Running Head: Steroid Exposure in COPD and Coronavirus Disease 2019
Funding Support: This study was funded by Boehringer Ingelheim, Inc. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations. To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the International Committee of Medical Journal Editors criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data.
Date of Acceptance: November 30, 2022 | Published Online Date: December 5, 2022
Abbreviations: AIC=Akaike Information Criterion; CI=confidence intervals; COPD=chronic obstructive pulmonary disease; COVID-19=coronavirus disease 2019; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; ICS=inhaled corticosteroid; IQR=interquartile range; KPNC=Kaiser Permanente Northern California; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; OR=odds ratio; PCR=polymerase chain reaction; PRINCIPLE=Platform Randomized Trial of Intervention Against COVID-19 in Older People study; SABA=short-acting beta2-agonist; SAMA=short-acting muscarinic antagonist; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2; STOIC=Steroids in COVID-19 study; VA=Veterans Affairs
Citation: Myers LC, Murray RK, Donato BMK, et al. Persistent steroid exposure before coronavirus disease 2019 diagnosis and risk of hospitalization in patients with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2023; 10(1): 64-76. doi: http://doi.org/10.15326/jcopdf.2022.0351
Introduction
A risk-benefit ratio exists with regard to steroid exposure in chronic obstructive pulmonary disease (COPD) patients. The Global initiative for chronic Obstructive Lung Disease (GOLD) recommends an inhaled corticosteroid (ICS) for patients with moderate to severe COPD who have a history of frequent COPD exacerbations.1-2 While studies show that ICSs can reduce COPD exacerbations in this population, there is also an increased risk of pneumonia from viral infections,3-12 which are known to cause as many as 60% of COPD exacerbations.13,14
The risk-benefit ratio of using steroids in COPD likely depends on several factors: the patient population, underlying pathophysiology, and timing of exposure to viruses. A retrospective study from the United Kingdom, OpenSAFELY, found a significantly higher risk of death from coronavirus disease 2019 (COVID-19) if individuals were on ICS therapy in the 4 months before infection compared with patients on other maintenance inhalers.15 However, systemic steroids have been shown to improve survival in patients hospitalized with some viral infections, such as COVID-19,16 but not with other viral infections, such as influenza.17 Moreover, the recent Steroids in COVID-19 (STOIC) and Platform Randomized Trial of Intervention Against COVID-19 in Older People (PRINCIPLE) clinical trials conducted to explore the efficacy and safety of inhaled budesonide in patients with early onset, mild COVID-19 (STOIC) or those at higher risk of complications (PRINCIPLE) have reported that budesonide was associated with quicker recovery and reduced the likelihood of requiring urgent medical care or hospitalization.18,19 In COVID-19 pneumonia, a 10-day course of dexamethasone improved the 28-day survival in patients hospitalized with hypoxemia.16 Patients requiring higher levels of respiratory support benefit the most from this intervention,16 suggesting that severe disease may have an underlying pathophysiology that is more inflammatory in nature and, thus, is more steroid responsive. An alternative explanation is that patients who develop severe disease may be later in the disease course when the virus is no longer actively replicating in the lung epithelium.
We sought to determine whether persistent steroid exposure in the year before a COVID-19 diagnosis is associated with a higher risk of COVID-19–related hospitalizations or hospice referral/death in COPD patients. Given the importance of the timing of steroid exposure in this disease, we defined the exposure of interest as persistent use (>6 months) within 1 year before a COVID-19 diagnosis. Understanding the risks associated with persistent steroid exposure before a COVID-19 diagnosis in the COPD population can help fill important clinical gaps such as: (1) whether to continue long-term ICS maintenance therapy in these patients during the pandemic, and (2) whether the risk-benefit ratio has tipped further against the use of ICS therapy in patients not meeting the GOLD criteria for ICS use.
Methods
This study was approved by the Kaiser Permanente Northern California (KPNC) Institutional Review Board (1659946-3). A waiver of informed consent was obtained.
