Running Head: Muscle Weakness in Current and Former Smokers
Funding support: The project described was supported by National Institutes of Health (NIH) and National Heart, Lung, & Blood Institute (NHLBI) Award Numbers U01 HL089897, U01 HL089856, and F32 HL164309. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the NHLBI. COPDGene is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Date of Acceptance: December 30, 2022 | Published Online Date: January 5, 2023
Abbreviations: β=standardized coefficient; 6MWD=6-minute walk distance; BMI=body mass index; CAT=COPD Assessment Test; COPD=chronic obstructive pulmonary disease; COPDGene®=COPD Genetic Epidemiology study; FEV1=forced expiratory time in 1 second; FVC=forced vital capacity; GH=General Health domain; GOLD=Global initiative for chronic Obstructive Lung Disease; HGS=handgrip strength; HR=hazards ratio; HU=Hounsfield unit; IQR=interquartile range; LAA -950=low attenuation area threshold of -950 Hounsfield units; PF=Physical Functioning domain; PRISm=preserved ratio-impaired spirometry; OR=odds ratio; SF-36=Short-Form-36; SGRQ=St George’s Respiratory Questionnaire; STS=sit-to-stand
Citation: Zou RH, Nouraie M, Rossiter HB, et al. Associations between muscle weakness and clinical outcomes in current and former smokers. Chronic Obstr Pulm Dis. 2023; 10(1): 112-121. doi: http://doi.org/10.15326/jcopdf.2022.0365
Online Supplemental Material: Read Online Supplemental Material (348KB)
Introduction
Extrapulmonary manifestations of chronic obstructive pulmonary disease (COPD) are well-recognized as important contributors to disease morbidity and mortality. Physical inactivity and reduced mobility are common features of both advanced aging and COPD that increase the risk of muscle weakness and dysfunction.1-4 Recent population-based studies estimate the prevalence of weakness to range between 20%-38% in individuals with COPD.5-6 Muscle weakness, which most commonly affects the thighs and upper limbs, contributes to clinical outcomes in COPD, including airflow obstruction, reduced functional status, decreased health-related quality of life, and mortality.2,7-9 Identifying muscle weakness in this population is critical for risk stratification and management, as early targeted interventions focusing on rehabilitation have been shown to improve exercise tolerance.10
While there are well-characterized studies describing weakness in COPD, there are limited data on associations between weakness measures and clinical outcomes in smokers with normal spirometry and smokers with preserved ratio-impaired spirometry (PRISm). Smokers with normal spirometry are at increased risk of respiratory symptoms and activity limitations compared with the general population.11-13 PRISm, a clinical phenotype characterized by reduced forced expiratory volume in 1 second (FEV1) and preserved ratio of FEV1 to forced vital capacity (FVC), is associated with higher rates of lung function decline and increased mortality compared with individuals with mild COPD.14-17 Therefore, it is important to understand whether weakness measures that have already been studied in classically defined COPD are generalizable to these other high-risk smoking subgroups.
Two commonly used measures to quantify muscle weakness are handgrip strength (HGS) and sit-to-stand (STS). The HGS test, which is a measure of upper limb strength and functionality, is reduced in individuals with COPD compared with healthy controls18,19 HGS is associated with lower pectoralis muscle area, which correlates with whole body lean muscle mass, as well as reduced health-related quality of life and greater exacerbation risk.20-22 The repeated STS test, which is a measure of lower limb function, strength, and fatiguability, is strongly associated with decreased functional capacity, reduced health-related quality of life, and increased mortality in individuals with COPD.23-26
The main objective of this study was to evaluate the prognostic validity of 30-second STS and HGS, 2 easy to perform measurements of weakness, on walk distance, health-related quality of life, severe exacerbations, and mortality in ever smokers with COPD, with normal spirometry, and with PRISm.
