guidance describing a process by which drug development tools (DDTs) may be qualified for regulatory decision-making; the final guidance was formally adopted in January 2014 and updated in November 2020.1 Concurrent with the publication of the draft guidance, the COPD Biomarkers Qualification Consortium (CBQC) was formed under the management of the COPD Foundation, with the following mandate: (1) to qualify biomarkers and clinical outcome assessments (COAs) through established regulatory processes to facilitate development of new treatments for chronic obstructive pulmonary disease (COPD); (2) to identify biomarkers and COAs for which sufficient data exist to warrant consideration for qualification; and (3) to fill knowledge gaps by facilitating collaborations among global consortia of investigators.2
In 2013, a CBQC working group evaluated opportunities for qualification of COAs as measures of exercise endurance. In a recent publication,3 members of the working group described a conceptual framework for a performance outcome measure associated with exercise endurance, showing: (1) how the disease process that characterizes COPD has meaningful effects on patient symptoms and physical functioning during everyday life; (2) how limitations in physical functioning are directly linked to exercise endurance among people with COPD; and (3) how endurance time during constant work rate cycle ergometry (CWRCE) can be objectively measured, yielding consistent interpretable results. Combining these elements, we concluded that endurance time during CWRCE is an appropriate candidate performance outcome for the measurement of exercise endurance in interventional studies.3
In this follow-up publication, we describe the process by which we assembled an integrated database of endurance time responses of individuals with COPD to potentially endurance-enhancing interventions. To accomplish this, we requested participant-level data from investigators of published studies that incorporated CWRCE as an outcome measure. The primary purpose of the integrated database was to assess the reliability, validity, responsiveness, and interpretability of change in endurance time (ET) during CWRCE, in support of a qualification dossier to be submitted to the FDA within the DDT qualification program. In the spirit of the CBQC mandate to facilitate collaborations among global consortia of investigators, a secondary purpose of the integrated database was to allow academic researchers to consider other research questions which may be addressed through analyses of the database. To this end, this introductory paper provides a detailed and transparent description of the demographics, characteristics, and responses of the analysis population, as a foundation for formal analysis supporting the validation /qualification process and other scientific questions that will be addressed in subsequent publications.
Methods
This analysis was performed using an integrated database consisting of existing data from interventional studies in patients with COPD employing constant work rate exercise tests performed to symptom limitation as an outcome measure, including pharmacological and non-pharmacological interventions.
Literature Search
Screening and Retrieval: This project implemented a literature search, full-text retrieval, and data extraction to provide a comprehensive library of all relevant studies and their reported test results. The search was focused on identifying studies utilizing constant work rate exercise as an outcome in interventional clinical trials.
Consistent with the COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN),4 our search included the primary databases MEDLINE® and EMBASE® as well as CINAHL® and the Cochrane Database®.
The following search strategy was used:
“pulmonary disease, chronic obstructive/ (no limits) OR free text terms (COPD or obstruct* pulmon* or airflow limitation).af.” in all fields
AND
“(constant power or constant work rate or constant-load or constant-power or constant-work rate or [exercise and time] or external* pace* or shuttle walk* or [exercise and intolerance] or [exercise and endurance] or [exercise and tolerance] or [high-intensity and exercise] or [submax* and exercise]).af.”
These terms used in the primary identification search provided a minimally restricted search for potentially relevant studies. We started with a search using MEDLINE® as the primary database. The titles and abstracts were examined. All records that showed the possibility that participants completed at least 1 high intensity constant work rate exercise test, regardless of modality or muscle activity, were marked as “keep.” The remainder were considered as irrelevant and “discarded.” “Kept” records were exported to an Access® database and an EndNote® library, duplicates discarded, and the records marked as “awaiting full text” examination. Using the same procedure, the search continued with EMBASE®, CINAHL®, and the Cochrane Database®. The search was completed in February 2016. The entire reference information was included in the database record including authors, title, journal, year of publication, and registry number/name of substance, registry number, and dates of study data collection. Full-text PDF versions of papers were included with the repository.
We screened (title and abstract) 2993 references and identified 553 papers for retrieval and evaluation for data extraction (see Figure 1).
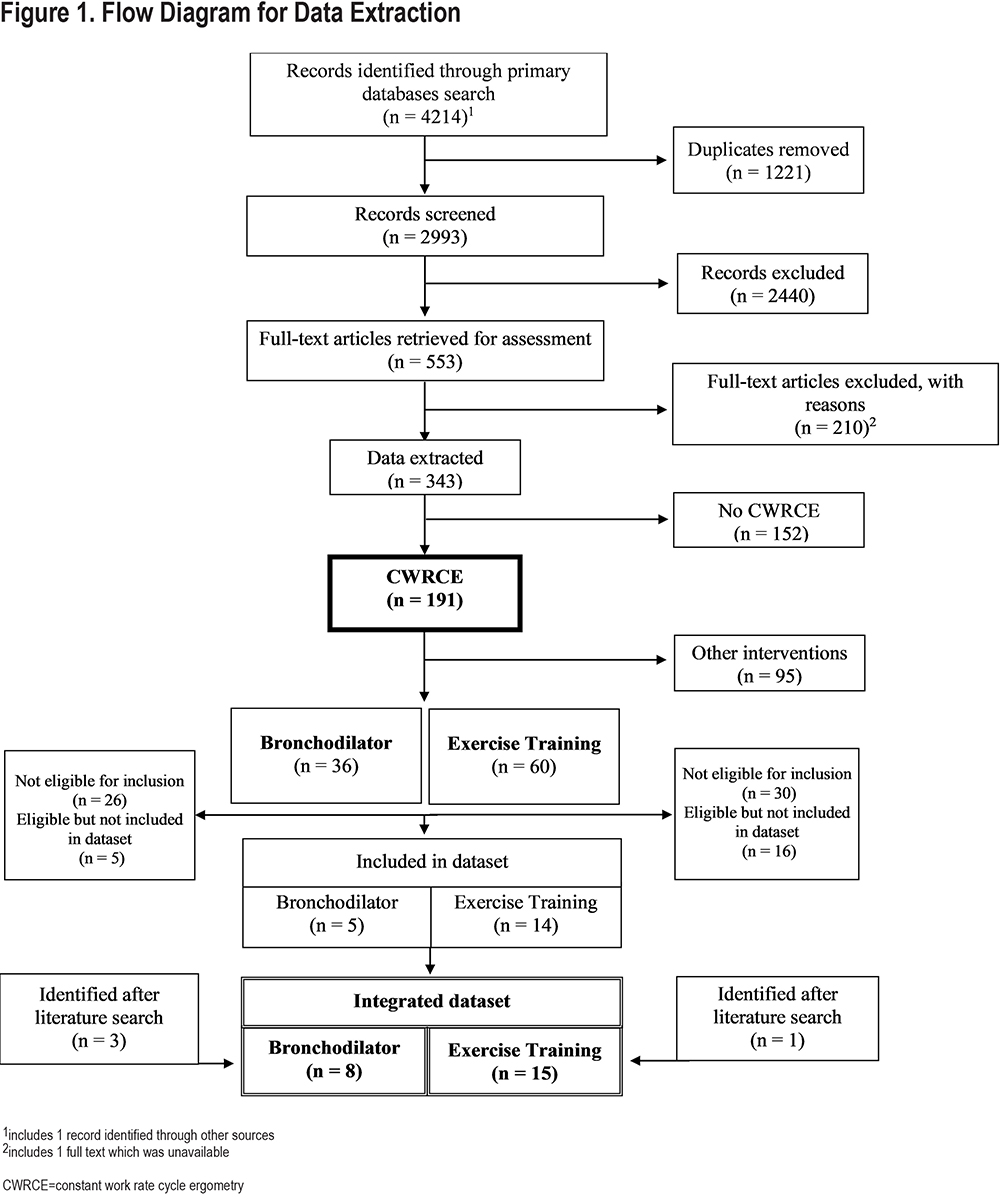
Data Extraction: Data were extracted and recorded in an electronic Access database. No studies were excluded based on quality. The data included codes to identify the type of constant work rate endurance tests used in the study including CWRCE, constant work rate treadmill test, and endurance shuttle walk test. Treatment durations and timing of assessments are also recorded when available.