Study Design and Data Source
This retrospective cohort study used longitudinal electronic medical records and claims data from KPNC, an integrated health care delivery system serving >4.3 million members, representing approximately 30% of the insured adult population of Northern California.20 KPNC members have been shown to be demographically similar to the background population in Northern California.20
Cohort Formation and Data Extraction
We identified adults (aged ≥18 years) within the KPNC health care system with a positive polymerase chain reaction (PCR) test result for severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) by the first nasopharyngeal swab sample between February 2, 2020, and September 30, 2020. We narrowed down the cohort to patients aged ≥40 years, which has been used as the age criterion in prior studies in COPD patients.21 We required that patients had continuous KPNC membership for ≥1 year before the COVID-19 diagnosis, except for a <3‑month gap in the membership or receipt of a health care service while not being a documented member. We further narrowed the sample to patients who had ≥2 encounters for COPD of any of the following types (outpatient, emergency department, or inpatient) using a 3-year lookback period before the COVID-19 diagnosis and the following International Classification of Diseases, 10th Revision diagnosis codes: J40, J41.0, J41.1, J41.8, J42, J43.0, J43.1, J43.2, J43.8, J43.9, J44.0, J44.1, and J44.9. The requirement of ≥2 encounters in the definition increases the specificity of identifying COPD patients.22 Because there is little turnover in the KPNC population (69% retention at 5 years), we used a 3-year lookback period.
We extracted demographic and clinical variables, including the Elixhauser comorbidity index,23 neighborhood deprivation index,24,25 number of health care encounters in the year before the COVID-19 diagnosis, pre–COVID-19 supplemental oxygen use, frequency of COPD exacerbations, pulmonary function tests, and last maintenance inhaler regimen before the COVID-19 diagnosis. The frequency of COPD exacerbations was defined using a previously published algorithm combining administrative diagnoses across care settings and prescription data.26,27 Frequent COPD exacerbators were defined as having ≥2 exacerbations per year, and infrequent COPD exacerbators were defined as having 0–1 exacerbation per year.
Exposure
The primary exposure was persistent oral and/or inhaled steroid exposure before the COVID-19 diagnosis. Patients who were on long-term oral or inhaled steroids (filled ≥6 months of prescriptions) in the year before the COVID-19 diagnosis were flagged as exposed. These fills did not have to be continuous or in close proximity to the COVID-19 diagnosis but could be for any dose and be alone or in combination with other medications in an inhaler. As medication adherence has been shown to be low for many chronic diseases, including COPD, we chose 6 months as the period of persistent steroid exposure in this study, which is based on previous studies.28-30 A sensitivity analysis was conducted assessing ≥8 months of steroid exposure.
Outcomes
The primary outcome was COVID-19–related hospitalizations, which was defined using combinations of administrative codes recommended by the Centers for Disease Control and Prevention. The positive SARS-CoV-2 PCR test result had to be 3 weeks before admission or during the hospitalization. The secondary outcome was a composite of death or hospice referral within 30 days of a positive test result, which has been used in a previous study to capture as many outpatient and impending deaths as possible.31 We also assessed the rate of 30-day, nonelective, any-cause rehospitalizations in the subgroup of patients who were hospitalized and survived.
Statistical Analysis
Categorical variables are described as numbers with percentages and continuous variables as medians with interquartile ranges (IQRs). Logistic regression was performed, and unadjusted and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are reported. For adjusted models, the groups of confounders were sequentially added. Because the timing of steroid exposure in COVID-19 is likely important,16 we adjusted for patients who filled a prescription for oral or inhaled steroids in the 1 month prior to before their COVID-19 diagnosis and included it as a covariate. First, the unadjusted OR for persistent oral or inhaled steroid exposure was examined (baseline model). Second, oral or inhaled steroid exposure in the month before the COVID-19 diagnosis was added (Model 1). Third, sociodemographic variables were added (Model 2). Fourth, confounders such as the Elixhauser comorbidity index, individual comorbidities, body mass index, smoking history, previous history of pneumonia, and markers of illness severity such as a need for pre–COVID-19 supplemental oxygen, history of frequent exacerbations, and fills for short-acting inhalers were added (Model 3). The Akaike Information Criterion (AIC) for each model and outcome were recorded to assess model fit.32
SAS (Version 9.4, Cary, North Carolina) was used for statistical analyses. Tests for significance were t2-tailed with p≤0.05.
Results
From >4.3 million adults in the KPNC population, 36,137 with a positive SARS-CoV-2 test result were identified during the study period, of whom 697 were aged ≥40 years and had COPD. Of these, 270 (38.7%) had a COVID-19–related hospitalization (Table 1).