Methods
Study Population
Participants from the multicenter COPD Genetic Epidemiology (COPDGene) study cohort were enrolled according to the previously published study design.27 All participants were between 45 and 80 years of age at time of enrollment and were current or former smokers with ≥10 total pack-year smoking history. Pertinent exclusion criteria included alternative primary lung disease, recent severe exacerbation requiring systemic corticosteroids or hospitalization within 4 weeks, active malignancy, and history of chest surgery or radiation. All participants completed pre- and post-bronchodilator spirometry, the 6-minute walk test (6MWT), and respiratory-specific questionnaires at each visit. For this study, we evaluated 1972 participants during their 10-year (phase 3) study visit. This timeline was selected as the weakness measurements of interest, 30-second STS and HGS, were performed only during this study visit. Participant comorbidities, including cardiovascular disease, congestive heart failure, chronic kidney disease, diabetes mellitus, malignancy, osteoarthritis, and joint pain were assessed by self-report. Local site institutional review boards approved all data acquisition procedures and written informed consent was obtained from all participants.
Definitions
COPD is defined by a post-bronchodilator spirometry FEV1/FVC ratio of <0.70. Airflow obstruction severity is characterized by post-bronchodilator percent predicted FEV1 (%predFEV1) using Global initiative for chronic Obstructive Lung Disease (GOLD) criteria, with mild, moderate, severe, and very severe disease defined by FEV1≥80%, 50%–79%, 30%–49%, and <30%, respectively.28 PRISm is defined15 as FEV1 ≤80% with FEV1/FVC ratio of ≥0.70. (Emphysema is quantified using a low attenuation area threshold of -950 Hounsfield units (HU) (%LAA -950).29
Sit-To-Stand Test
STS was measured by asking participants to stand from a seated position with their arms held across their chest and to sit back down for as many repetitions as possible over a 30-second period. Trained personnel monitored participant form, with incorrectly executed repetitions removed from the final count. All participants wore their prescribed supplemental oxygen during testing.
Handgrip Strength Test
HGS was measured using a standardized protocol with a Jamar hand dynamometer (Fabrication Enterprises, White Plains, New York), where 3 separate repeated measurements were taken using the participant’s dominant, unsupported hand.19,21 The highest value was recorded.
6-Minute Walk Test
The 6MWT is a validated functional exercise performance measure used to assess physical fitness and performance status in COPD.30 Participants were asked to walk as far as possible along stretches of an unimpeded 30-meter corridor for a total of 6 minutes, with standardized instructions at each minute. The participant could terminate exercise for fatigue or symptoms. The primary measurement is walk distance in meters. All participants prescribed oxygen therapy utilized supplemental oxygen therapy during testing.
Respiratory Questionnaires
The St George’s Respiratory Questionnaire (SGRQ) is a COPD-specific validated questionnaire used to assess respiratory health status and health-related quality of life.31 SGRQ scores range between 0–100, where higher scores indicate greater respiratory symptom burden. The Short-Form-36 (SF-36) questionnaire is a validated measure of functional health and overall well-being using questions spread out over 8 domains with score ranges between 0–100, where lower scores indicate poorer health.32 For this study, we focused on the General Health (GH) and Physical Functioning (PF) domains. The COPD Assessment Test (CAT) is a patient-reported questionnaire evaluating the impact of COPD on health status with score ranges between 0–40, where higher scores indicate greater respiratory symptom burden.33 History of severe exacerbations, defined by those requiring hospitalization in the year prior to the study visit, was self-reported and dichotomized as absent or present. Prospective severe exacerbations following the study visit were assessed by phone at regular 6-month intervals and dichotomized as absent or present. A total of 1571 participants had prospective severe exacerbation data, with a median follow-up of 384 days (interquartile range [IQR] 206–487 days) and a maximum follow-up of 883 days.
