Running Head: AECOPDs, Inpatient Rehabilitation, and COVID-19
Funding support: The current study was co-funded by the Public-Private Partnerships allowance made available by Health~Holland, Top Sector Life Sciences and Health (LSHI19003) and ZonMWAQ10 (ERACoSysMed grant #90030355).
Date of Acceptance: December 22, 2022 | Published Online Date: January 3, 2023
Abbreviations: AECOPDs=acute exacerbations of chronic obstructive pulmonary disease; ANOVA=analysis of variance; BMI=body mass index; CAT=COPD Assessment Test; FEV1=forced expiratory volume in 1 second, FVC=forced vital capacity, GOLD=Global initiative for chronic Obstructive Lung Disease; IPC=infection prevention and control; IQR=interquartile range; mMRC=modified Medical Research Council; NIV=non-invasive ventilation; PCR=polymerase chain reaction, PR=pulmonary rehabilitation; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2; SD=standard deviation
Citation: Waeijen-Smit K, Houben-Wilke S, Posthuma R, et al. Impact of coronavirus disease 2019-related infection prevention and control measures on the occurrence of COPD exacerbations during inpatient pulmonary rehabilitation. Chronic Obstr Pulm Dis. 2023; 10(2): 127-138. doi: http://doi.org/10.15326/jcopdf.2022.0345
Online Supplemental Material: Read Online Supplemental Material (532KB)
Introduction
During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced coronavirus disease 2019 (COVID-19) pandemic, a worldwide reduction in the number of hospital-admitted acute exacerbations of chronic obstructive pulmonary disease (AECOPDs) ranging between 44% to 78% has been observed.1-8 COVID-19–related infection prevention and control (IPC) measures, including social distancing, wearing face masks, and overall enhanced hygiene,9,10 may explain this reduction in hospitalizations by also affecting the transmission of respiratory viruses other than SARS-CoV-2. Since viral infections are an important cause of AECOPDs,11-13 reducing transmission with IPC measures will likely reduce the number of AECOPDs. Avoidance of hospital care by patients due to fear of contracting a SARS-CoV-2 infection may also contribute to the reduction of AECOPD-related hospitalizations.14-16 However, health care avoidance would not reduce the number of AECOPDs per se, but rather presentation to the hospital. This would reconcile the increased numbers of AECOPDs managed in community settings15 with the decreased hospitalization rates. At present, it is unclear whether IPC measures or health care avoidance by patients have been driving the lower AECOPD hospitalization rates. It is important to distinguish between these 2 different causes as it may hold important implications for the post-pandemic management of COPD and AECOPDs.
The COVID-19 pandemic has placed an immense burden on health care systems. Adjusted, remote, postponed, or canceled care are examples of such challenges introduced by COVID-19. The care provided at Ciro, a pulmonary rehabilitation (PR) center located in Horn, the Netherlands, was equally affected by COVID-19 and measures were taken correspondingly. COVID-19–related IPC measures were introduced during the first COVID-19 wave, in addition to pre-existing IPC measures (e.g., no handshaking policy), and included social distancing, wearing face masks, and weekly SARS-CoV-2 polymerase chain reaction (PCR) testing, amongst others. The current study was designed to explore the impact of COVID-19–related IPC measures on the occurrence of AECOPDs in patients with COPD admitted for inpatient PR. In addition, drop-out and mortality rates were assessed given their close relationship with AECOPDs.17,18 Because of the daily nursing, and continuous access to AECOPD-related care at Ciro, the potential effect of health care avoidance could be ruled out. It was hypothesized that the inpatient AECOPD rate would reduce during the COVID-19 pandemic compared to previous years as a result of the implementation of COVID-19–related IPC measures.
Methods
Study Setting
This retrospective study was conducted at Ciro, a specialized center for comprehensive PR (Horn, the Netherlands). PR at Ciro consists of a state-of-the-art, multidisciplinary, patient-tailored program (Monday to Friday; patients in need of medical or nursing care also receive inpatient care during weekends),19 Patients are supervised by the medical staff including chest physicians throughout the PR program.