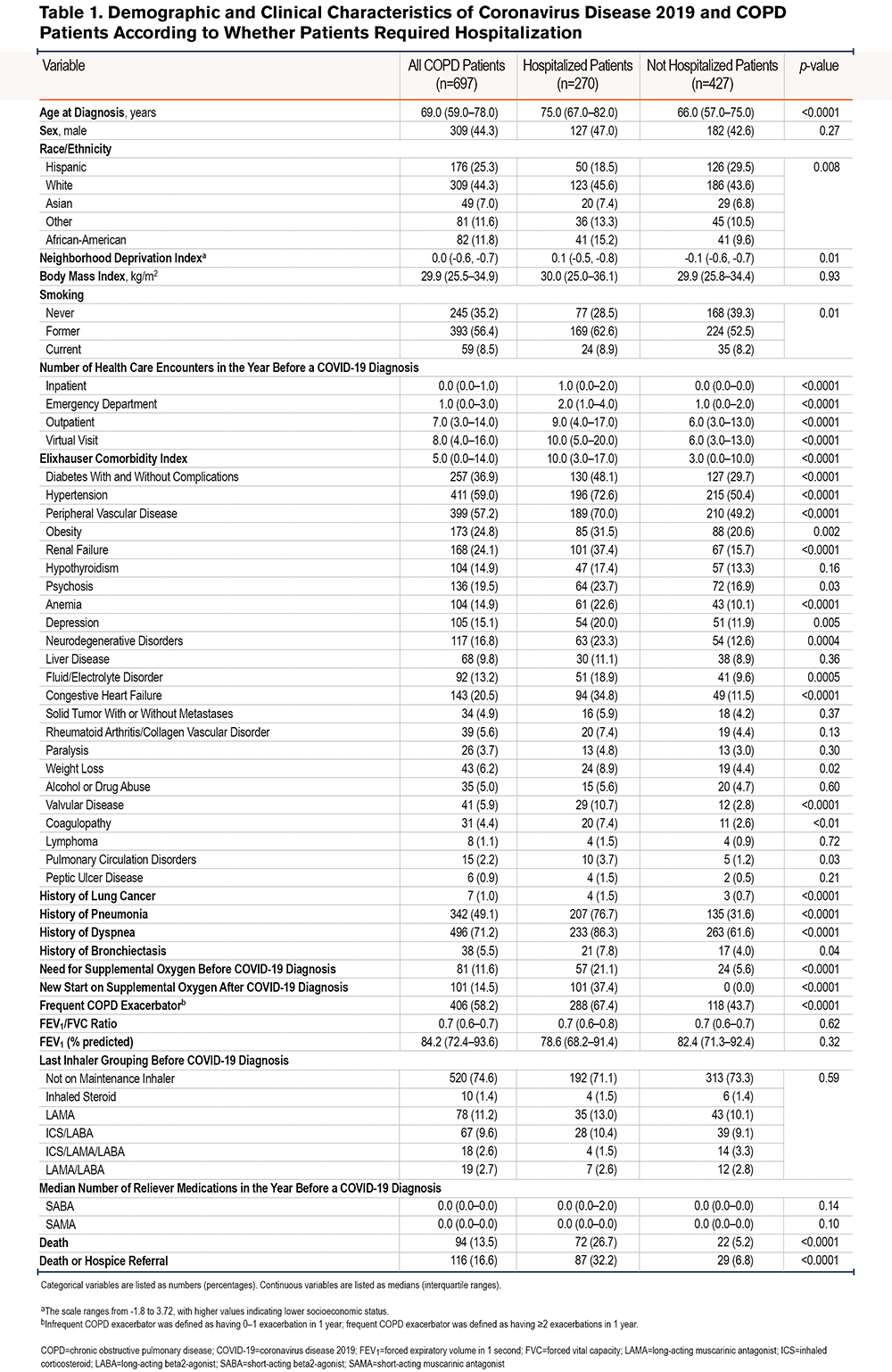
The median age of COVID-19 and COPD patients was 69.0 years (IQR 59.0–78.0) (Table 1). COPD patients hospitalized for COVID-19 were significantly older than those not hospitalized (75.0 versus 66.0 years, p<0.0001) and had a higher comorbidity burden (Elixhauser index of 10.0 versus 3.0, p<0.0001). Additionally, COPD patients hospitalized for COVID-19 had a higher neighborhood deprivation index, indicating lower socioeconomic status, than those not hospitalized (0.1 versus -0.1, p=0.01). The median number of baseline outpatient (9 versus 6, p<0.0001), emergency department (2 versus 1, p<0.0001), and inpatient (1 versus 0, p<0.0001) visits in the COVID-19 and COPD cohort was higher in the hospitalized than in the not-hospitalized population.
