Running Head: Physical Activity and Symptom Burden in COPD
Funding support: The Canadian Cohort Obstructive Lung Disease (CanCOLD) study is funded by the Canadian Institutes of Health Research (CIHR/ Rx&D Collaborative Research Program Operating Grants- 93326); the Canadian Respiratory Research Network (CRRN); the Respiratory Health Network of the Fonds de recherche du Québec - Santé; industry partners: Almirall; Astra Zeneca Canada Ltd; Boehringer Ingelheim Canada Ltd; GlaxoSmithKline Canada Ltd; Merck; Novartis; Nycomed; Pfizer Canada Ltd; and Theratechnologies; and the Jo Kolk Study Fund Foundation
Date of Acceptance: December 19, 2022 | Published Online Date: December 22, 2022
Abbreviations: ALE=active living environment; BMI=body mass index; Can-ALE=Canadian Active Living Environment; CanCOLD=Canadian Cohort Obstructive Lung Disease study; CAT=COPD Assessment Test; CHAMPS=Community Health Activities Model Program for Seniors; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CPET=cardiopulmonary exercise testing; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; MVPA=moderate-to-vigorous physical activity; mMRC=modified Medical Research Council; PA=physical activity; peak VO2=exercise capacity; PFT=pulmonary function test/testing
Citation: Oostrik L, Bourbeau J, Doiron D, et al. Physical activity and symptom burden in COPD: the Canadian Obstructive Lung Disease study. Chronic Obstr Pulm Dis. 2023; 10(1): 89-101. doi: http://doi.org/10.15326/jcopdf.2022.0349
Online Supplemental Material: Read Online Supplemental Material (295KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease that is characterized by airflow limitation and is accompanied by a high symptom burden and mortality rates.1 COPD affected 215 million individuals worldwide in 2017 and the incidence is increasing due to the continued exposure to smoking- and non-smoking-related risk factors and the ageing of many populations.2,3 In Canada, the prevalence of COPD is over half a million patients with an estimated equal number of undiagnosed individuals.4
The most common symptom of COPD is dyspnea made worse by exertion including daily physical activity (PA).2,4-6 PA levels have been shown to be lower in individuals with COPD, although heterogeneity is high.7 Decreased PA in individuals with COPD is of concern as it has been shown to be associated with lung function decline, increased exacerbations, frequent hospital admissions, and most importantly, higher mortality.5,6,7
Studies on determinants of PA in COPD populations have provided limited information due to small sample sizes and lack of control groups.8 Cross-sectional and longitudinal studies of PA in COPD have included, predominantly, patients with moderate to severe COPD attending outpatient clinics. The Canadian Cohort Obstructive Lung Disease study (CanCOLD) tracks more than 1500 participants and is one of the rare cohorts specific to COPD that has recruited its participants from the general population rather than more convenient sampling in clinical settings. This has the advantage to better mirror the prevalence of COPD including those with physician-diagnosed and undiagnosed disease.9 Although individuals with undiagnosed COPD may be less symptomatic and impaired, even if they experience less exacerbations than those with physician-diagnosed COPD, overall burden on the health care system can be considerable.10,11 Furthermore, from the same CanCOLD cohort, it has been demonstrated that individuals with mild COPD were more likely to report significant dyspnea and worse health-related quality of life than non-COPD individuals.11,12
The present study explored the relationship between symptom burden and PA in COPD patients followed in the CanCOLD study. The primary objective was to investigate the relationship between the impact of COPD symptoms on moderate-to-vigorous intensity PA (MVPA) in individuals with mild to moderate COPD from a random sampling that mirrors the COPD population at large. The secondary objectives were: (1) to investigate MVPA in individuals with physician-diagnosed mild to moderate COPD compared to those with undiagnosed COPD, and (2) to assess factors that are associated with MVPA in people with mild to moderate COPD compared to those without COPD. We hypothesized that individuals sampled from the general population with mild to moderate COPD having significant symptom burden accounted by the COPD Assessment Test (CAT) would have lower levels of MVPA than those with low symptom burden.