Cycle Ergometer Study Identification: For the purposes of this paper, only those studies featuring CWRCE were selected for analysis. Too few constant work rate treadmill tests (~500 participants over 2 studies) were found to be suitable for detailed analysis; endurance shuttle walk test studies are the subject of a separate analysis. Of the 343 studies extracted, 191 were found to employ CWRCE (Figure 1). Of these studies, subdivision was performed to identify the interventions employed. Thirty-six studies in which bronchodilator(s) were the intervention(s) and 60 studies in which exercise training was the intervention were identified. Other interventions identified included hyperoxia, non-invasive ventilation, and heliox; since these interventions were acute (single timepoint) in nature and because the number of participants in these studies was relatively low (138, 112, and 35, respectively), they were not included in the analysis.
Of the 36 bronchodilator studies, 26 were judged not suitable for inclusion. Of the 60 exercise training studies, 30 were judged not suitable for inclusion. Reasons for exclusion and identification of excluded studies are provided in the online supplement.
We sought to contact key authors for each of the remaining 10 bronchodilator and 30 exercise training studies. A request was made to provide participant-by-participant anonymized data, in as much detail as available. For 5 bronchodilator studies and 16 exercise training studies, investigators either could not be contacted or reported that participant-by-participant data could not be provided. The online supplement lists all excluded studies, with classification of reasons. For 5 bronchodilator studies and 14 exercise training studies, investigators agreed to participate in data submission.
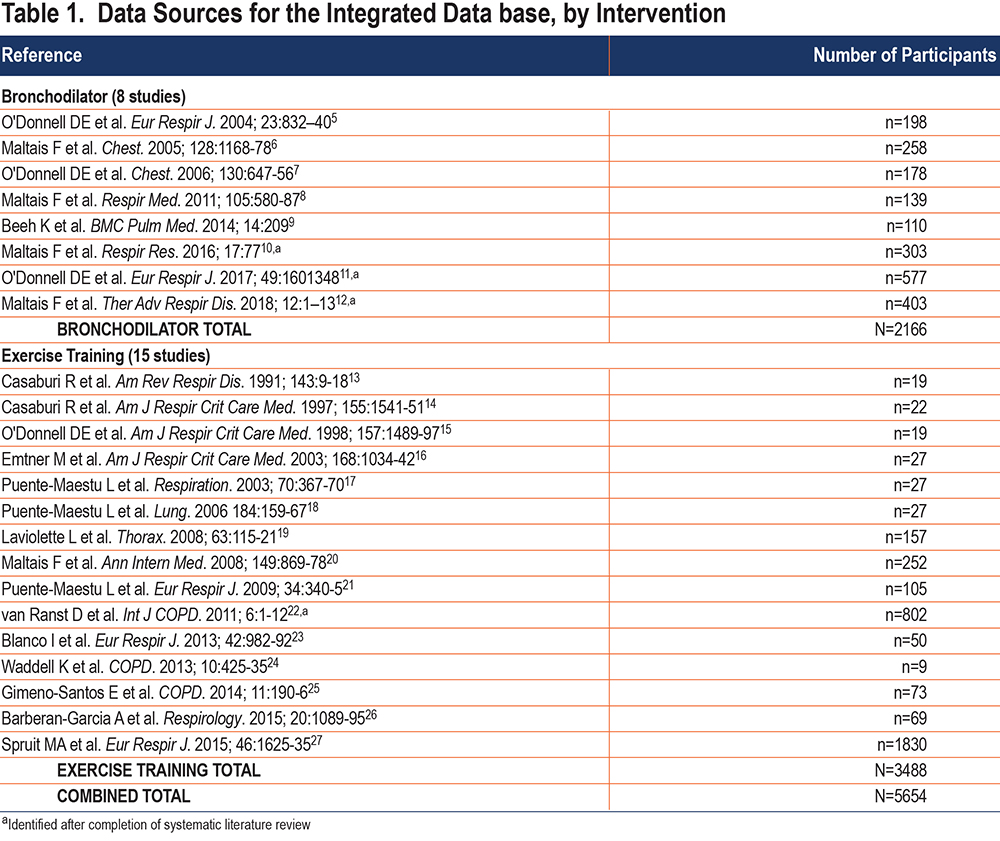
In the course of this process, 3 additional bronchodilator studies (whose data was in the process of being published) and 1 additional exercise training study were identified and included. Table 1 identifies the 8 bronchodilator and 15 exercise training studies included in the final data base.5-27
Integration of Study Data into a Unified Data Base
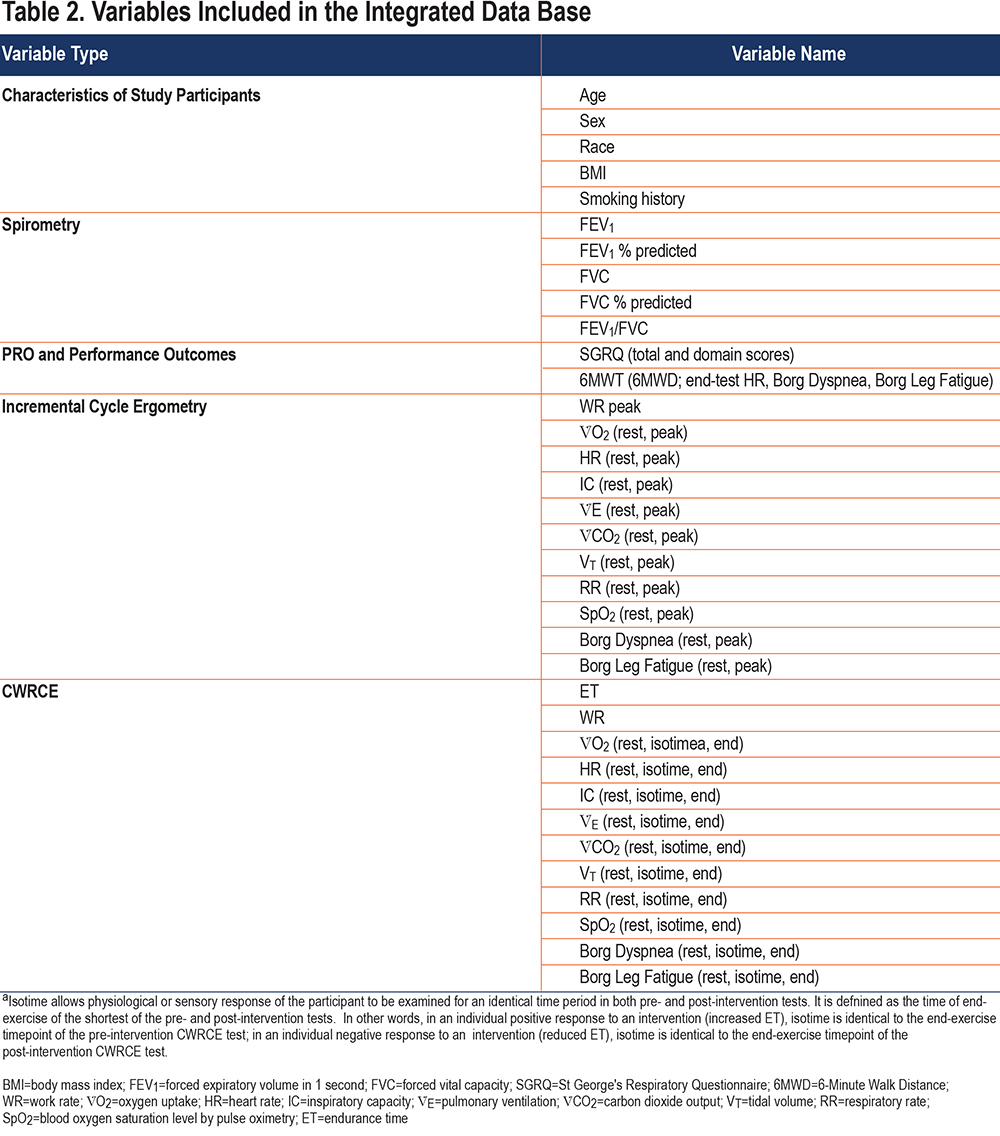
Individual investigators submitted participant-by-participant data, usually in the form of Excel files. Syneos Health (formally INC) was contracted to receive these data files. Syneos Health, assisted by authors R.Casaburi and A. Hamilton, created a data dictionary for all relevant variables and mapping of each source data set to the unified data set was performed. The study team conducted multiple quality control checks to verify that all anticipated variables were included in the database. By consensus of the study team, a set of variables was chosen for further analysis (Table 2). Omitted variables were those that were either present in only a small minority of data sets (e.g., arterial blood gases) or were considered not to be of