Study Design
Patients admitted between October 1, 2020, and March 1, 2021, the first winter season with COVID-19–related IPCmeasures in place,were compared to patients admitted during the same period in previous years (2017–2018, 2018–2019, and 2019–2020). The inclusion period was carefully selected given the known rise in AECOPDs during the autumn and winter months in the northern hemisphere.20-23 Electronic medical records were retrospectively screened for the occurrence of AECOPDs, drop-out, and mortality rates during PR. The Medical Ethical Teaching Committee of the Maastricht University Medical Centre confirmed that the Medical Research Involving Human Subjects Act does not apply to the current study. Therefore, approval was waived by the committee (METC 2021-2631). This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Study Population
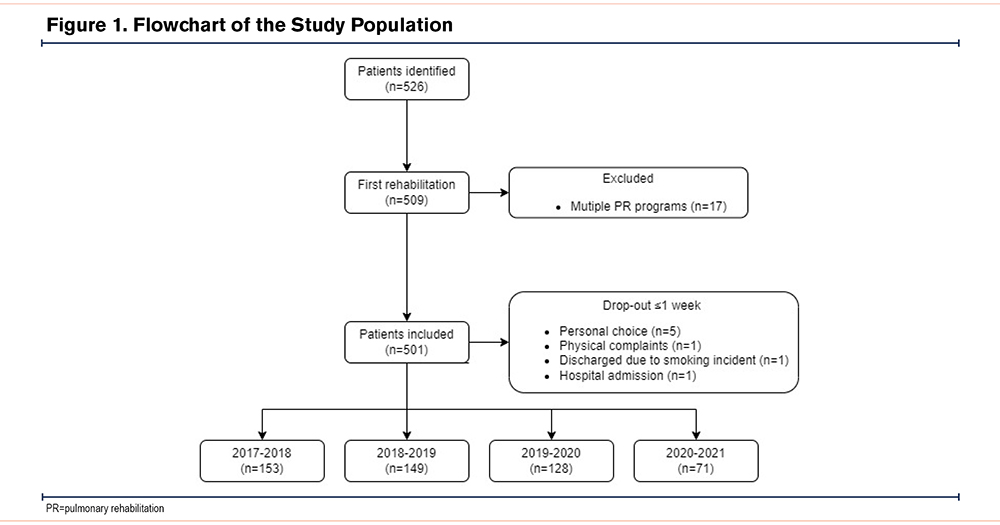
Patients with a diagnosis of COPD established by a chest physician, and confirmed by spirometry (i.e., the post-bronchodilator ratio of forced expiratory volume in 1second (FEV1) to forced vital capacity (FVC) of less than 0.70),24 admitted for an 8-week inpatient PR program during the observation periods were eligible for inclusion. Patients may attend PR multiple times over several years; if patients were admitted for multiple PR programs during the observation period, only the first course was recorded. Furthermore, patients who dropped out during the first week of PR (≤5 days) were excluded. Due to the real-life retrospective electronic medical records-based study design, no sample size calculation was performed.
Outcomes
Exacerbations were defined by an increase in respiratory symptoms and the need for additional pharmacological treatment according to the Global initiative for chronic Obstructive Lung Disease (GOLD) document.24 Exacerbation-like events were evaluated by a chest physician or physician assistant. If indicated, chest X-ray, electrocardiography, and venous blood were collected as part of a routine AECOPD diagnosis to rule out differential diagnoses such as pneumonia, pneumothorax, and cardiac abnormalities. AECOPDs were scored according to treatment intensity: moderate AECOPDs were defined as AECOPDs necessitating treatment with systemic glucocorticoids, antibiotics, or both.24 Severe AECOPDs were defined as AECOPDs necessitating treatment with (enhanced) oxygen therapy, intensification of long-term home non-invasive ventilation (NIV), or escalation to hospital care facilities >24 hours for care not available in Ciro such as the need for intravenous treatment, mechanical ventilation, initiation of acute NIV, or continuous monitoring.