Statistical Analysis
Our primary objective was to evaluate the prognostic validity of the weakness measures, 30-second STS and HGS, on clinical outcomes in ever smokers with COPD, with normal spirometry, and with PRISm. Multivariable linear regression modeling was used to determine relationships between weakness measures and the 6MWD, the SGRQ, the SF-36 General Health questionnaire, and the SF-36 Physical Functioning questionnaire in the entire cohort, with results represented as standardized coefficients (β). Subgroup analyses stratified by sex (male, female) and spirometry (airflow obstruction, normal spirometry, PRISm) were also performed. Multivariable logistic regression modeling was used to determine relationships between weakness measures and history of severe exacerbation and prospective severe exacerbation, with results represented as odds ratios (ORs) with 95% confidence intervals. Multivariable proportional hazards regression modeling was used to determine relationships between weakness measures and mortality since the study visit, with results represented as hazard ratios (HRs) with 95% confidence intervals. Time-to-event survival analyses using Kaplan-Meier survival function and Cox proportional hazards regression modeling were used to depict prospective mortality since the study visit to the recorded time of death. The generalized Wilcoxon/Gehan-Breslow weighted log-rank test using Bonferroni adjustment for multiple comparisons was used to evaluate survival differences between subgroups, as proportional hazards assumptions were not met. For all analyses, models were adjusted for age, sex, body mass index (BMI), and FEV1% predicted. Sex was included as a covariate due to differences in muscle mass and muscle distribution between sexes. BMI was included as a covariate due to the inverse correlation between BMI and acute exacerbation of COPD.34,35 FEV1% predicted was included as a covariate due to its strong association with weakness, independent of airflow obstruction.36 We evaluated cardiovascular health (self-reported cardiovascular disease and congestive heart failure) as a potential confounder between weakness measures and clinical outcomes of interest in multivariable regression modeling, using a 10% change in coefficients cutoff as meaningful. All analyses were performed using Stata 17.1 (StataCorp, Inc. College Station, Texas).
Results
Study Cohort Characteristics
The study cohort had a mean age of 69.0±8.4 years, equal sex distribution (49% male, 51% female), BMI of 29.0±6.2kg/m2, and substantial smoking history (median 40 pack years, IQR 28–54 pack-years) (Table 1). A total of 923 (47%) participants met GOLD criteria for COPD, with the majority demonstrating mild (26%) to moderate (42%) airflow obstruction (data not shown). A total of 173 (9%) participants reported a history of severe exacerbations in the year prior to the study visit. Participants had a mean 30-second STS of 10.7±4.2 repetitions and HGS of 29.0±9.9kg. Health-related quality of life measures were notable for a median SGRQ score of 18 (IQR 6–37), an SF-36 General Health score of 67 (IQR 47–82), and an SF-36 Physical Functioning score of 70 (IQR 40–90). There were no substantial clinical measurement differences in sex-stratified analyses, with the exception of greater mean HGS (35.4±9.0kg versus 22.7±6.1kg) and SF-36 Physical Functioning score (75, IQR 45–90 versus 65, IQR 40–88) in males compared with females (supplemental Table 1 in the online supplement).