The majority of patients with COVID-19 and COPD (n=496, 71.2%) had a history of dyspnea diagnosis during an encounter in the year before the COVID-19 diagnosis. COPD patients hospitalized for COVID-19 were more likely to have a history of dyspnea than those not hospitalized (86.3% versus 61.6%, p<0.0001). Additionally, 342 patients (49%) had a history of pneumonia, and those with a history of pneumonia were more likely to be hospitalized for COVID-19 than those without a history of pneumonia (76.7% versus 31.6%, p<0.0001).
Overall, 81 (11.6%) patients required supplemental oxygen before the COVID-19 diagnosis. All patients who newly required supplemental oxygen after the COVID-19 diagnosis (14.5%) required hospitalization. Over half of the cohort (58.2%) was considered frequent COPD exacerbators in the baseline period. COPD patients hospitalized for COVID-19 were more likely to be frequent exacerbators than those not hospitalized (67.4% versus 43.7%, p<0.0001).
A total of 94 patients (13.5%) died and 116 (16.6%) died or had hospice referral. COPD patients hospitalized for COVID-19 were more likely to die than those not hospitalized (26.7% versus 5.2%, p<0.0001).
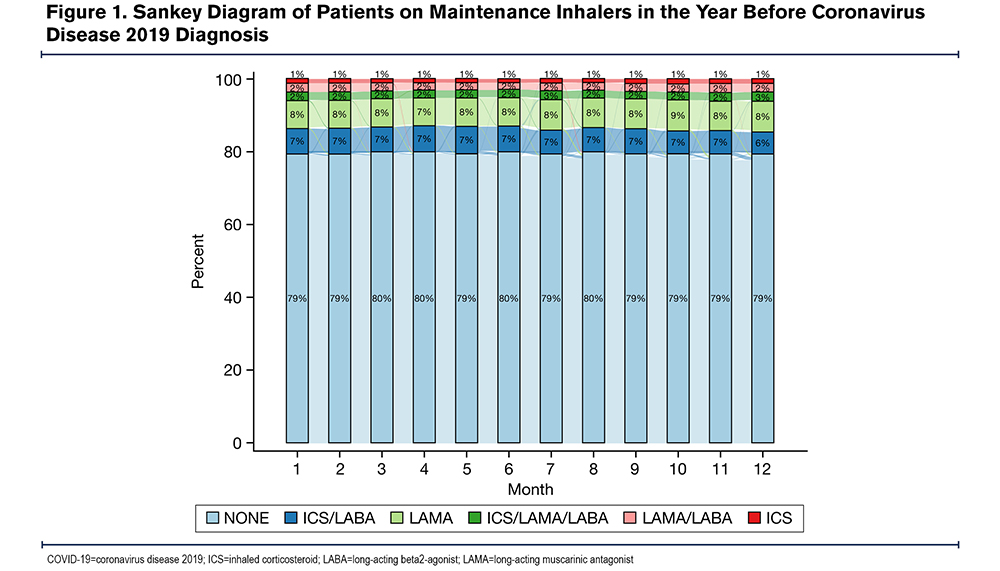
A majority of the cohort (71.7%) were not on any maintenance inhalers in the month before the COVID-19 diagnosis (Table 1). For those on maintenance medications in the year before the COVID-19 diagnosis, the regimen tended to remain stable over time (Figure 1). There were 84 patients (12.1%) who experienced 1 change in their inhaler regimen and 19 (2.7%) who experienced 2 changes in their inhaler regimen in the year before the COVID-19 diagnosis. Overall, 538 (77.2%) were neither exposed to steroids in the month before the COVID-19 diagnosis nor persistently exposed, 53 (7.6%) were exposed in the month before but not persistently, 23 (3.3%) were exposed persistently but not in the month before, and 83 (11.9%) were exposed both persistently and in the month before (Table 2).
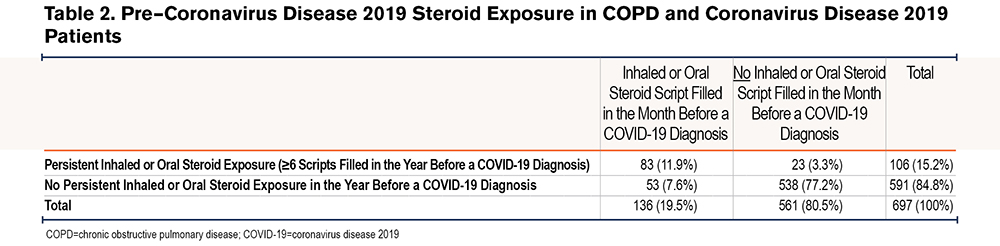
To quantify the dose of steroids received, the median daily dose was 40 mg (IQR 22–40) of prednisone equivalents among those exposed to oral steroids and 500 mcg (IQR 500–1000) of fluticasone propionate equivalents among those exposed to inhaled steroids in the month before COVID-19 diagnosis.
