Running Head: Association of Hoover’s Sign With MEP/MIP Ratio
Funding Support: No funding to report.
Date of Acceptance: November 9, 2022 | Published Online Date: November 16, 2022
Abbreviations: BMI=body mass index; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; IC=inspiratory capacity; MEP=maximal expiratory pressure; MIP=maximal inspiratory pressure; PFT=pulmonary function test; RV=residual volume; TLC=total lung capacity; VA=alveolar volume
Citation: Maloney TG, Anderson ZS, Vincent AB, Magiera AL, Slocum PC. Association of Hoover’s sign with maximal expiratory-to-inspiratory pressure ratio in patients with COPD. Chronic Obstr Pulm Dis. 2023; 10(1): 1-6. doi: http://doi.org/10.15326/jcopdf.2022.0341
Introduction
Patients with chronic obstructive pulmonary disease (COPD) may have abnormalities of chest wall motion. One of the oldest described abnormalities of chest wall motion is Hoover’s sign, first described by Charles Franklin Hoover,1 and is classically described as lung hyperinflation leading to a flattened diaphragm which causes the lower rib cage to move paradoxically during inhalation, in an inward direction rather than outward.2 The sign is reported to have a sensitivity of 58% and specificity of 86% for detecting obstructive airway disease3 which may make it a useful sign in the diagnosis of COPD. Previous studies have shown a frequency of Hoover’s sign of 36%, 43%, and 76% in Global initiative for chronic Obstructive Lung Disease (GOLD)4 moderate, severe, and very severe stages of COPD, respectively.5
In describing a phenotype of patients with Hoover’s sign, previous studies found that the presence of Hoover’s sign is associated with older age, higher body mass index (BMI), and more severe airflow obstruction. Studies have not shown that Hoover’s sign is associated with a higher degree of hyperinflation.6,7 The presence of Hoover’s sign in individuals with COPD may have important clinical implications because it is associated with higher exacerbation frequency,8 higher dyspnea symptoms with exercise,9 and an increased frequency of hospitalizations and emergency department visits.8
There are various tests to screen for respiratory diaphragm dysfunction including maximal inspiratory pressures with or without phrenic nerve stimulation, positional change in vital capacity from seated to supine, transdiaphragmatic pressure, electromyography, dynamic evaluation of diaphragmatic movement using fluoroscopy for unilateral diaphragm dysfunction, chest radiograph, and ultrasound.10,11 Many of these tests are invasive or require specially trained technicians to complete. The maximal expiratory pressure (MEP) to maximal inspiratory pressure (MIP) ratio has been used as a screening test for diaphragm paralysis.12 Ultrasound has been validated in detecting diaphragm dysfunction in COPD,11 however, this is an additional test requiring a specially trained technician that patients with COPD must undergo to assess diaphragm function. Given that patients with COPD in our institution routinely undergo static pressure measurements during pulmonary function testing (PFT), our study aims to see if Hoover’s sign is associated with a change in the MEP/MIP ratio in patients with COPD. Since diaphragm dysfunction in Hoover’s sign could be described as incomplete paralysis, we hypothesized that there would be an observed increase in the MEP/MIP ratio in patients with COPD who had Hoover’s sign.
Methods
This observational, prospective, single-center cohort study was performed in an outpatient pulmonary clinic from October 2021 to May 2022. This study was approved by the Freeman Health System Institutional Review Board (approval number 2022001). Internal medicine resident physicians performed Hoover’s sign examinations and had previously attended clinic and were trained by a pulmonologist in detecting Hoover’s sign. No patients were previously known by the examiners. Inclusion criteria were the following: age >18 years old, known diagnosis of COPD, had PFTs and respiratory pressures performed during the study period, had a forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio < 70%, had an FEV1 of <50%, and had stable COPD without signs of acute exacerbation. Patients were excluded if they chose not to undergo examination. PFTs and respiratory pressure measurements were performed by trained respiratory therapy technicians who were blinded to any other data. Maximal static pressures were obtained in the seated position as per American Thoracic Society Guidelines.13 Individuals who met inclusion criteria based on data recovered from the medical registry were invited to return to the pulmonary clinic for Hoover’s sign examinations. Examiners were blinded to PFT and respiratory pressure data. Examinations for Hoover’s sign were performed by 2 internal medicine resident physicians while the patient was in the seated position.
The observer agreement of the findings was calculated with k statistic and was interpreted as follows: 0–0.2, slight agreement; 0.2–0.4, fair agreement; 0.4–0.6, moderate agreement; 0.6–0.8, substantial agreement; and 0.8–1.0, almost perfect agreement.14,15 The 2 comparison groups were those with the presence of Hoover’s sign and those with the absence of Hoover’s sign. Continuous variables and PFT variables were not normally distributed, therefore, we report the median (range) as a measure of central tendency. Categorical variables were reported as the percentage of persons in each category. The Mann-Whitney U Test, a non-parametric method for comparing medians, and the χ2 tests were used to determine statistical significance of the continuous, categorical, PFT, and respiratory pressure variables. A p value threshold of 0.05 was used to determine statistical significance. We expressed MEP/MIP and MIP as an absolute value to aid in clinical utility.