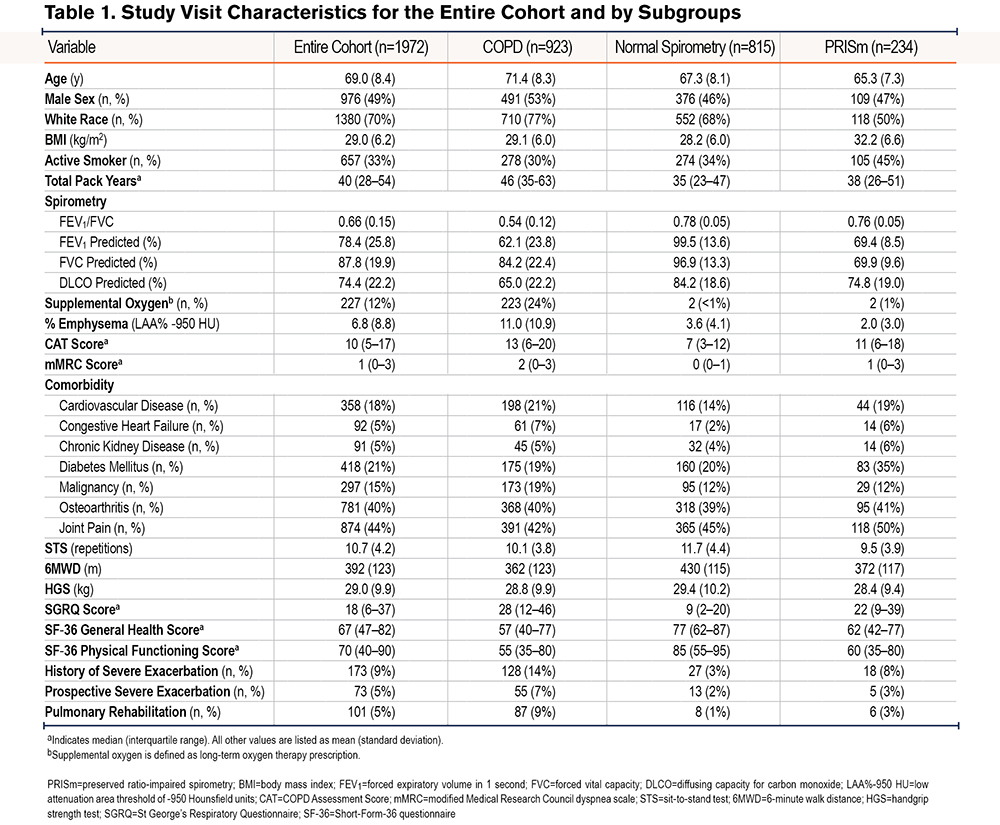
Compared with participants with normal spirometry, participants with COPD tended to be older (71.4±8.3 years versus 67.3±8.1 years), have lower FEV1% predicted (62.1±23.8 versus 99.5±13.6), and have higher frequency of prior severe exacerbations (14% versus 3%) (Table 1). The COPD subgroup also had a lower 6MWD (362±123m versus 430±115m), SF-36 General Health score (57, IQR 40–77 versus 77, IQR 62–87), and SF-36 Physical Functioning score (55, IQR 35–80 versus 85, IQR 55–95), as well as a higher SGRQ score (28, IQR 12–46 versus 9, IQR 2–20) compared with the normal spirometry subgroup. On average, participants with PRISm had clinical measurements falling between participants with COPD and with normal spirometry, most notably with regards to FEV1% predicted, walk distance, health-related quality of life, and severe exacerbation frequency. In subgroup analyses, there were no substantial differences in HGS and STS performance between current and former smokers in the entire cohort (data not shown); these subgroups had similar total pack-year smoking history.
Weakness Measures Correlate with Clinical Outcomes
STS and HGS correlated with walk distance, health-related quality of life, history of severe exacerbations, and mortality (Table 2). The overall magnitudes of effect size for clinical outcomes were larger for STS than for HGS. Self-reported cardiovascular disease and congestive heart failure were not meaningful confounders between weakness measures and clinical outcomes, with absolute changes in coefficients of <8% (data not shown). Subgroup analyses comparing SGRQ, and SF-36 subdomains demonstrated similar magnitudes of effect sizes for STS and HGS (supplemental Table 2 in the online supplement). In sex-stratified analyses, females had larger magnitude effect sizes between STS and health-related quality of life, history of severe exacerbations, and mortality compared with males (supplemental Table 3 in the online supplement), without substantial interaction between sex and clinical outcomes.