substantial importance for the subsequent analysis (e.g., end-tidal gas concentrations). Variables included: (1) participant demographics; (2) spirometry results; (3) physiologic and perceptual responses to incremental exercise (recorded at rest and peak exercise); (4) physiologic and perceptual responses to constant work rate exercise (recorded at rest, isotime, and end-exercise, where isotime is defined as the response at the end of the shorter of the baseline and post-intervention tests); (5) St George’s Respiratory Questionnaire (SGRQ) results; and (6) 6-minute walk test (6MWT) responses (Table 2). Transferred data were examined and not included in the analysis if not within physiologically plausible ranges.
Analysis Population
The full analysis population included all participants who had at least 1 pre-intervention (baseline) CWRCE assessment of ET. All CWRCE tests must have been conducted at the same absolute work rate pre- and post-intervention; participants with a baseline and post-intervention test performed at different work rates were excluded from analyses.
Data in the bronchodilator intervention clinical trial dataset was further organized and analyzed according to treatment groups based on the available data and sample size, as indicated below:
- Placebo: study assignment to placebo group
- Active treatment: includes long-acting bronchodilator therapy as bronchodilator monotherapy (long-acting muscarinic antagonist [LAMA] or long-acting beta-agonist [LABA]), bronchodilator combination therapy (LAMA/LABA combination), or bronchodilator in combination with an inhaled corticosteroid (ICS) (LABA/ICS). By agreement with the individual investigators and owners of these data, analyses according to specific pharmacologic agents (or combination of agents) were not performed.
Studies with both parallel and cross-over designs were solicited. However, for cross-over studies, only the first period was included in the analysis, essentially converting them to parallel studies.
Since only 2 exercise training studies included a usual care control group, usual care control group data from the exercise training studies was not included in the integrated database.
Statistical Considerations
Descriptive statistics for continuous variables included mean, standard deviation (SD), median, interquartile range, and range (minimum to maximum). For categorical variables, the percentage distribution by category was used.
Quality Assurance of Analytic Database
All variables were reviewed for quality assurance. Individual study mapping files were used to compare accuracy with the data in the analytic database. Variables were examined to ensure values were within physiologically plausible ranges (see Table A1 in the online supplement). Outliers were examined for unit consistency and possible mislabeling of study data; in almost all cases, outliers were less than 1% of all values. No data received were permanently removed from the database.