Methods
This study was a cross-sectional sub-study embedded in the CanCOLD study, an ongoing prospective cohort study across 9 study sites in Canada with general population sampling. Full details on this cohort study design can be found in a previously published report.13
Population
The study population of the CanCOLD cohort consists of non-institutionalized adults above the age of 40 recruited in 9 Canadian cities. COPD diagnosis and severity were determined by post-bronchodilator spirometry according to the Global initiative for chronic Obstructive Lung Disease (GOLD)2 and divided into 4 sub-groups: (1) healthy individuals (never-smokers and normal spirometry; n=347), (2) individuals at-risk for COPD (ever-smokers and normal spirometry; n=474), (3) GOLD 1 or mild COPD (n=406), and (4) GOLD 2 or moderate COPD (n=287). Individuals in group GOLD 3 and GOLD 4 or severe COPD (n=44) were not included due to the small sample size and because the emphasis of our study was on mild to moderate disease. Individuals with spirometrically-defined COPD who reported having received a previous physician diagnosis of COPD, chronic bronchitis, or emphysema on entering the CanCOLD study were identified as “diagnosed,” and those with spirometrically-defined COPD, who reported not having a previous physician diagnosis of COPD before entry in the CanCOLD study, were identified as having “undiagnosed” COPD.
All demographic variables, clinical characteristics, outcomes, and other factors analyzed in this cross-sectional sub-study were provided by the CanCOLD group in February 2020. Data analyzed in this sub-study were collected during baseline CanCOLD on-site visits between 2009-2015. Demographics (age, body mass index, sex, marital status, working status, and smoking habits) and clinical data such as symptom burden, past exacerbation habits, diagnosis status, comorbidities, and respiratory medication were collected using a questionnaire. Perceived respiratory disability was collected by the McGill COPD questionnaire for both COPD groups and the at-risk group. It was not required for participants from the healthy group. However, if they did agree to fill out the questionnaire, the data was saved in our database. Pulmonary function and exercise capacity were measured according to standard pulmonary function testing (PFT) and cardiopulmonary exercise testing (CPET) methods, respectively. The Canadian Active Living Environment (Can-ALE) index walkability dataset was provided by the Canadian Urban Environmental Health Research Consortium14 and linked to 6-digit residential postal codes of CanCOLD participants. Can-ALE index is a composite score of different factors associated with walking rates such as intersection density, dwelling density, and points of interest.15
Physical Activity and Symptom Burden
The primary outcome was MVPA expressed in energy expenditure (kcal/week). This was estimated by the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire.16,17 The CHAMPS is a reliable and valid questionnaire to estimate PA in people with COPD.16,17 It consists of 40 items of which 28 items assess weekly frequency and duration of different physical activities. Caloric expenditure associated with these physical activities are then calculated.16 This caloric or energy expenditure is calculated by a combination of the duration of an activity with the load of that activity, and therefore, a more comprehensive estimation than frequency of PA. Stewart et al tested known-groups validity and recognized 3 physical activity levels: initially sedentary (mean energy expenditure 1057[SE149]), somewhat active (mean 1136 [SE125]), and already active (2328[SE181]).18 Data on frequency and duration of MVPA are presented in the online supplement.
Symptom burden was measured using the CAT. Symptom burden was classified categorically by the cut-off points (CAT: low <10, high≥10) consistent with the original recommendations and the GOLD reports for clinical practice.2,19
Other possible factors included forced expiratory volume in 1 second (FEV1), exercise capacity (peakVO2), season (winter, spring, summer, autumn), past exercise habits (>3/week, ≤3/week, ≤1–3/month, no exercise), and walkability. For walkability, living areas were mapped for active living environment (ALE) and linked to participants by postal code.14 ALE-Class represents the intersection density, dwelling density, and points of interest. ALE-Transit class includes transit points for the greater cities as well. Both ALE outcomes are scored from 1 to 5, with a higher ALE value representing a more favorable environment for active living.14
Statistical Analysis
Descriptive statistics report on demographic variables and other characteristics, using means ±SD or percentages, as appropriate, for the total study population and for the sub-groups separately. Normative values of MVPA by the CHAMPS within the CanCOLD cohort were calculated based on the results in the CanCOLD healthy controls based on the following exclusions: (1) cigarette smoking history of ≥ 5 pack years, (2) spirometry with a fixed ratio of FEV1 to forced vital capacity (FVC) < 0.7 and less than lower limit predicted criteria, (3) self-reported chronic bronchitis, COPD, diabetes, angina, or myocardial infarction (participants who self-reported systemic hypertension with no other cardiovascular disease were not excluded), and (4) one or more non-ambulatory functional status attributed to comorbidities that limit the mobility of the individual such as musculoskeletal comorbidities, neurological conditions, or nonspecific chronic pain. Complete case analysis was performed as missing values were below 2%. Missing data are reported per characteristic.