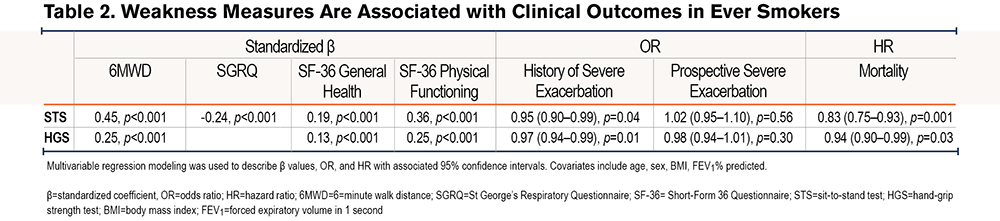
Lower Sit-to-Stand Quartile Associates with Increased Mortality
The median duration of prospective mortality follow-up since the study visit was 384 days (IQR 206–487 days) and a maximum follow-up of 883 days. Participants in the lowest STS quartile (quartile 1: mean 6.2±2.0 repetitions) had lower survival compared with participants in the higher STS quartiles (quartile 3: mean 11.9±0.8 repetitions; quartile 4: mean 16.8±3.3 repetitions) (Figure 1).
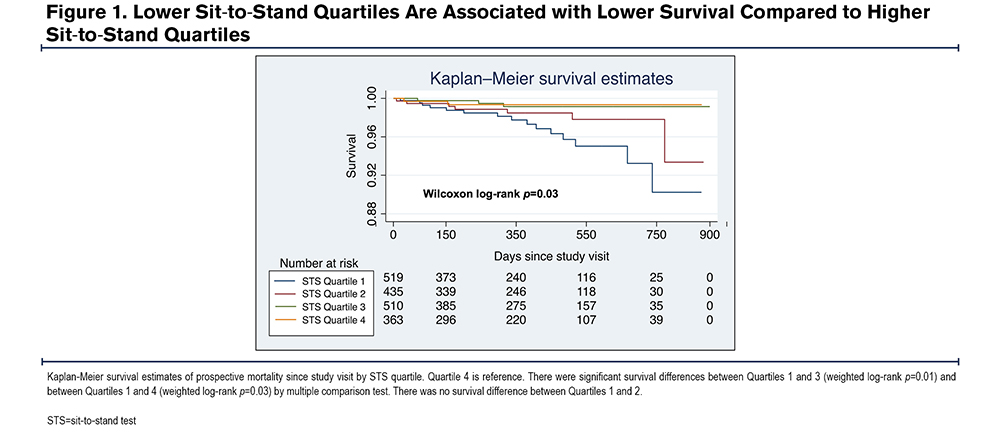
Associations Are Independent of Spirometric Subgroup
When stratified by COPD, normal spirometry, or PRISm status, STS and HGS retained associations with the 6MWD, SGRQ, SF-36 General Health, and SF-36 Physical Functioning in adjusted analyses (Figure 2). The PRISm subgroup had similar or larger magnitude effect sizes for clinical outcomes compared with the other subgroups. Compared with males, females had an overall trend towards larger magnitude effect sizes between STS and walk distance and SF-36 scores (supplemental Figure 1 in the online supplement). There were no consistent subgroup associations or trends between weakness measures and severe exacerbations and mortality (data not shown).
Discussion
In this study, we demonstrated that higher 30-second STS and HGS performances are associated with improved clinical outcomes in current and former smokers in the COPDGene study cohort. These associations are independent of airflow obstruction and suggest that STS and HGS, 2 easy to perform measures of weakness in the outpatient setting, can provide valuable prognostic information about functional performance, health-related quality of life, severe exacerbations, and mortality in a high-risk population.
We reported the important clinical finding that the magnitudes of effect size between weakness measures and functional capacity and health-related quality of life were similar between spirometric subgroups after adjusting for age, sex, BMI, and FEV1% predicted. With increasing recognition that smokers with normal spirometry and smokers with PRISm have substantial respiratory symptom burden and functional limitations, our results demonstrated that the correlations observed in these subgroups were similar to those seen in smokers with airflow obstruction. Respiratory symptom burden in participants with PRISm and with normal spirometry by CAT subscore (breathlessness) and mMRC score, even in the absence of a reflection of FEV1/FVC, may have contributed to reduced physical activity and consequent deconditioning. This suggests that respiratory symptom burden and functional limitations in smokers may not be primarily driven by underlying lung disease, but rather by a combination of lung disease and comorbidity burden, most notably cardiovascular fitness. These results add to prior literature describing weakness in lung disease, which has focused primarily on individuals with COPD, and demonstrate the generalizability of STS and HGS in the evaluation of functional performance and health-related quality of life in high-risk subgroups that may not have been routinely considered for weakness evaluation.