Endpoints and Missing Data
Using the protocol described in our previous publication,3 endurance times in the integrated database are reported in minutes. Some studies recorded responses at more than 1 post-intervention time point. Based on a review of the published studies included in the database, end of treatment was chosen as the study visit closest to Week 6 for bronchodilator studies, and closest to Week 8 for exercise training studies. The rationale for this choice is that, for bronchodilator studies, 6 weeks is commonly used to assure steady-state of response; 8 weeks is a common duration of an exercise training program. Other imputations are based on either well-defined algorithms (e.g., body mass index [BMI], forced expiratory volume in 1 second [FEV1] % predicted based on European Community of Coal and Steel normative values28) or on methodology descriptions from the published studies (e.g., fraction of peak work rate in the incremental test consistently used for the CWRCE test, sex of single-sex studies). Missing data for endurance time were not imputed.
Results—Descriptive Statistics
Participant demographics are reported in Table 3. A total of 5654 participants, all with COPD, who had a baseline ET were included in the study database from 23 individual studies, including 8 bronchodilator studies (n=2166) and 15 exercise training studies (n=3488).
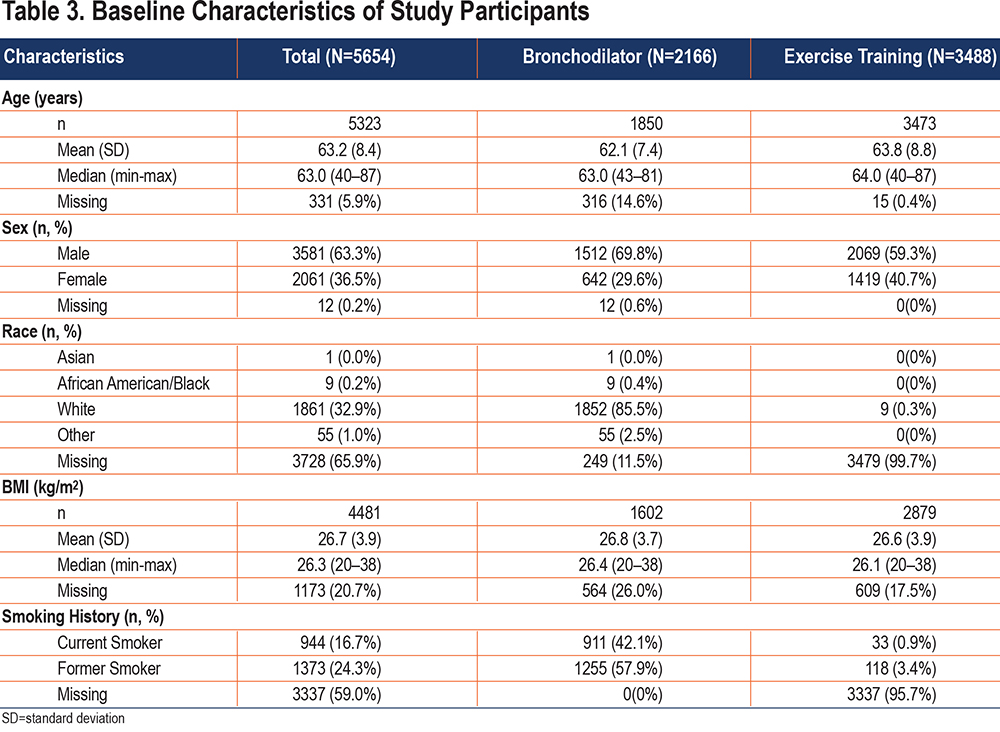
For the total sample, the mean (SD) participant age was 63.2 (8.4) and ranged from 40–87 years. More than half of the total sample was male (63.3%), with slightly more males in the bronchodilator group compared to the exercise training group (69.8% and 59.3%, respectively). Of those with identified race, participants in the bronchodilator group were predominantly White (96.6%); data for race was not recorded for almost all participants in exercise training studies (99.7%). For the total sample, the mean (SD) BMI was 26.7 (3.9) kg/m2 and similar by intervention group. Among those in the bronchodilator group, 42.1% were current smokers and 57.9% were former smokers; current smoking status was not reported for almost all participants in the exercise training group.
Spirometry
Descriptive data for FEV1 and FEV1 % predicted are reported in Table 4. Mean (SD) FEV1 at baseline was 1.30 (0.51) L for the total sample, with a range from 0.5 to 3.0 L. FEV1 was 1.24 (0.50) L for the exercise training group and 1.43 (0.50) L for the bronchodilator (active) group, indicating that the exercise training cohort had, on average, somewhat more severe disease. As expected, change in FEV1 from baseline to the post-intervention assessment was greater for the bronchodilator (active) group (mean increase 0.14 [0.24] L) compared to the bronchodilator (placebo) group (mean increase 0.02 [0.21] L). It should be noted that only a small number of exercise training studies assessed pulmonary function post-intervention, with FEV1 at post-intervention missing for 94.8% of this intervention group; however, for the 181 participants who had post-intervention spirometry, FEV1 change was small (mean 0.04 [0.18] L), as expected. Baseline mean FEV1 % predicted for the total population averaged 47.2 (16.8)% demonstrating severe airflow obstruction on average, with substantial numbers of participants with moderate, severe, and very severe obstruction, which is representative of the COPD population seen in clinical practice.