To investigate the relationship between symptom burden and MVPA, descriptive statistics produced the distribution of PA levels in all participants with COPD according to low and high symptom burden groups (CAT: low <10, high≥10). MVPA energy expenditure for both high and low symptom burden was reported for the total COPD population and separated by subsets of GOLD 1 and GOLD 2. The difference in MVPA was tested using general linear models (GLM). For the association of symptom burden with MVPA, parameter estimates, and 95% confidence intervals (CI) were generated, adjusted for age, sex, body mass index (BMI), smoking status, and musculoskeletal disorders. In addition, MVPA was reported according to diagnosis status for the total COPD study population and separate for GOLD 1 and GOLD 2. Differences and associations were tested by GLM according to the same methods.
To explore other potential factors associated with MVPA, multivariate analyses with a stepwise approach was used. All variables explored in the univariate analyses with p<0.05 were considered as continuous variables in the multivariate model, with age, sex, smoking status, and BMI included as covariates in all models. SAS software version 9.4 was used (SAS Institute) for all data analyses.
Results
Data from 1514 participants were included in this sub-study. Characteristics of the study population are shown in Table 1. Less than 20% of the GOLD 1 and 40% of the GOLD 2 individuals had a physician diagnosis of COPD before enrolling in the CanCOLD study. High symptom burden (CAT: high≥10) was reported in about 40% of the GOLD 2 individuals, 2 times more than any of the 3 sub-groups. Musculoskeletal diseases, such as osteoporosis and connective tissue diseases, were the most common comorbidities across all groups, followed by hypertension, cataracts, and any cardiovascular disease (CVD).
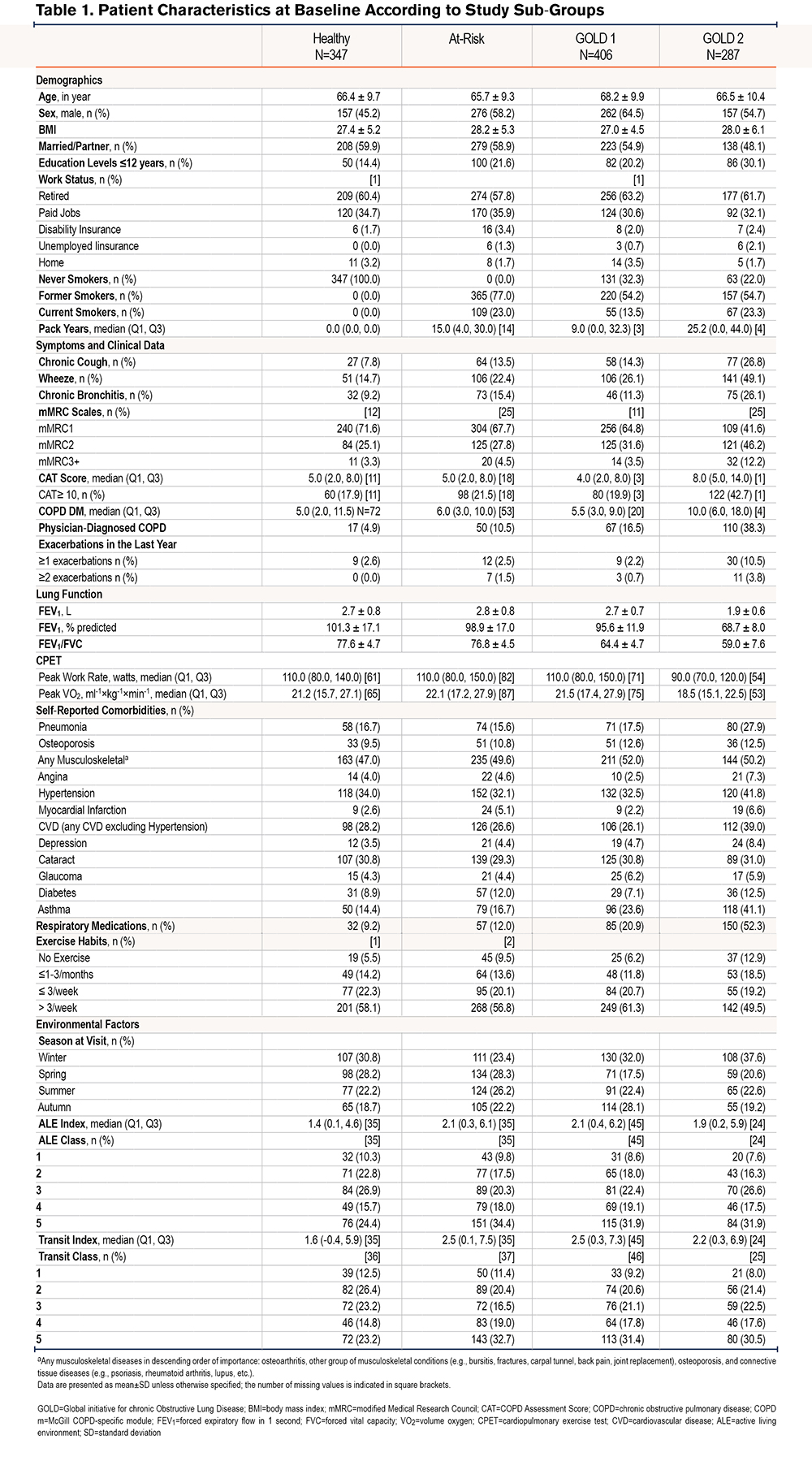
Physical Activity Levels
MVPA outcomes of the CHAMPS questionnaire in energy expenditure for the 4 sub-groups are shown in Figure 1. Normative values of MVPA by CHAMPS within the CanCOLD cohort and the outcomes of duration and frequency for MVPA are shown in Figure 1 and in Table S-I and Table S-II in the online supplement.