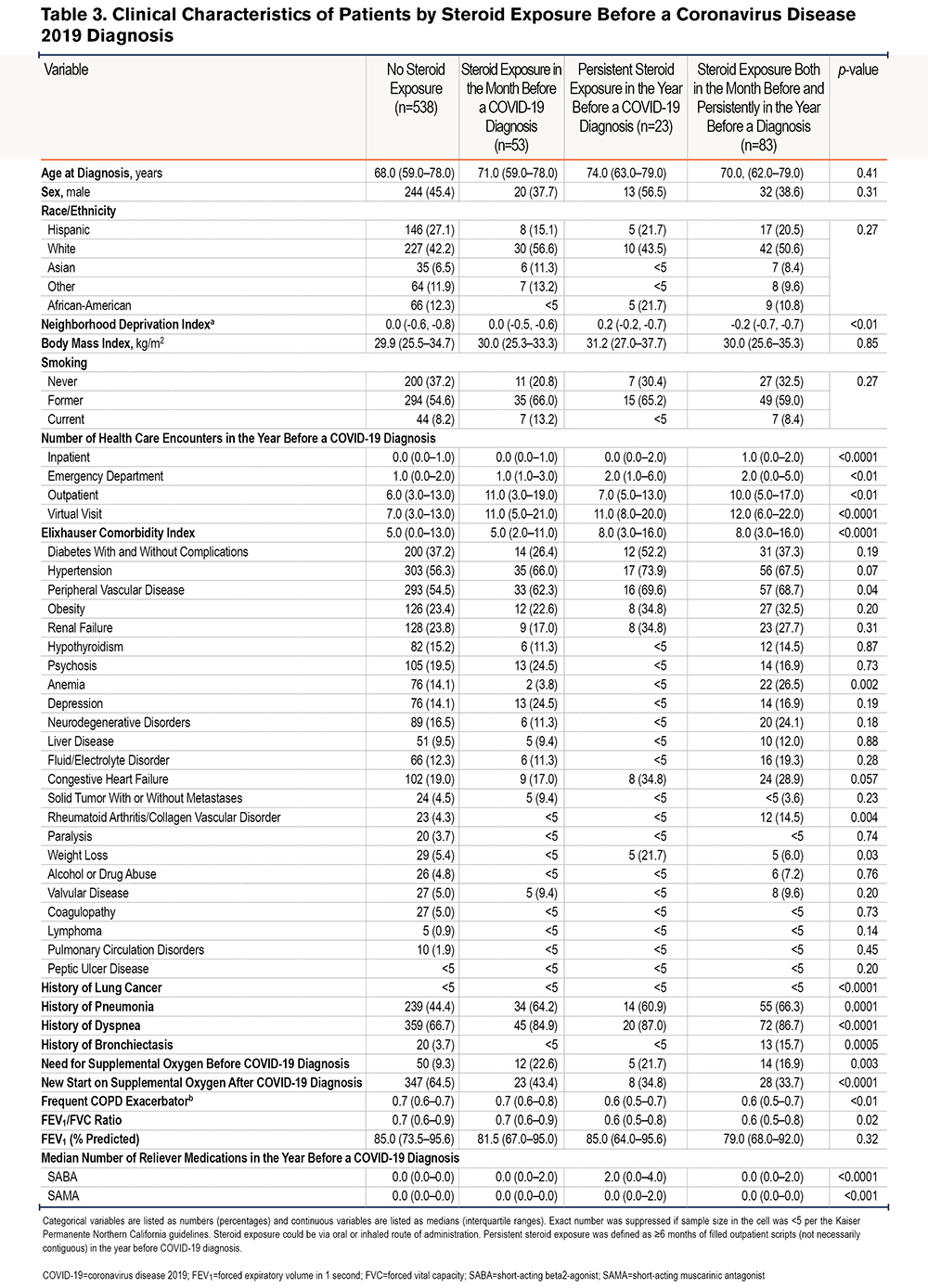
Because the receipt of steroids can be a marker of the severity of COPD disease, we examined whether clinical characteristics were different in patients according to their degree of steroid exposure (Table 3). There were no differences in demographic characteristics (age, sex, race, neighborhood deprivation index, body mass index, or smoking status) by degree of steroid exposure. However, there were differences in health care utilization before the COVID-19 diagnosis. Patients who had received steroids persistently and 1 month before their COVID-19 diagnosis tended to have a higher median number of hospitalizations (1; IQR 0–2) compared with patients who had neither exposure (0; IQR 0,1). Additionally, the median Elixhauser comorbidity index in patients who had received steroids persistently and 1 month before COVID-19 diagnosis tended to be higher (8; IQR 3–16) compared with patients who had neither exposure (5; IQR 0, 13); the median forced expiratory volume in 1 second (FEV1) was similar between patients exposed to steroids persistently and 1 month before their COVID-19 diagnosis (79.0% percent of predicted; IQR 68.0%, 92.0%) compared with patients who had neither exposure (85.0%; IQR 73.5%, 95.6%). Patients with both persistent and steroid exposure 1 month prior had a higher incidence of pneumonia than those without steroid exposure (66.3% versus 44.4%). Comparisons of individual comorbidities are limited by the small sample size within each cell (Table 3).