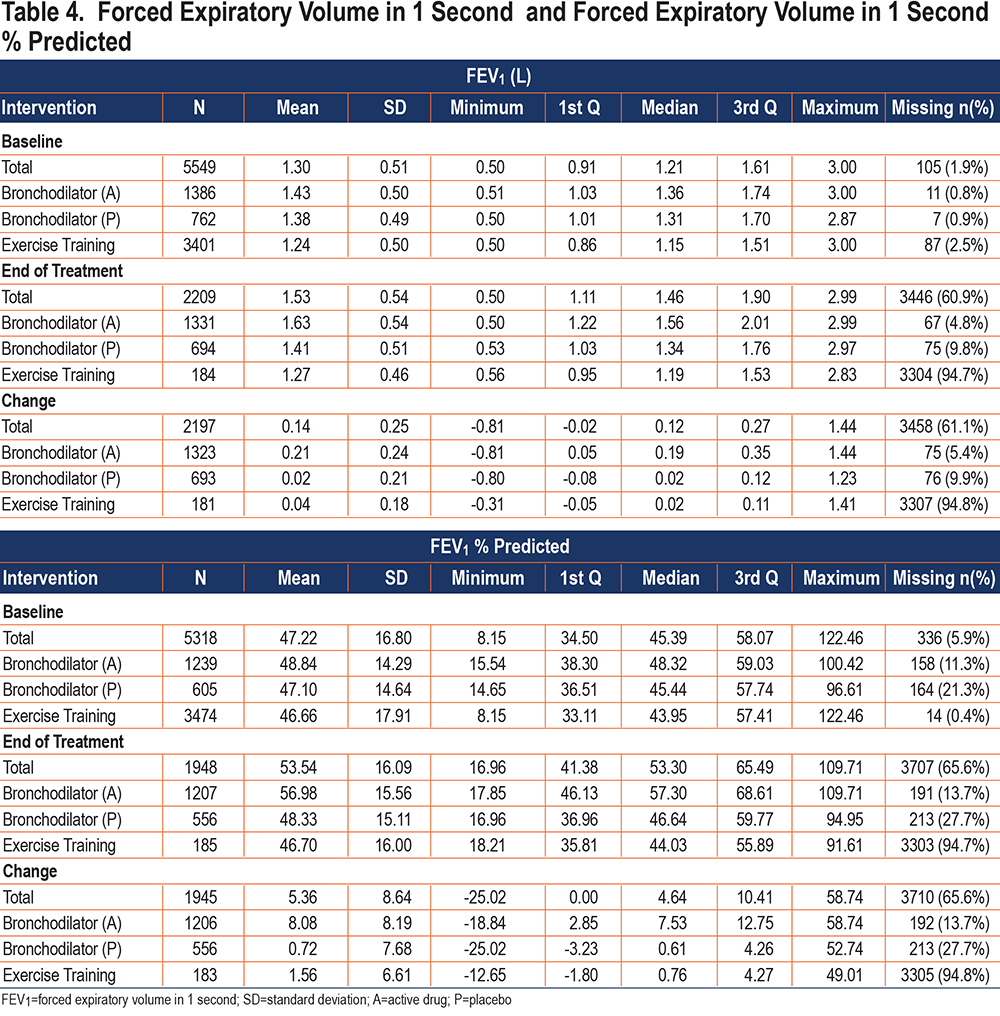
Descriptive data for forced vital capacity (FVC), FVC % predicted, and FEV1/FVC (%) are presented in the online supplement (Tables S1.1 and S1.2) and show similar trends observed with FEV1 by intervention group.
Incremental and Constant Work Rate Cycle Ergometry: Work Rates
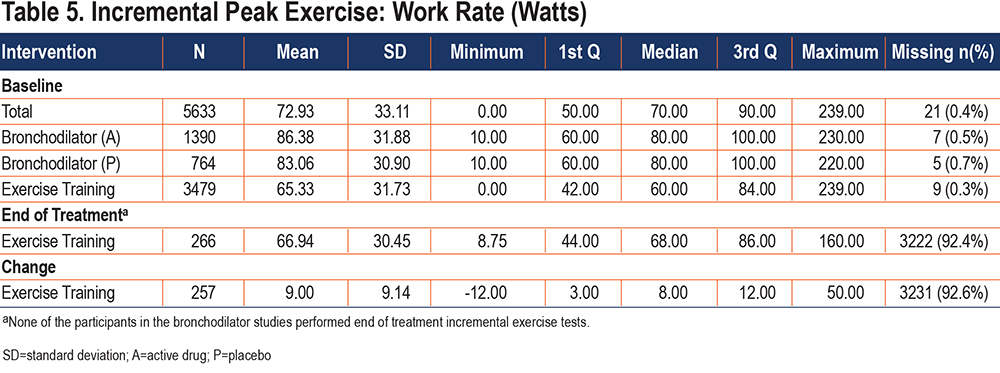
At baseline, the mean (SD) incremental peak exercise work rate for the total sample was 72.9 (33.1) watts (Table 5). Incremental peak exercise work rate was higher for bronchodilator studies (85.2 [31.6] watts) than exercise training studies (65.3 [31.7] watts), likely, in part, related to the somewhat more severe spirometric impairment in the exercise training cohort. Baseline incremental exercise studies were a requirement to allow determination of the CWR target. Post-intervention incremental tests were performed in only a minority of participants (n=257), all involving exercise training. At end of treatment, the average increase in incremental peak exercise work rate in exercise training studies was 9.0 (9.1) watts.
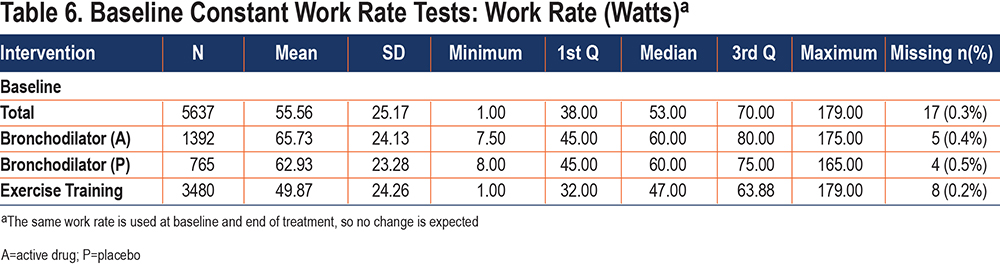
At both baseline and end of treatment, mean CWR was 55.6 (25.2) watts (Table 6). Mean CWR was higher for bronchodilator studies (64.7 [23.9] watts) than exercise training studies (49.9 [24.3] watts) but was a similar fraction of peak work rate in the incremental test (averaging 76.0 [3.1] % and 76.3 [4.9] %, respectively).
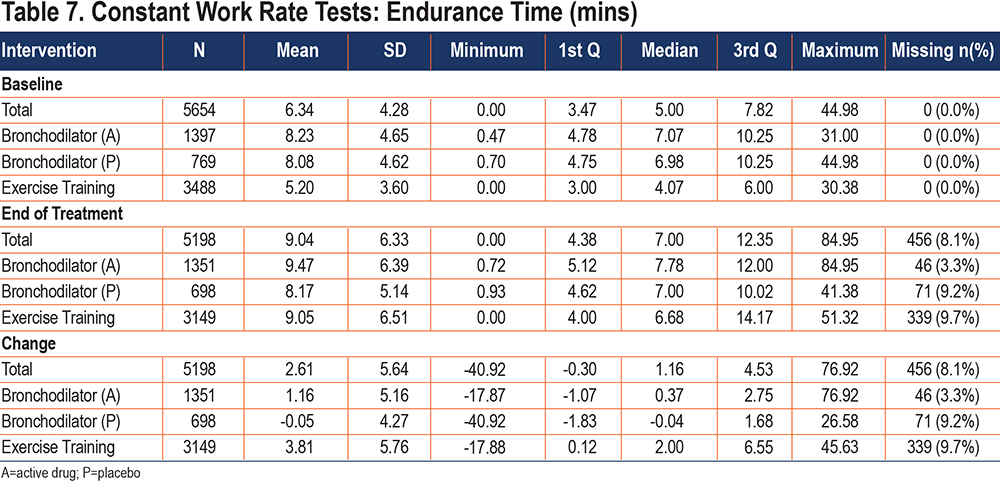
Endurance Time During Constant Work Rate Cycle Ergometry
Endurance time characteristics of the study population are presented in Table 7. In the bronchodilator studies, a total of 769 participants were in the placebo treatment arm and the remaining 1397 participants were on active treatment. In the exercise training studies, all 3488 participants were on active treatment. At baseline, mean (SD) ET for the total sample was 6.34 (4.28) min, ranging from 0.0 minutes to 44.98 minutes. Average baseline ET was longer for the bronchodilator group compared to the exercise training group (8.18 mins versus 5.20 mins, respectively). Change from baseline to end of treatment was higher for exercise training (3.81 [5.76] min) compared to those in the bronchodilator group on active treatment (1.16 [5.16] min); ET change for those receiving placebo was very small (-0.05 [4.27] min).