Symptom Burden and Physical Activity
Figure 2 shows MVPA categorized by high and low symptom burden. Of all participants with COPD, 32% had a high symptom burden according to the CAT (≥10). When considering all participants with COPD, mean MVPA was significantly lower for those with a high symptom burden. When considering the sub-groups of COPD participants (GOLD 1 and GOLD 2), MVPA was lower in the participants of the high symptom burden group compared to those of the low symptom burden group. However, for both group GOLD 1 and GOLD 2, associations lacked in statistical power (Figure 2). Data on duration and frequency of MVPA are shown in Table S-III in the online supplement.

We found a negative association between symptom burden and MVPA, for the participants in the whole group with COPD after adjusting for age, sex, BMI, smoking status, and any musculoskeletal disorders (Table 2). Within COPD sub-groups, there was no statistically significant association between symptom burden and MVPA for the GOLD 1 sub-group.
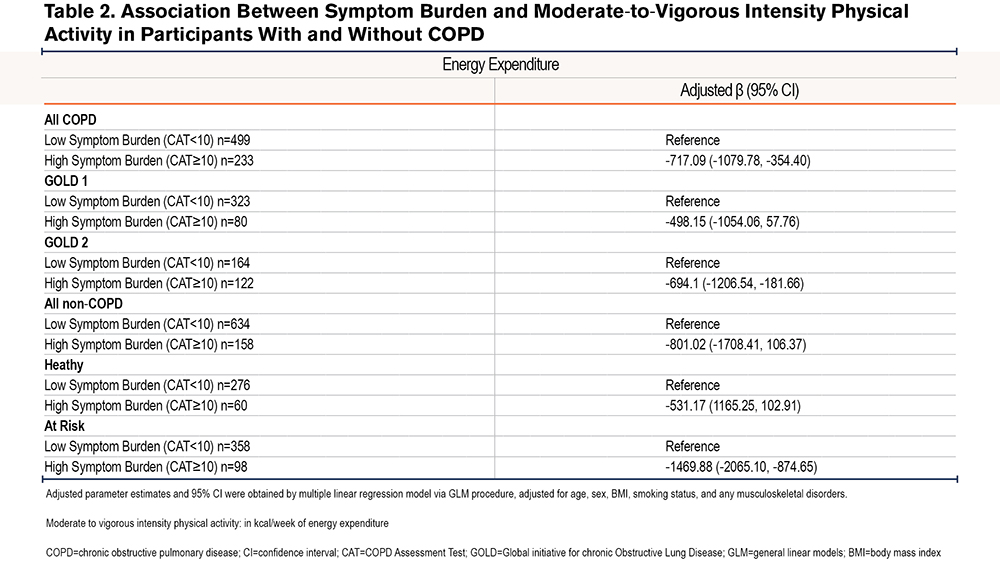
Diagnostic Status and Physical Activity
Of the total group of participants with COPD, 72% were undiagnosed before entering the study. Within the diagnosed group 67.2% used respiratory medication; for the undiagnosed group this was 22.5%. Participants in the undiagnosed group had significantly higher MVPA than those of the diagnosed group (Figure 3) when considering all COPD groups. This corresponded with the other outcomes of the CHAMPS, frequency and duration (Table SIV in the online supplement). There was a significant negative association between being diagnosed and experiencing lower MVPA in the group with all COPD (Table 3). This association is attributed mostly to the GOLD 2 sub-group as MVPA was significantly different between diagnosed and undiagnosed in this sub-group (Figure 3). For the GOLD 1 sub-group, there was no association between diagnostic status and MVPA.