The magnitudes of effect size were larger for STS than for HGS for clinical outcomes. Puhan and colleagues reported similar findings in a smaller Dutch and Swiss outpatient cohort with moderate to severe COPD.23 The stronger magnitude associations may be partially due to differences in the underlying characteristics of the tests: STS relies primarily on coordination of lower extremity strength and truncal stability, as well as muscle flexor and extensor strength, whereas HGS relies primarily on isometric contraction of the dominant hand. Therefore, it is possible that STS may be a stronger predictor of overall mobility and, therefore, functional status and health-related quality of life. It is noteworthy that we cannot make direct comparisons between hazard ratios for mortality as these values are not standardized, as opposed to the standardized coefficient values for the 6MWD, SGRQ, and SF-36. The simplistic nature of the STS test, which does not rely on specialized equipment or repeated measurements, makes it an ideal measurement of weakness in the outpatient setting.
We demonstrated sex-specific differences in weakness correlations with clinical outcomes in our heterogeneous study cohort. STS was more strongly associated with health-related quality of life, history of severe exacerbations, and mortality in females compared with males in spite of similar study visit clinical characteristics. Despite this, we did not observe sex-specific associations for HGS even though substantial sex differences were found in this measure (35.4±9.0kg in males, 22.7±6.1kg in females). It is unclear how comorbidity burden affects the sex-specific magnitude strength of associations between STS and clinical outcomes, as females reported lower frequency of cardiovascular disease but higher frequencies of osteoarthritis and joint pain. Sex differences in lean muscle mass, skeletal muscle distribution, and body fat composition may contribute to these findings. Sex-specific predictive differences between weakness measures and clinical outcomes are complicated and require further analyses.
The PRISm subgroup had strong correlations between weakness measures and functional capacity and health-related quality of life. In our study cohort, participants with PRISm had clinical measurements in between participants with COPD and participants with normal spirometry. This is consistent with prior studies suggesting that PRISm is a transitional stage between normal spirometry and COPD, with 12%–38% of PRISm individuals eventually progressing to airflow obstruction.13,16,17,37,38 The strong associations between weakness measures and clinical outcomes may be explained by the substantial heterogeneity within the PRISm subgroup itself. When PRISm participants were stratified by FEV1% predicted, those in the lowest quartile demonstrated higher weakness measures and comorbidity burden, as well as lower walk distance and health-related quality of life scores, compared with those in the highest quartile (supplemental Table 3 in the online supplement). This suggests that, perhaps, the heterogeneous PRISm phenotype should be further stratified by FEV1% predicted for risk stratification, similar to GOLD staging for COPD. The associations between weakness measures and clinical outcomes in this subgroup may have been influenced by increased prevalence of metabolic disease with diabetes mellitus and peripheral neuropathy.39 Additionally, the higher prevalence of self-reported osteoarthritis and joint pain in participants with PRISm may have contributed to lower strength measures and increased functional status limitations in this subgroup. Our results reiterate the importance of screening former and current smokers who have spirometric abnormalities beyond airflow obstruction for weakness.
While pulmonary rehabilitation is known to be associated with improved exercise capacity and health-related quality of life in COPD, this intervention is generally underutilized in the outpatient setting; only 5% of participants in this cohort participated in pulmonary rehabilitation at the time of the study. Importantly, evaluation of the impact of the pulmonary rehabilitation intervention on measures of muscle weakness, exercise capacity, and health-related quality of life in smokers with normal spirometry and with PRISm has not been evaluated well and is an important area of future research.