Results
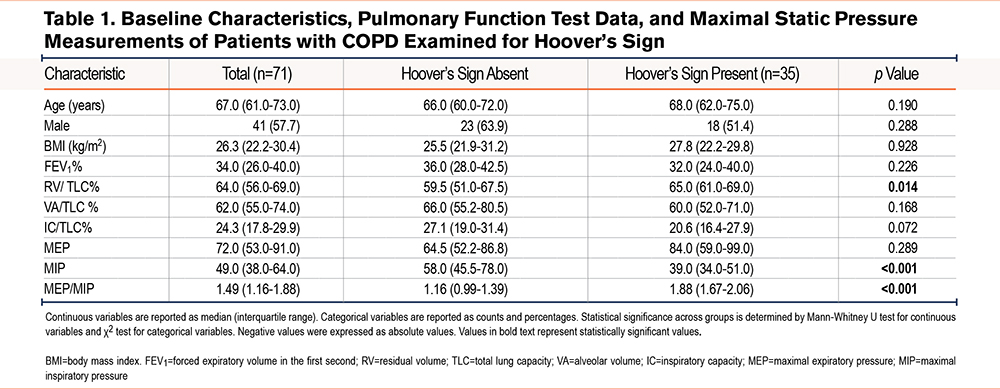
A total of 71 patients who met inclusion criteria agreed to undergo Hoover’s sign examinations. There were 41 males (57.7%) and 30 females (42.3%) with a median age of 67.0 years (61.0–73.0 interquartile range, range 44–91). Thirty-five (49.2%) of the patients examined exhibited Hoover’s sign. Observer agreement as demonstrated by the κ statistic was 0.8 indicating substantial to almost perfect agreement.
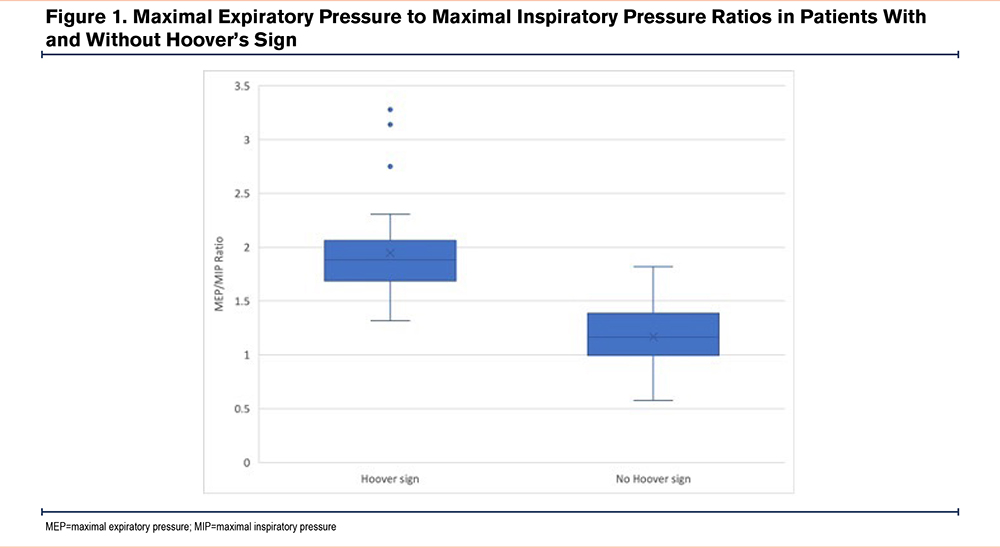
Between the 2 comparison groups, age, sex, and BMI were not statistically different. Selected PFT and static pressure measurements are presented in Table 1. In patients with and without Hoover’s sign, the median FEV1, median ventilation heterogeneity as measured by the alveolar volume (VA) to total lung capacity (TLC) ratio, median degree of hyperinflation as measured by the inspiratory capacity (IC) to TLC ratio, and median MEP were not statistically significant between the 2 groups. For individuals with Hoover’s sign, the median MEP/MIP ratio was significantly higher than those without Hoover’s sign (1.88 versus 1.16, p<0.001), which was driven by a statistically significant lower difference in MIP (39.0 versus 58.0, p<0.001). The difference in the median MEP/MIP ratio is displayed in Figure 1. The median degree of air trapping, as measured by the residual volume (RV) to TLC ratio, was higher among individuals with Hoover’s sign (65.0 versus 59.5, p<0.014), albeit not as strongly significant as the difference between the MEP/MIP and MIP.