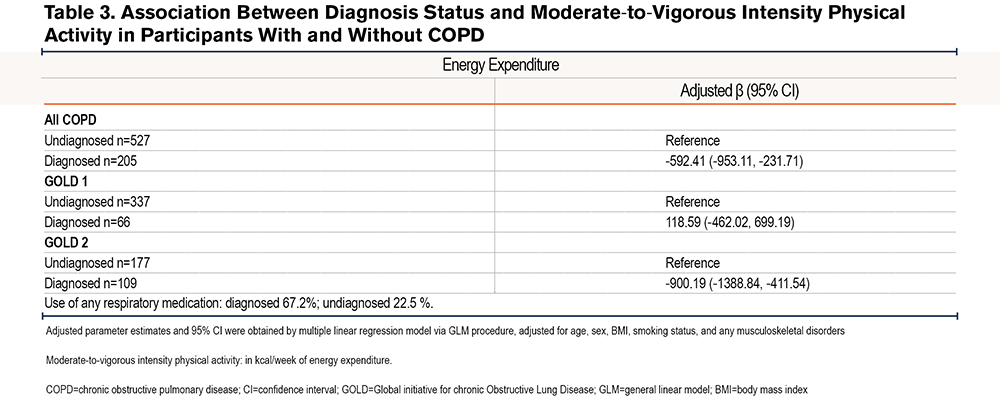
Other Factors Associated with Moderate-to-Vigorous Intensity Physical Activity in People With and Without COPD
For the participants of all COPD groups, the continuous score for symptom burden measured by the CAT showed significant negative associations with MVPA (Table 4). Other factors that were significantly associated with MVPA in the final model were FEV1, winter, autumn, and past exercise habits. The latter showed high β-coefficients and a significant relationship with all MVPA outcomes Table SVI in the online supplement).
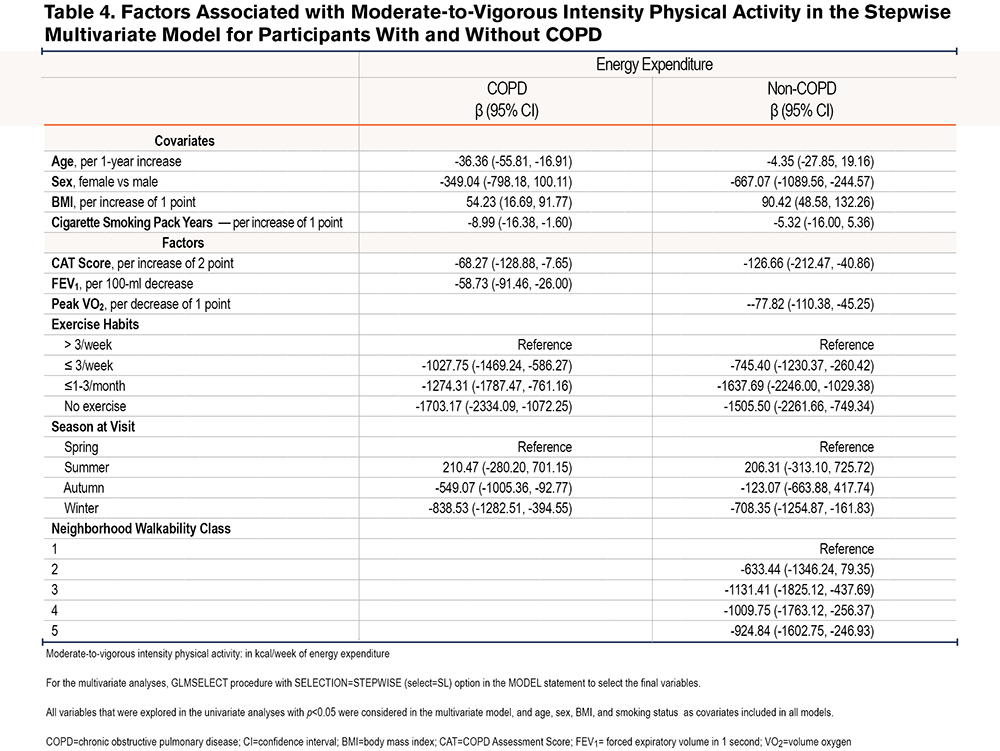
Symptom burden and winter showed high to modest β-coefficients for the MVPA. ALE-Class showed negative non-sequential β-coefficients for every higher value (representing a more favorable environment for active living) on the 5-point scale: higher walkability showed an association to lower MVPA. Past exercise habits and exercise capacity were shown to be significantly associated with MVPA for the non-COPD group with high β-coefficients, of which exercise capacity was not included in the multivariate model for participants with COPD due to high correlation with symptom burden by the CAT.