Physiologic and Perceptual Variables
Incremental Exercise: In the online supplement,Tables S2.1 through S2.10present descriptive statistics for baseline, end of treatment, and change in peak incremental exercise values for the physiologic and perceptual variables of Borg Dyspnea, Borg Leg Fatigue, heart rate, inspiratory capacity (IC), pulmonary ventilation (V̇E), oxygen uptake (V̇O2), blood oxygen saturation (SpO2), carbon dioxide uptake (V̇CO2), tidal volume (VT), and respiratory rate (RR). The baseline incremental test peak values for these variables are of value in determining whether the CWR tests bring patients to their physiologic maximal values. Post-intervention incremental tests were not conducted for bronchodilator studies. For the exercise training intervention, post-intervention peak exercise values are available in a subset of participants; in this subset, Borg dyspnea, Borg leg fatigue, SpO2, and RR are modestly lower, while peak V̇E, V̇O2, and V̇CO2 values are modestly higher, and peak heart rate shows little change (no post-treatment IC values are available).
Constant Work Rate Cycle Ergometry: Descriptive statistics for CWR physiologic variables are presented in the online supplement (Tables S3.1 – S3.30) for Borg Dyspnea, Borg Leg Fatigue, heart rate, IC, V̇E, V̇O2, V̇CO2, VT, RR, and SpO2. Overall, resting values are not present in most participants, most markedly in exercise training participants. Further, end-exercise values for exercise training participants have a higher fraction of missing values than do bronchodilator participants. However, even in cases where a higher fraction of participants have missing values for these variables, hundreds of participant studies contain these variables, so that useful analysis is possible. Further, isotime data are limited for both bronchodilator and exercise training groups, with missing rates generally in the 80%–90% range. Here, too, because of the large size of the data set, several hundred participants are available for most isotime variable analyses both for bronchodilator and exercise training studies (the average number of participants having changes in isotime responses available for these variables is ~750).
Tables S3.1 through S3.6 in the online supplement present values at rest, end exercise, and isotime at baseline, end of treatment, and change from baseline to end of treatment for the Borg Dyspnea (Tables S3.1 – S3.3) and Leg Fatigue scales (Tables S3.4 – S3.6). Both measures are rated on a 0–10 scale, with higher scores representing greater severity. Baseline values at rest average 1.30 (1.39) for Borg Dyspnea and 1.27 (1.47) for Borg Leg Fatigue and at end-exercise average 6.70 (2.29) for Borg Dyspnea and 6.16 (2.61) for Borg Leg Fatigue. For the exercise training intervention (but not for bronchodilator), post-intervention averages are lower: end-exercise and isotime Borg Dyspnea values by 0.77 and 1.96, respectively and end-exercise and isotime Borg Leg Fatigue values by 0.80 and 1.98, respectively.
Tables S3.7 through S3.9 in the online supplement present values at rest, end exercise, and isotime at baseline, end of treatment, and change from baseline to end of treatment for heart rate (b/min). At baseline, end-exercise heart rate averages 121.7 (18.9) b/min, with little change post-intervention. At isotime, post-intervention heart rate is higher for bronchodilator by 2.4 (11.6) b/min, while heart rate is lower for exercise training by 3.8 (10.9) b/min.
Tables S3.10 through S3.12 in the online supplement present values at rest, end, and isotime for baseline, end of treatment, and change from baseline to end of treatment for IC (L). For bronchodilator active group (but not placebo), IC values are, on average, higher post-intervention at rest, peak exercise, and isotime, suggesting reduction in hyperinflation. In the exercise training studies, increase in IC post-intervention is seen prominently in isotime values, suggesting reduction in isotime hyperinflation.
Tables S3.13 through S3.15 in the online supplement present rest, end-exercise, and isotime values for V̇E. At baseline, end-exercise V̇E averages 40.4 (13.2) L/min. Consistent with a bronchodilator’s ability to increase ventilatory capacity, both average end-exercise and isotime V̇E values are higher post-intervention. Consistent with exercise training’s ability to lower ventilatory requirement for exercise, average isotime V̇E values are lower post-intervention.
Tables S3.16 through S3.21 in the online supplementpresent rest, end-exercise, and isotime values for V̇O2 (Tables S3.16 – S3.18) and V̇CO2 (Tables S3.19 – S3.21). At baseline, end-exercise V̇O2 averages 1.14 (0.37) L/min. For exercise training (but not bronchodilator) end-exercise (but not isotime) V̇O2 increased mildly.
Tables S3.22 through S3.27 in the online supplement present descriptive statistics for VT (Tables S3.22 – S3.24) and RR (Tables S3.25 – S3.27). End-exercise and (to a lesser extent) isotime VT are higher for both the bronchodilator and exercise training interventions. End-exercise and (quite distinctly) isotime RR are lower for the exercise training intervention. For exercise training, the isotime increase in VT (by 0.04 [0.21] L) and fall in RR (by 2.39 [4.76] breaths/min) document a shift toward a slower, deeper breathing pattern.
Tables S3.28 through S3.30 in the online supplementpresent rest, end-exercise, and isotime values for SpO2. Only a modest fraction of participants manifest clinically important exercise desaturation (<88% saturation), most of whom are in the exercise training group. For both bronchodilator and exercise training, on average only small change is observed for SpO2.