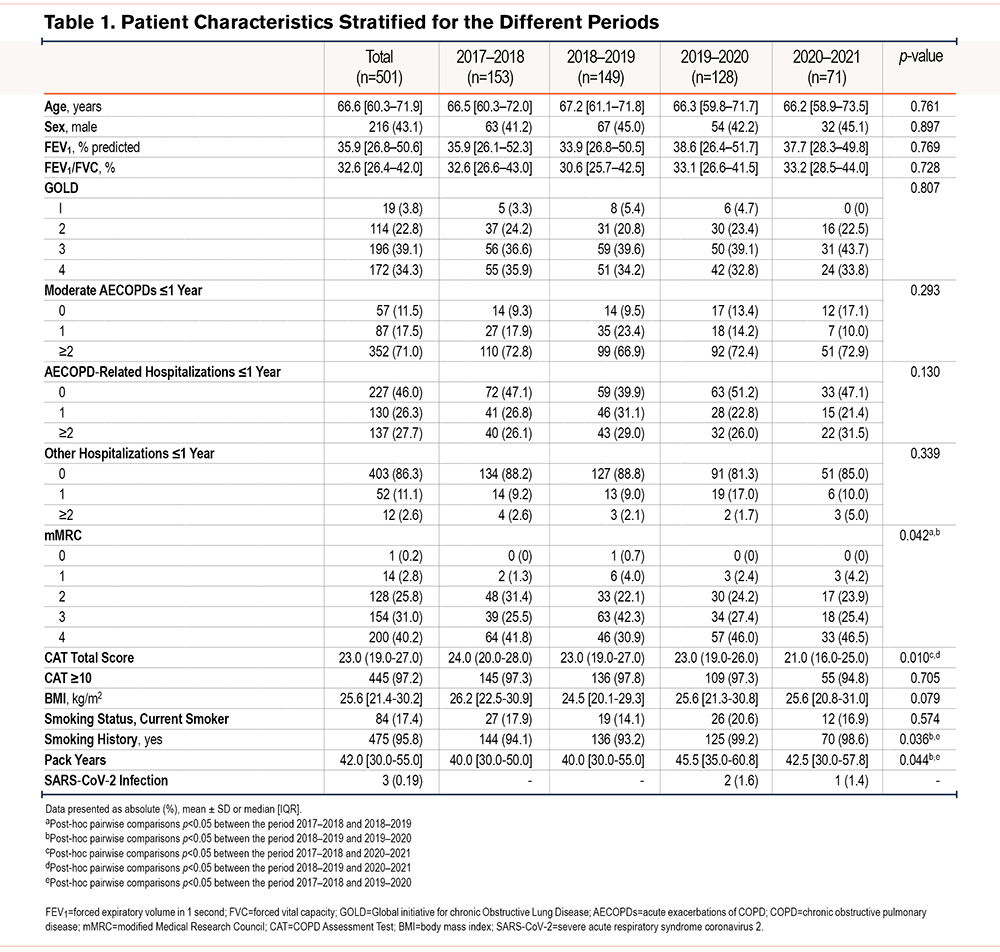
Baseline characteristics included age, sex, smoking status (current, former, and never-smoker), pack years, body weight (kg), height (m), and body mass index (BMI [kg/m2]). Disease-specific measures included lung function (FEV1, FVC, and FEV1/FVC [absolute and % predicted]), GOLD grade (1–4), and the total number of moderate AECOPDs and AECOPD-related hospitalizations in the year prior to admission for PR. Furthermore, the modified Medical Research Council (mMRC) scale (0–4 points), and the COPD Assessment Test (CAT [0–40 points]) were assessed. Equivalent cut-off scores of ≥2 for mMRC and ≥10 for CAT were used to stratify patients based on dyspnea severity or health status, respectively.24 The use of maintenance therapies (inhalation therapy, systemic antibiotics, systemic corticosteroids, long-term oxygen therapy, and home-based NIV) were recorded. These outcomes were collected during the 2.5-day baseline pre-rehabilitation assessment.19
Coronavirus Disease 2019-Related Infection Prevention and Control Measures
COVID-19–related IPC measures were introduced in Ciro from March to May of 2020 (during/after the first national COVID-19 wave)25 and included social distancing (i.e., 1.5 meters), strictly using single-patient rooms, enhanced hygiene measures, and indoor ventilation, as well as training group size limits and introduction of eating meals in shifts. Furthermore, all newly admitted patients were tested for SARS-CoV-2 using SARS-CoV-2 antigen tests (Abbott PanbioTM). Patients could only commence PR after having obtained a negative test result. In addition, patients were isolated during PR when a SARS-CoV-2 infection was suspected and remained in isolation if confirmed by PCR testing. During these events, patients were also tested for influenza. During the early stages of the second national COVID-19 wave,25 further COVID-19–related IPC measures (in addition to the previously administered COVID-19–related IPC measures) were introduced at Ciro as of October 1, 2020. These measures included wearing surgical IIR face masks in public areas and weekly routine testing using SARS-CoV-2 RT-PCR tests. Hence, October 2020 to March 2021 marks the first winter season with full COVID-19–related IPC measures in place. Some IPC measures had already been established at Ciro as part of routine clinical care before the COVID-19 pandemic. These included aerogenic isolation (i.e., patient isolation and wearing surgical IIR face masks) in situations where an influenza or human metapneumovirus infection was suspected and/or confirmed. Moreover, a no-handshaking policy was introduced in January 2018 in response to the national influenza epidemic.26