Discussion
The data from this study showed that the group of individuals with high COPD symptom burden was negatively associated with MVPA including those with mild disease. In addition, we demonstrated that undiagnosed COPD participants from the GOLD 2 sub-group were significantly more active than those who had physician-diagnosed COPD. Finally, the other most important factors that were indicative of a lower MVPA in non-COPD and COPD participants were past exercise habits and winter season.
Our sample showed high mean MVPA estimated by the CHAMPS questionnaire with a wide range and high variability within all sub-groups and the normative group. In comparison, Mak and colleagues showed mean scores of 1011 for energy expenditure in Canadians with COPD using the CHAMPS, which is much lower than the mean score of 2318 reported for the CanCOLD cohort.17 This could be explained by the fact that they included more participants with severe COPD. In addition, this study’s mean MVPA is higher than the 3 physical activity levels stated by Stewart and colleagues: respectively sedentary, somewhat active, and active. Our high mean MVPA could be due to our study population, which was relatively younger.18,20 The mean scores of the at-risk, healthy, and mild COPD groups are very similar between groups in this study, in contrast with other studies that stated patients with mild COPD already show some physical impairment in comparison with healthy individuals.21 The difference may come from the selection of individuals in those studies. Most previous studies have used a convenient clinical sample which consisted of participants who are attended by clinicians and are more likely to be symptomatic than the general population. The results from our more general sample could emphasize the difficulty and need for identification of individuals within the mild COPD group who are less active.
The inverse relationship of symptom burden with MVPA is in line with previous studies on patients with COPD.2,6,22,23 This present study extends on these prior findings by showing, for the first time, that symptom burden is also related to MVPA in patients with mild to moderate COPD from a population-based sample, almost three-quarters of whom were not physician diagnosed. Demeyer and colleagues found an association between lower PA levels and higher symptom burden in a clinical COPD sample in different study sites in Europe, measured by the modified Medical Research Council (mMRC) Dyspnea Scale, and highlighted a minority of patients with mild COPD who showed reduced PA.22 However, the PA outcome in that study was in steps per day and the authors did not report on MVPA specifically. In the present study, patients in the mild COPD sub-group with a high symptom burden reported lower MVPA levels, which emphasizes the importance of identifying this sub-group early. Despite this association not being statistically significant, it could indicate there may be a relatively inactive sub-group within the wide range of MVPA levels in the mild COPD group. Identification by the assessment of symptom burden could, therefore, be useful.
To our knowledge, this is the first study to investigate the relationship between MVPA and diagnostic status (physician-diagnosed and undiagnosed COPD) in a large general population cohort. MVPA was significantly higher for undiagnosed individuals in the entire COPD group, especially for moderate COPD (although the score remained below the normative values from our total study population) and could be due to a lower impact of disease state. However, within the mild COPD sub-group, no significant relationship between diagnostic status and MVPA levels could be demonstrated. To investigate this further, we performed a post-hoc analysis within this subgroup to assess whether patients with a high symptom burden were less active than patients with a low symptom burden. A trend was observed for every CHAMPS outcome. This is in line with the results of the study of Remoortel and colleagues, who reported a reduction in PA levels (daily steps and MVPA) in a small study population of undiagnosed patients and additionally found that a reduction in PA levels started early in the disease.24 However, our analysis failed to reach statistical significance, which could be due to lack of statistical power. Also, despite not having a diagnosis, 22.5 % of the undiagnosed group used respiratory medication, which makes it harder to interpret results. Follow-up data is needed to study whether this trend of a negative association of MVPA levels with symptom burden could lead to deterioration in the onset of COPD.