Other Measures of Relevance
6-Minute Walk Test Walking Distance: Descriptive statistics are presented in Tables S4.1 through S4.4 in the online supplement for physiologic and perceptual responses to the 6MWT. Data are available for the exercise training group only. Mean (SD) 6MWT walking distance (WD) was 440.3 (109.0) meters at baseline and increased to 465.6 at end of study, an increase of 24.9 (53.5) meters. Baseline end-exercise heart rate (HR) averaged 110.3 (15.7) b/min. In baseline tests, end-exercise Borg Dyspnea and Borg Leg Effort scores averaged 4.77 and 4.07, respectively, which corresponds to a description of “somewhat severe” to “severe.”
St George’s Respiratory Questionnaire: Descriptive statistics are presented inTable S5 in the online supplement. Scores range from 0–100, with higher scores indicating worse COPD-specific health-related quality of life. Data are available for the exercise training group only. Mean SGRQ total score was 53.9 (19.3) at baseline, indicating substantially impaired quality of life. Exercise training was associated with a mean change from baseline in total score of -5.4 (16.3), which is well above the established minimal clinically important difference (4 units). Baseline scores for the activity domain were particularly high (70.6 [23.0]), demonstrating the debility in activities of daily living that COPD patients experience. Mean scores in all SGRQ domains fell substantially post-intervention.
Discussion
The primary purpose of the CBQC constant work rate exercise project is to conduct a formal, comprehensive analysis, including the assessment of reliability, validity, responsiveness, and interpretability of change, to support the qualification of ET during CWRCE as a COA for COPD interventional clinical trials that may be used for regulatory decision making. This aim necessitated the creation of an integrated database consisting of previously collected data from interventional studies in patients with COPD.
Previous studies have focused on the evaluation of test-retest repeatability of ET during constant work rate cycle ergometry29,30 as well as an initial evaluation of concurrent validity by comparing with other exercise tests.29 A meta-analysis was recently conducted to support the European Respiratory Society (ERS) statement on the use of exercise testing in the evaluation of interventional efficacy and concluded that ET during constant work rate cycle ergometry is responsive to rehabilitative and pharmacologic interventions.31 By creating an integrated database of participant-level data, we will build on the meta-analysis by conducting the participant-by-participant correlational analyses required for a complete analysis in support of regulatory qualification.
To lay the foundation for the creation of the integrated database, we conducted a rigorous literature search following well-established COSMIN standards3 to gain a full appreciation of the number of interventional studies that have incorporated constant work rate exercise testing (not only constant work rate cycle ergometry, but also constant work rate treadmill walking and the endurance shuttle walk test); the search results captured all of the publications contained in the literature search conducted as part of the ERS exercise testing statement,31 which confirmed the comprehensiveness of our search strategy. While it was not our intention to include data from all studies in the integrated database (which would not have been logistically feasible), the results of the literature search did provide valuable direction in terms of highlighting a list of relevant investigators to contact and request transfer of study data into the integrated database. Finally, a total of 5654 participants with a baseline ET were included in the integrated database from 23 individual studies, including 8 bronchodilator studies (n=2166) and 15 exercise training studies (n=3488). This is, by far, the largest participant-by-participant interventional constant work rate exercise data base ever collected.
Patient and clinical characteristics of participants included in the analysis are representative of the COPD population with moderate to very severe airflow obstruction. Mean (SD) participant age was 63.2 (8.4) and ranged from 40–87 years old; more than half of the total sample was male (63.3%). Baseline mean FEV1 % predicted for the total population was 47.2% (16.8) demonstrating severe airflow obstruction on average, with substantial numbers of participants with moderate and very severe obstruction.
A strength of this data base is the inclusion of a range of physiologic and perceptual variables that will be valuable in elucidating mechanistic links to the observed changes in ET during CWRCE. It is notable that a number of these variables are not available for a substantial fraction of the cohort, as individual studies did not record all variables. Nevertheless, the presence of several hundred to several thousand individual values for these variables will allow a well-powered analysis of the linkages underlying ET changes. A further strength of this data base is its inclusion of participants widely representative of the population clinically impacted by COPD. Sex balance is reasonable. A weakness is the lack of racial diversity, with the overwhelming majority of participants, in whom it is specified, being of White background; this is a reflection of a long-standing issue in COPD interventional trials.
In developing the integrated database, care was taken to include all relevant data required to support the primary validation analysis for regulatory qualification of ET during CWRCE. In light of the significant amount of resources and effort required to create an integrated database, during the planning phase of the database development, we also considered other potential uses of the database beyond the primary validation analysis for regulatory qualification. To this end, we: (1) included data from studies with interventions that were not used in the primary validation analysis, such as heliox, hyperoxia, non-invasive ventilation, and (2) included data from studies that included other measures of exercise endurance, namely constant work rate treadmill walking and the endurance shuttle walk test. We developed this separate, stand-alone publication to highlight the unique background and processes involved in the creation of an integrated database that offers the opportunity to explore a number of relevant research questions in addition to the specific validation analyses to support regulatory qualification, and potentially will be the foundation for a number of future publications.
The integrated database includes studies from 2 interventions, namely inhaled bronchodilator medication and rehabilitative exercise training, for the evaluation of the responsiveness of ET during CWRCE. To remain true to the non-competitive nature of this novel industry/academia collaboration, it was agreed that the 8 industry-sponsored bronchodilator studies would be included in the analysis database without specific product identification. Rather, all bronchdodilator active treatment arms were labeled as “bronchodilator.” Each bronchodilator included has been shown to have an effect on endurance time and, as such, this grouping of different bronchodilators under 1 “active treatment” category was justifiable as a means to evaluate the responsiveness of ET during CWRCE.
Even though the specific purpose of the validation analysis was for regulatory (FDA) qualification of drugs via the DDT qualification pathway, we decided to also include data from studies that included exercise training as an intervention. The measurement properties of a COA should focus on the concept of interest to be measured and should be agnostic to the intervention, such that a validated measurement instrument may be used for the evaluation of treatment benefit for both pharmacological and non-pharmacological interventions. Furthermore, the neuromuscular and cognitive impacts of a pulmonary rehabilitation intervention may be relevant to pharmaceutical interventions that target non-pulmonary manifestations of COPD. We believe that it is important that the same standards for validation are used across different classes of intervention.32 In building on the development of a conceptual framework this project created for exercise endurance,4 we encourage the further evolution of a unified, patient-centric organizing framework that seeks to identify a core outcome set in patients with COPD that represents a full description of meaningful treatment benefit for patients with COPD (with the possibility to extend to other chronic lung diseases).