Discussion
Our study showed that patients with COPD in our study population who had Hoover’s sign had a higher MEP/MIP ratio than those without Hoover’s sign. The presence of Hoover’s sign was also associated with a higher MIP and RV/TLC ratio. Because of the k value in our study, we demonstrated that Hoover’s sign can be detected with precision in trained examiners.
The MEP/MIP ratio in our study was lower than the reported value12 for those with unilateral diaphragm paralysis which was reported to be 2.1. In addition, those in our study group without Hoover’s sign had a lower median MEP/MIP ratio than the median MEP/MIP ratio of 1.5 in the normal diaphragm function group in the Koo et al study.12 It is unclear why there was a difference between their normal diaphragm function group and ours, but it could be confounded by our study population who had advanced COPD, whereas, their study population had unexplained dyspnea with our assumption that COPD was ruled out in their study participants. In addition, Hoover’s sign pathology results from diaphragm flattening leading to an increased radius of curvature, which increases muscle tension and causes the force vector on the lower aspects of the ribs to move inward rather than cephalad. This culminates in the lower rib cage motion being directed inward on inspiration instead of outward.16 With this physiology, Hoover’s sign can be described as incomplete diaphragm dysfunction rather than paralysis, so the MEP/MIP ratios between Hoover’s sign individuals and those with diaphragm paralysis cannot be directly compared. Therefore, since all of our study participants had advanced COPD, and since Hoover’s sign is not synonymous with diaphragmatic paralysis, comparing any of our data and participant groups with the data and participant groups of the Koo et al study should be done with caution since there may be significant confounders. Regardless, we show that the MEP/MIP ratio can accurately predict the presence of diaphragm dysfunction manifesting as Hoover’s sign in patients with COPD.
Although participants with Hoover’s sign had a significantly lower MIP than those without Hoover’s sign, the values of MIP in both groups were heterogenous with wide ranges, making its utility in diagnosing diaphragm dysfunction less reliable. Therefore, we propose that the MEP/MIP ratio be used in place of MIP to determine whether patients with COPD have diaphragm dysfunction manifesting as Hoover’s sign. It is important to keep in mind that the Hoover’s sign examinations were performed in an outpatient pulmonary clinic in stable COPD patients. While we believe this has near universal value in the adult outpatient setting, we acknowledge validation needs to be confirmed in the primary care setting, emergency departments, and tertiary care centers before it is universally used in these settings. In the primary care setting, its role may have limitations due to the technical challenges in obtaining respiratory pressure measurements, such as good lip seal and holding cheeks to minimize oropharyngeal influence.
In this study, we demonstrated that Hoover’s sign can be detected with precision in trained examiners and that respiratory pressures can be used as an adjunct to diagnose Hoover’s sign in patients with COPD. The agreement between observers in our study was similar or slightly higher than the k values previously reported.3 The prior training of the examiners and the characteristics of the patients included can probably explain the high degree of agreement in the series.
Compared to prior studies, our study did not show differences in baseline characteristics between those with and without Hoover’s sign. Specifically, we detected no difference in age or BMI between the 2 groups, whereas prior studies showed those with Hoover’s sign to have a higher BMI and older age.5 It is unclear whether this is population specific or due to other unforeseen factors not considered in our study. Congruent with another study,5 we found that Hoover’s sign was not associated with hyperinflation. We observed a novel finding of higher degree of air trapping in patients with Hoover’s sign than those without Hoover’s sign. This finding has not been previously reported. It is unclear whether this is an association in the disease process, or whether air trapping plays a role in the development of diaphragm dysfunction. Further studies would need to determine the role of air trapping in diaphragm function in patients with COPD.
Later in our study period, a small number of participants disclosed that they had participated in pulmonary rehabilitation, which was not a question assessed in our study. Interestingly, none of these participants had Hoover’s sign on exam. It is unknown whether patients might have had diaphragm dysfunction prior to rehabilitation. Pulmonary rehabilitation has been shown to partially revert diaphragmatic impairments as assessed by ultrasound.17 Whether pulmonary rehabilitation would lead to resolution of Hoover’s sign on examination or air pressure measurements could be assessed in future studies.
In conclusion, we found that an increased MEP/MIP ratio is correlated with the presence of Hoover’s sign in patients with COPD with stage 3 and 4 GOLD criteria disease and provides a simple alternative to assess for diaphragm dysfunction in this population.
Acknowledgements
Author contributions: TGM and PCS take full responsibility for the article, including for the accuracy and appropriateness of the reference list. TGM and PCS conceived the study. TGM, PCS, and ZSA were involved in protocol development, researching literature, and gaining ethical approval. TGM and ZSA were involved in patient recruitment. TGM, ZSA, ABV, and ALM performed patient examinations. TGM, ZSA, ABV, ALM, and PCS contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. All authors approved the version of the manuscript submitted for publication.
Declaration of Interest
There is no financial arrangement or other relationship to disclose for any of the authors that could be construed as a conflict of interest.