In addition to symptom burden, poor past exercise habits and winter season were significantly positively associated with lower MVPA in participants with and without COPD, with greater impact in the COPD group. We do not know of other studies that report on past exercise habits in relation with current MVPA levels. In a study by Koreny et al, patients with COPD who were more physically active at the beginning of a PA intervention program, were more likely to finish the program and stay more active afterwards.25 And although their previous physical activity was not stated as past exercise habits, this could indicate that activity habits in general can be an important factor in physical activity levels of individuals with COPD. Follow-up on the participants from the CanCOLD cohort could give insight in the relationship of (past) exercise habits to physical activity and deterioration, especially in formerly undiagnosed patients.
The positive association of winter with lower MVPA in non-COPD and COPD groups is consistent with other findings and may be due to cold and rainfall. The latter could also explain the negative association of autumn with MVPA in the COPD group only, due to the difficulty of breathing in humid weather caused by rain, specifically for the COPD group.26 This is also the time of year when respiratory tract infections and COPD exacerbations are most common, which may also have negatively impacted PA levels.26
Lung function was shown to be significantly associated with MVPA in the COPD group only. Previous reports on lung function and PA have been inconsistent, as some studies failed to demonstrate an association, possibly due to their small sample sizes.5,27,28 Positive associations of exercise capacity with MVPA were only demonstrated in the non-COPD group. Exercise capacity in the healthy population is known to be only moderately associated with PA.4
Neighborhood walkability, here reported in sequential classes, surprisingly showed a negative association for non-COPD participants. Although other findings on walkability of neighborhoods and PA are indeed inconsistent,29,30 an inverse association does not seem logical and could not be explained by the data available. The CHAMPS questionnaire targets multiple (moderate) activities and may not be the right instrument to find a relationship of neighborhood walkability and MVPA levels. While accelerometer data is likely to be more valid, this was beyond the feasibility of the present study.
Strength and Limitations
This study has some strengths and limitations. A strength of the current study is the inclusion of a general population sample. The sample size and inclusion of individuals with spirometrically-confirmed COPD, but not having physician-diagnosed COPD provided insightful associations and a novel basis to demonstrate the impact of symptom burden and other factors on PA levels across the spectrum of severity of disease, including mild to moderate COPD and pre-clinical disease. One of the limitations includes the multiple PA outcomes of the CHAMPS, as they are difficult to compare with measurements in other studies. Direct measurements of PA such as using accelerometers compared to questionnaires are known to be more reliable, yet they have a motivational effect with the potential to artificially increase PA levels.31 The CHAMPS is a self-reported questionnaire, which may increase the risk of reporting socially acceptable answers. However, the CHAMPS targets different activities, and is reliable in patients with COPD.17 It is also shown to be able to detect changes over time and therefore, appropriate in large population studies such as the ongoing CanCOLD longitudinal cohort.22 The cross-sectional design of the present study does not allow for claiming causal effects of determinants on the progression of COPD. However, these findings generate a novel basis and new hypotheses to further investigate possible associations of symptom burden and PA in individuals with COPD from mild to moderate and not only severe disease.
Conclusion
MVPA was found to be inversely related to symptom burden in a general population sample that included a large proportion of individuals with COPD who have not previously received a diagnosis by a physician and a high number of mild to moderate COPD individuals. Assessment of symptom burden may help identify patients with lower MVPA, especially for moderate COPD and for relatively inactive individuals with mild COPD.
Acknowledgements
Author contributions: LO assisted with designing the study, analyzed and interpreted the data, and wrote the manuscript. TJ and JB co-designed the study, assisted with interpretation of the data, and provided critical feedback on the manuscript. DD and BR provided feedback on the study design, assisted with interpretation of the data, and provided critical feedback on the manuscript. PZ assisted with data analysis. SA, KC, PH, FM, DM, DO’D, WT, and BW assisted with interpretation of the data and provided critical feedback on the manuscript. All authors reviewed and approved the final version of the manuscript.