The unadjusted OR for the risk of hospitalization in the exposed compared with the unexposed cohort in the baseline model was 1.51 (95% CI 0.99–2.28; Table 4). The unadjusted OR for death or hospice referral in the baseline model was 1.59 (95% CI 0.96–2.63). After adjusting for all variables (Model 3), the OR for hospitalization was 0.77 (95% CI 0.41–1.46); which was considerably lower than an OR of 1.46 for death or hospice referral (95% CI 0.66–3.25). Only Model 1 for the secondary outcome of death or hospice referral produced a significant result with the lower CI barely above 1 (OR 2.00, 95% CI 1.01–3.95). The overall AIC decreased for both primary and secondary outcomes with the addition of more variables. Among patients who had survived COVID‑19–related hospitalizations, there was a higher risk of 30-day, nonelective, any-cause rehospitalization for COPD patients after adjusting for the covariates in Model 3, although with a wide upper CI (OR 3.75, 95% CI 1.25–11.26).
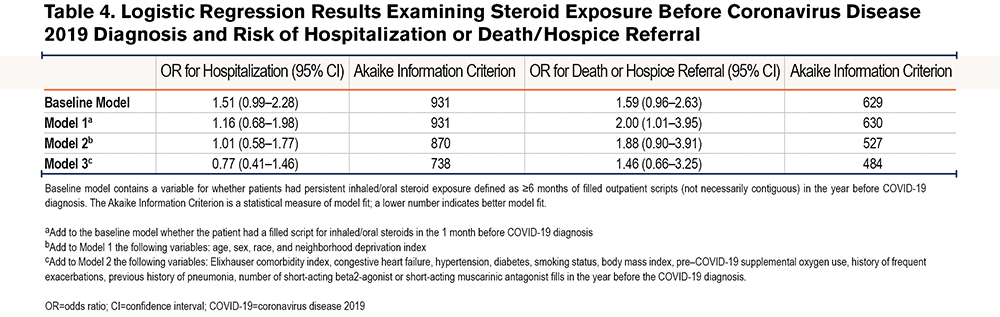
The requirement of a higher threshold (≥8 months) to flag a patient as persistently exposed to steroids in the year before their COVID-19 diagnosis decreased the number of patients considered exposed to 17 (2.4%), without a change in the regression result for hospitalization.
Discussion
We evaluated, in COPD patients, the association between persistent steroid exposure in the year before a COVID-19 diagnosis and risk of hospitalization. We found that patients hospitalized with COVID-19 were more likely to have a history of pneumonia than those who did not require hospitalization and that patients exposed to steroids before a COVID-19 diagnosis were also more likely to have a history of pneumonia than those who were not exposed to steroids, either persistently or in the month before their COVID-19 diagnosis. We did not detect associations between persistent steroid exposure and either hospitalization or death/hospice referral after adjusting for covariates, including steroid exposure in the month before the COVID-19 diagnosis. Our null findings could be due to low power or a mixed result in which steroids may be beneficial in some patients to control their baseline COPD and harmful in others who may have prolonged viral shedding (>14 days) as a result of being immunocompromised.33 The statistically significant association between COPD and 30-day rehospitalization could reflect COPD patients’ pre–COVID-19 tendency to engage in more health care encounters, which could reflect unmeasured confounders (e.g., anxiety and comorbid disease severity), although the wide CI should limit overinterpretation of this finding. Overall, this study addresses a clinically important question that is relevant to patient care during an evolving respiratory pandemic.