Statistical Analysis
Baseline characteristics were reported as mean and standard deviation (SD) or as the median and interquartile range (IQR) for continuous variables, as appropriate, and as count and percentage for categorical characteristics. The primary outcome of interest was the occurrence of AECOPDs during each observational period. Pearson’s Chi-squared test was performed to compare the number of patients experiencing an AECOPD (yes/no) between the different periods. The one-way analysis of variance (ANOVA) was performed to compare the number and severity of AECOPDs between the different periods. Pearson’s Chi-squared test, ANOVA, and the Kruskal-Wallis test were performed to assess differences between patient characteristics, as appropriate. Post-hoc pairwise comparisons were performed and adjusted by the Bonferroni correction for multiple tests. Statistical analyses and visualization were performed using IBM SPSS Statistics 25 (SPSS Inc., Chicago, Illinois) and Excel (Microsoft Excel 2007, Redmond, Washington). A priori, p-values ≤0.05 were considered statistically significant.
Results
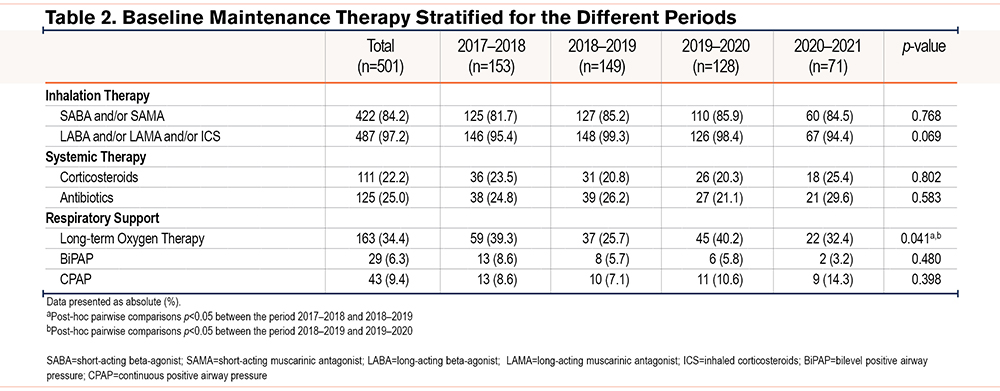
A total of 526 patients with COPD were admitted for inpatient PR at Ciro between October 1 and March 1 during the years 2017–2021. A total of 501 patients were included in the analyses (Figure 1). Patient characteristics were comparable between the different periods (Table 1). Briefly, the median age of the total population was 67 years and 43% was male. The majority of patients were classified as GOLD 3–4 (73%), and most patients reported moderate to very severe breathlessness (mMRC≥2). A total of 71% of the patients had experienced at least 2 moderate AECOPDs, and 54% of the patients were admitted to the hospital at least once for an AECOPD in the year before admission for PR. The use of maintenance therapies is shown in Table 2. Apart from long-term oxygen therapy, no significant differences were observed between the different periods. Long-term oxygen therapy use was significantly higher in 2017–2018 versus 2018–2019 (Chi-square, p=0.013) and in 2019–2020 versus 2018–2019 (Chi-square, p=0.014). No differences in drop-out and mortality rates were observed (see the online data supplement).