There are several strengths of this study worth highlighting. First, our cohort was comprised of a large percentage (53%) of current and former smokers with normal spirometry and with PRISm, which discriminates it from previous studies focusing primarily on participants with spirometrically-defined COPD. In particular, the clinical and physiologic heterogeneity between these subgroups is important and provides critical information about different populations of ever smokers at risk for weakness, morbidity, and mortality. Second, this study demonstrated the prognostic validity of 2 easy to perform weakness measures, 30-second STS and HGS, on walk distance, health-related quality of life, history of severe exacerbations, and mortality in ever smokers. Our results indicate that simple test measures, which can be rapidly performed in the outpatient setting without the time- or labor-intensive testing required for many current weakness models, have important clinical implications. Third, this was a large, multicenter study cohort with well-characterized data on almost 2000 heterogenic participants.
There are several limitations of this study. First, the majority of participants with airflow obstruction had mild to moderate lung disease, which may limit generalizability to individuals with severe COPD who may have higher frequencies of severe exacerbations. However, it is worth noting that individuals with severe COPD are generally more likely to be screened for weakness and enrolled in rehabilitation programs to improve functional status and respiratory symptoms. Second, this study focused primarily on cross-sectional measures and only included several longitudinal measures with limited follow-up data since the study visit. This contributed to right censoring when prospective severe exacerbations and mortality events were either unknown or not observed. Third, we focused this study on weakness measures but did not have readily available neurocognitive, psychometric, or health care utilization scores that contribute to overall frailty in this population, which is associated with COPD morbidity and mortality. Fourth, participant responses may be subject to recall bias and response bias for self-reported answers about severe exacerbation history. Fifth, we did not have quadriceps dynamometry available for quantification of isokinetic lower extremity muscle function measurements due to the need for specialized equipment. This limits direct comparisons between upper and lower extremity clinical testing.
In conclusion, our study showed that poorer 30-second STS and HGS performance was associated with adverse clinical outcomes in COPDGene participants. STS and HGS are 2 easy to perform measurements of weakness with the potential to be used for earlier risk stratification and referral to pulmonary rehabilitation. Our findings highlight the importance of screening all current and former smokers for weakness in the outpatient setting.
Acknowledgements
Author contributions: RHZ is the guarantor of the content of this manuscript and takes overall responsibility for the manuscript. RHZ, RC, EAR, and JB were responsible for the initial study concept and design. RHZ and SMN performed statistical analyses. RHZ, SMN, HBR, MLM, DLD, SM, GRW, PKS, BJM, RC, EAR, and JB contributed to data interpretation, manuscript preparation, manuscript revisions, and were responsible for the decision to submit the manuscript.
Declaration of Interest
HBR reports grants and contracts from the NIH and Tobacco-Related Disease Research Program, consulting fees from Omniox, and is involved in contracted clinical research with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Astellas, United Therapeutics, Genentech, and Regeneron; he is a visiting Professor at the University of Leeds, United Kingdom. DLD reports grants and contracts from the NIH and Bayer. GRW reports grants and contracts from the NIH, Department of Defense, and Boehringer Ingelheim, consulting fees from Pulmonx, Vertex, and Janssen Pharmaceuticals, participation on a data safety monitoring board for Pulmonx and is a co-founder and equity shareholder in Quantitative Imaging Solutions, a company that provides consulting and software development services for image and data analytics; GRW’s wife works for Biogen. BJM reports grants and contracts from NHLBI, American Lung Association, Department of Defense, AstraZeneca, and Pearl, consulting fees from AstraZeneca and Third Pole, royalties from Wolters Kluwer Health (UpToDate), and participation on data safety monitoring boards for Spiration, Mylan, Quintiles, Mount Sinai, University of Wisconsin, and Baystate Medical Center; he serves on medical advisory boards for GlaxoSmithKline, Boehringer Ingelheim, and AstraZeneca. RC reports grants and contracts from Regeneron and speaker bureau participation with GlaxoSmithKline and Boehringer Ingelheim; he serves on the medical advisory board and has stock ownership with Inogen. RHZ, SMN, MLM, SM, PKS, EAR, and JB have no conflicts to disclose.