Our study adds to the current literature on steroid exposure before a COVID-19 infection. A large, randomized controlled trial previously examined the use of inhaled budesonide in adults within 7 days of the onset of mild COVID-19 symptoms.18 In the intention-to-treat analysis, the primary outcome of an emergency department visit or hospitalization occurred in 2 (3%) patients in the treatment arm and 11 (15%) in the usual care arm.18 Clinical recovery was 1 day shorter in the treatment arm (median: 7 days in the budesonide group).18 The trial was conducted in the general adult population, not COPD patients specifically, who have unique respiratory microbiomes, inflammation patterns, and physiologic responses to viruses.34 Nevertheless, it provides some evidence to suggest that short-term steroid exposure at the time of COVID-19 infection may be beneficial.
A retrospective United Kingdom–based study by Schultze et al, OpenSAFELY, examined 148,557 COPD patients who had been prescribed an inhaled steroid in the 4 months before a COVID-19 diagnosis, either as monotherapy or in combination with other medications.15 Although they report a higher risk of COVID-19–related deaths in patients prescribed ICSs than those prescribed nonsteroid inhalers after full risk adjustment (hazard ratio 1.39, 95% CI 1.10–1.76), the authors argue that this harmful association of steroids could be due to confounding that could not be adjusted for using electronic health data.15 They reason that: (1) they observed a higher risk of death in patients on triple therapy (ICS/long-acting beta2-agonists[LABA]/long-acting muscarinic antagonists[LAMA]), which contains the same amount of steroids as ICS-containing combination therapy but is given to patients with a more severe baseline disease, and (2) they observed a higher risk of non–COVID-19–related deaths in patients receiving inhaled steroids, which could reflect a more severe baseline disease. An alternative interpretation, however, could be that patients exposed to steroids before their COVID-19 diagnosis are at a higher risk of hospitalization due to more rapid viral proliferation early in the disease course. There is also a suggestion of a dose-response relationship of a higher ICS dose with higher mortality in the asthma cohort of the OpenSAFELY study. We report a null but directionally similar finding to Schultze et al’s study. Although our sample size was much smaller, we had more granular variables for risk adjustment (for example, the need for supplemental oxygen before the COVID-19 diagnosis). There were differences in how we defined steroid exposure (at least 6 filled prescriptions in 1 year before the COVID-19 diagnosis versus 1 prescription written [not filled] in the 4 months before the COVID-19 diagnosis).
Given the inconclusive evidence to date regarding the risk or benefit of exposure to a steroid-containing inhaler before the COVID-19 infection, GOLD has recommended that COPD patients continue their currently prescribed maintenance inhalers during the COVID-19 pandemic.35 Previous studies have shown that before the pandemic, patients were frequently either undertreated or overtreated with maintenance inhalers. The proportion of COPD patients who are on no maintenance therapy has been reported36-40 to range between 37% and 74%. Conversely, it has been reported that almost 60% of patients are receiving triple therapy (ICS/LABA/LAMA), even though GOLD recommends reserving triple therapy for moderate to severe patients, which is estimated to be only 10%–15% of the population.36,37 Given the evolving nature of evidence-based medicine during this pandemic, being on the correct maintenance inhaler is even more critical to the management of COPD patients given the potential risk of being on an unnecessary ICS if it were found to be harmful.
Our sample size of COPD patients who developed COVID-19 is small but consistent with that in previous literature. In a meta-analysis of 15 studies, Alqahtani et al report that only 2% of COVID-19 patients have COPD.41,42 Multiple studies from the Veterans Affairs (VA) reported a drop in COPD exacerbations during the pandemic, which could reflect masking and social distancing behaviors during the pandemic that prevented infections in this potentially vulnerable population.43,44 Baum et al reported a 48.4% total reduction in admissions with a principal diagnosis of COPD to the VA in the first 4 months of the pandemic.43 Other authors postulate that there is a protective effect of chronic steroids taken by COPD patients. Milne et al’s study showed that inhaled steroids downregulate the expression of ACE2, which is the receptor that SARS-CoV-2 uses to gain entry into cells.45
Our cohort of COVID-19 patients had mild COPD overall, with a median FEV1 of 84.2% predicted and only 25% were prescribed any maintenance inhaler before their COVID‑19 diagnosis. Trujillo et al report that COPD patients had high self-reported compliance with preventative measures during the pandemic and that patients with more severe COPD were more adherent to preventative measures.44 We believe our cohort represents a mild fraction of the COPD population due to behavioral modifications made by patients with a more severe baseline lung disease during the pandemic. Patients with severe baseline COPD may feel especially vulnerable and be more inclined to wear masks and socially distance themselves to avoid being infected in the first place.