There are some data management challenges that need to be addressed when retrospectively developing an integrated database from previously conducted studies, with each study designed without specific attention to future integration of the data with other studies. This may be contrasted with the prospective development of an integrated database that occurs, for example, with the planning of a Phase 3 program for a new pharmaceutical. In this latter situation, where the data management is fully under the oversight of an individual sponsor, it is relatively straightforward to develop data consistency requirements a priori, such as the use of the same instructions for the exercise testing protocol across studies, consistent methodology training across all sites, consistent inclusion of all relevant variables using the same terminology, use of the same inclusion/exclusion criteria across studies, pre-specification of the same intervention duration and timepoints for outcome assessment. These are important considerations when the goal of the studies is to evaluate the efficacy of the intervention. However, we would assert that differences across studies should be viewed as a strength rather than a limitation when the specific focus of the analysis is the validation of the outcome measure rather than the evaluation of effectiveness of an intervention, as it allows for the evaluation of the robustness of the primary endpoint of interest (in this case, endurance time during CWRCE) under varying study design conditions.
The full analysis population included all participants who had at least 1 pre-intervention (baseline) CWRCE assessment of ET. It is notworthy that we did not stipulate an additional requirement for at least 1 post-intervention CWRCE of ET for the full analysis set. Some of the analyses to be conducted (construct validity, content validity) require baseline ET, while other analyses (repeatability, responsiveness) require both pre-intevention and post-intervention ETs (repeatability examines the pre- and post-intervention ET in the placebo group; responsiveness examines the pre- and post-intervention ET in the active treatment group). Restricting the full analysis set requirement to only a pre-intervention ET ensured that we optimized the data available for content/construct validity analysis, with the repeatability and responsiveness analyses being conducted on a subset of the full analysis dataset where both pre- and post-intervention ETs are available.
In summary, we assembled a large participant-by-participant data base suitable for the evaluation of endurance time during CWRCE as a clinical outcome assessment for use in COPD clinical trials. Having previously established this outcome as a patient-centered, meaningful treatment benefit, the stage is now set for the analysis supporting the validation/qualification process and the assessment of other relevant questions, which will be described in subsequent publications.
Acknowledgements
Author Contributions: RC, AH, DM, and GH designed the project with the input of the Working Group. RC, AH, GH, and RY were involved in the design of the analysis. RC and GH had full access to the data and assure the accuracy of the results. TD and RG designed and completed the literature review. GH and RY completed the analyses. RC, DM, and AH contributed to the drafting of the manuscript. TD, JG-A, MF, RG, GH, NKL, FM, DOD, JP, LP-M, SR, FS, MS, RT-S, KT, and AvtH were involved in the review and editing of the manuscript. All authors have given final approval of the version that has been submitted for publication.
The authors would like to thank the the following members of the CBQC Constant Work Rate Exercise Working Group for their support and contributions during the project: Christopher B. Cooper, MD, University of California, Los Angeles; Nick Hopkinson, FRCP, Brompton Hospital, London, United Kingdom; Nicholas Locantore, PhD, GlaxoSmithKline, Collegeville, Pennsylvania, (at the time of the work); Ubaldo Martin MD, AstraZeneca (at the time of the work), Divya Mohan, MRCP, PhD, GlaxoSmithKline, Collegeville, Pennsylvania (at the time of this work); Alberto Neder, MD, Queens University, Kingston, Canada; Andrea Noronha, BSc, Syneos Health, Morrisville, North Carolina; Michael Polkey, PhD, FRCP, Brompton Hospital, London, United Kingdom; Danielle Rodriguez, PhD, Evidera, Seattle, Washington; Harry B. Rossiter, PhD, the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, California; Sally J. Singh, PhD, University of Leicester, United Kingdom; Martyn Walker,BSc, Syneos Health, Ebchester, England, United Kingdom; and Susan Ward, PhD, Crickhowell, Wales, United Kingdom.
Data Sharing Statement: This manuscript describes the development of an integrated database to be used in secondary analyses of shared data. The authors attest that the use of the shared data was in accordance with the terms agreed to upon their receipt; the sources of the data are described in Table 1. The original data was used to evaluate the effects of the intervention (either bronchodilation or exercise training) on exercise endurance. In contrast, the secondary analysis uses the data to support the validation of endurance time during CWRCE as a clinical outcome assessment in clinical trials. Consistent with the recommendations of the International Committee of Medical Journal Editors’ statement regarding data sharing, the co-chairs of the CBQC Constant Work Rate Exercise Working Group (Rich Casaburi, Alan Hamilton) acknowledge the efforts required to generate the original clinical trial data by actively encouraging the original investigators and company representatives to collaborate as part of the working group. Furthermore, academic investigators who shared data, and a representative from each pharmaceutical company that shared data, are included as authors.
Declaration of Interest
Richard Casaburi reports consulting fees/honoraria from Boehringer Ingelheim and GlaxoSmithKline. He is involved in contracted clinical research with Regeneron and United Therapeutics. Debora Merrill is a former employee (retired) of Merck and a current shareholder. Malin Fageras is a full-time employee of AstraZeneca with salary and stock shares in the company. Gale Harding and Ren Yu are employed by Evidera, a health care research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In their salaried positions, they work with a variety of companies and organizations and receive no payment or honoraria directly from these organizations for services rendered. Nancy Kline Leidy is employed by Evidera, a health care research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In this salaried position, Dr. Leidy works with a variety of companies and organizations. She receives no payment or honoraria directly from these organizations for services rendered, with the exception of honoraria received for her advisor role on several National Institutes of Health and FDA-funded programs: PATIENTS, PCAR, and NUCOAT. Denis O’Donnell reports receiving research funding via Queen's University from AstraZeneca, Lung Health Foundation, and Boehringer Ingelheim Canada during the conduct of the study. There are no conflicts of interest to declare with regards to this study. Stephen Rennard reports a Patient-Centered Outcomes Research Institute grant, consulting fees from GlaxoSmithKline, Boehringer Ingelheim, Sanofi, Verona, and NovoVentures not related to the submitted manuscript, 2 patents not related to the submitted work, and shares awarded from GlaxoSmithKline as part of compensation while employed there 2015 to2019. He is also president and founder of Great Plains Biometrix. Ruth Tal-Singer is a former employee (retiree) and current shareholder of GlaxoSmithKline. She reports personal fees from Immunomet, Vocalis Health, Teva, and ENA Respiratory before 2021. She is a member of ENA Respiratory Board of Directors on behalf of the COPD Foundation and owns share options. Kay Tetzlaff is a full-time employee of Boehringer Ingelheim. Alan Hamilton was an employee of Boehringer Ingelheim during the development of the integrated database. The rest of the authors have nothing to declare.