The authors thank CanCOLD study participants and individuals in the CanCOLD Collaborative Research Group: Executive Committee: Jean Bourbeau (PI) (McGill University, Montreal, Quebec, Canada); Wan C. Tan (co-PI) (University of British Columbia, Vancouver, British Columbia, Canada); Shawn Aaron (University of Ottawa, Ottawa, Ontario, Canada); Kenneth R. Chapman (University of Toronto, Toronto, Ontario, Canada); J. Mark FitzGerald (University of British Columbia, Vancouver, British Columbia, Canada); Paul Hernandez (Dalhousie University, Halifax, Nova Scotia, Canada); François Maltais (University of Laval, Quebec City, Quebec, Canada); Darcy D. Marciniuk (University of Saskatchewan, Saskatoon, Saskatchewan, Canada); Denis E. O'Donnell (Queen's University, Kingston, Ontario, Canada); Don D. Sin (University of British Columbia, Vancouver, British Columbia, Canada); Brandie Walker (University of Calgary, Calgary, Alberta, Canada).
International Advisory Board: Jonathon Samet (the Keck School of Medicine of University of Southern California, Los Angeles, California); Milo Puhan (John Hopkins School of Public Health, Baltimore, Maryland ); Qutayba Hamid (McGill University, Montreal, Quebec, Canada); James C. Hogg (University of British Columbia, James Hogg Research Centre, Vancouver, British Columbia, Canada).
Operations Centre: Jean Bourbeau (PI), Dany Doiron, Palmina Mancino, Pei Zhi Li, Dennis Jensen, Carolyn Baglole (University of McGill, Montreal, Quebec, Canada), Yvan Fortier (Laboratoire telematique Respiratory Health Network, FRQS, Montreal, Quebec, Canada); Wan C. Tan (co-PI), Don Sin, Julia Yang, Jeremy Road, Joe Comeau, Adrian Png, Kyle Johnson, Harvey Coxson, Miranda Kirby, Jonathon Leipsic, Cameron Hague (University of British Columbia, James Hogg Research Centre, Vancouver, British Columbia, Canada).
Economic Core: Mohsen Sadatsafavi (University of British Columbia, Vancouver, British Columbia, Canada).
Public Health Core: Teresa To, Andrea Gershon (University of Toronto, Toronto, Ontario, Canada).
Data Management and Quality Control: Wan C.Tan, Harvey Coxson (University of British Columbia, Vancouver, British Columbia, Canada); Jean Bourbeau, Pei-Zhi Li, Zhi Song, Andrea Benedetti, Dennis Jensen (McGill University, Montreal, Quebec, Canada); Yvan Fortier (Laboratoire telematique Respiratory Health Network, FRQS, Montreal, Quebec, Canada).
Field Centres: Wan C. Tan (PI), Christine Lo, Sarah Cheng, Elena Un, Cynthia Fung, Wen Tiang Wang, Liyun Zheng, Faize Faroon, Olga Radivojevic, Sally Chung, Carl Zou (University of British Columbia, James Hogg Research Centre, Vancouver, British Columbia, Canada); Jean Bourbeau (PI), Palmina Mancino, Jacinthe Baril, Laura Labonte (McGill University, Montreal, Quebec, Canada ); Kenneth Chapman (PI), Patricia McClean, Nadeen Audisho (University of Toronto, Toronto, Ontario, Canada); Brandie Walker, (PI), Curtis Dumonceaux, Lisette Machado (University of Calgary, Calgary, Alberta, Canada); Paul Hernandez (PI), Scott Fulton, Kristen Osterling, Denise Wigerius (Dalhousie University and Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada); Shawn Aaron (PI), Kathy Vandemheen, Gay Pratt, Amanda Bergeron (University of Ottawa, Ottawa, Ontario, Canada); Denis O'Donnell (PI), Matthew McNeil, Kate Whelan (Queen's University, Kingston, Ontario, Canada); François Maltais (PI), Cynthia Brouillard (University of Laval, Quebec City, Quebec, Canada); Darcy Marciniuk (PI), Ron Clemens, Janet Baran, Candice Leuschen (University of Saskatchewan, Saskatoon, Saskatchewan, Canada).
The Can-ALE, indexed to DMTI Spatial Inc., postal codes, were provided by the Canadian Urban Environmental Health Research Consortium.
Declaration of Interest
The authors report no conflict of interest related to the publication of this paper.