Our study has multiple strengths. First, because the timing of steroid exposure is important in COVID-19, we accounted for steroid exposure both persistently and in the 1 month before a COVID-19 diagnosis. It is well established16 that a 10-day regimen of dexamethasone 6 mg daily confers a survival benefit to hypoxemic patients hospitalized for COVID-19. Current practice is to treat hospitalized patients with any degree of hypoxemia with this regimen but not those without hypoxemia or outpatients who may be earlier in their disease course.46 Treating patients early in their disease course with steroids could potentially cause harm given that the virus may still actively be replicating at day 10 after infection.47 Furthermore, we performed a sensitivity analysis of persistent steroid exposure and demonstrated that few patients changed maintenance inhaler regimens over time. The latter point confirms the stability of patients’ medication regimen (in turn, exposure) over time. Second, we used rich, longitudinal data such that we could capture variables as detailed as steroid dosing and hospice referrals, regardless of whether they occurred in the inpatient or outpatient setting. Third, we examined the AIC to assess model fit as we adjusted for increasing numbers of variables. This is the first study conducted in the United States that assesses the association between steroid use and the risk of hospitalizations in COPD patients.
However, several limitations must be considered. First, steroid exposure in COPD can be a marker of disease severity so it is challenging to study steroid exposure retrospectively. We directly compared the characteristics of patients receiving various forms of steroids before their COVID-19 diagnosis to clarify how they may be different. There were no differences in demographic characteristics, but patients with steroid exposure had a higher Elixhauser comorbidity index, a longer history of dyspnea and pneumonia, and higher previous health care utilization. Fortunately, these are characteristics that we could adjust for in regression modeling, but it is possible that other confounders exist that could not be adjusted for. Second, in defining patients with steroid exposure in the month before a COVID-19 diagnosis, this prescription could have been filled 1 day or 29 days prior and for any dose/duration. We quantified the median daily dose to describe the exposure group. However, we did not have the power to delineate other aspects of steroid exposure, which may be important for understanding whether there is an association with COVID-19–related hospitalization. For example, there may be a particular point in time before a COVID-19 diagnosis when steroid exposure flips from harmful to protective. Finally, a majority of the population did not have persistent steroid exposure, resulting in a small sample size for regression. As electronic medical records and claims data were used, it is unclear how many patients actually complied with the ICS regimen prescribed after acquiring the medication. Recently published literature suggests that the undertreatment of COPD with maintenance medications is common; hence, this finding is likely not unique to our cohort at KPNC.36-40 Lastly, we studied the relationship of steroids with COVID-19, which is a steroid-responsive viral infection, but the relationship with other viral infections (influenza, rhinovirus) may be different.
In conclusion, this retrospective cohort study did not detect an association between persistent steroid exposure before a COVID-19 diagnosis and hospitalization, which may have been limited by the sample size. While it is advisable to avoid oral steroids if possible due to the known side effects, for patients with moderate to severe COPD who meet the GOLD recommended criteria, the common practice has been to continue ICS therapy during the pandemic. Prevention measures, such as mask wearing, social distancing, and vaccination are indispensable for patients at a higher risk of contracting severe COVID-19 during the pandemic.
Acknowledgments
Author contributions: LCM, BMKD, and JFE conceived of the idea. LCM performed data analysis. VXL oversaw the data extraction. PK provided statistical consultation. RKM provided clinical consultation. All authors interpreted the results and critically reviewed the manuscript.
The authors wish to acknowledge Colleen Plimier, MPH, for programming assistance. We also wish to acknowledge Melissa Parker, MS, and Marvin Yan, MBA, for help with medication data extraction.
Data sharing statement: In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Declaration of Interest
BMKD, AS, and JFE are employees of Boehringer Ingelheim Pharmaceuticals, Inc. RKM is the Chief Medical Officer of Spire Health, serves as the Chairman of the Board for the Allergy and Asthma Foundation of America, and reports consulting fees from Boehringer Ingelheim Pharmaceuticals, Inc. LCM, VXL, and PK are employees of Kaiser Permanente Northern California and have received funding from Boehringer Ingelheim Pharmaceuticals, Inc., for this study. The original manuscript was written by lead author, Dr. Myers. Cactus editing service was used to assemble co-authors' edits and respond to requested revisions.