Severe Acute Respiratory Syndrome-Coronavirus-2 Infections
One patient (1.4%) admitted during 2020–2021, and 2 patients (1.6%) admitted during 2019–2020 became infected with SARS-CoV-2. Two out of the 3 patients experienced mild COVID-19 and restarted PR after a negative SARS-CoV-2 RT-PCR test result was shown. One patient was admitted to the hospital and died of the consequences of COVID-19. Of note, as a direct consequence of the COVID-19–related IPC measures, a 50.5% reduction was seen in the total number of admissions for inpatient PR between October 1, 2020, and March 1, 2021, compared to the average of the previous 3 periods. This was mainly related to the introduction of single-patient rooms, and thus, a reduction in the overall number of available beds.
Acute Exacerbations of COPD Rates During Inpatient Pulmonary Rehabilitation
During 2020–2021, 22 patients (31.0%) experienced at least one AECOPD during inpatient PR compared to 43 patients (33.6%) in 2019–2020, 55 patients (36.9%) in 2018–2019, and 83 patients (54.2%) in 2017–2018, Figure 2A. This represents a non-significant reduction of 25.4% in the number of patients experiencing at least one AECOPD during 2020–2021 (31.0%) compared to the average of the previous 3 periods (41.6%) (Chi-square, p=0.077). Significant differences were, however, found in the number of patients experiencing an inpatient AECOPD between 2017–2018 versus 2018–2019 (Chi-square, p=0.002), 2017–2018 versus 2019–2020 (Chi-square, p=0.001) and 2017–2018 versus 2020–2021 (Chi-square, p=0.001).
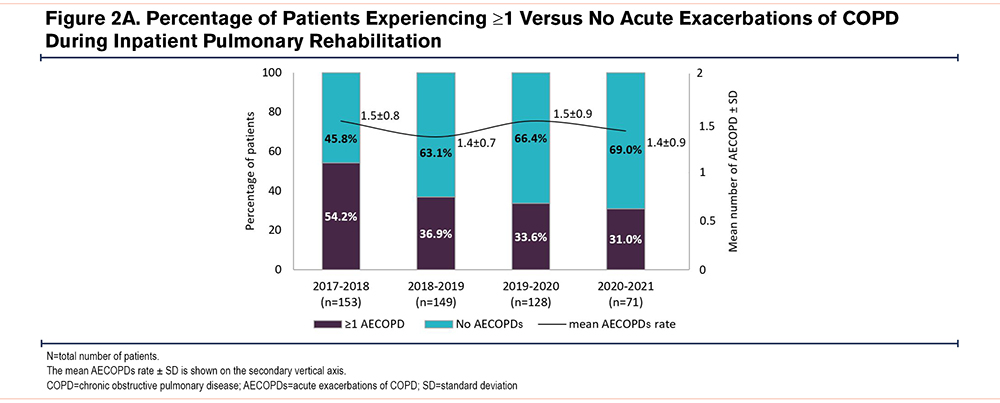
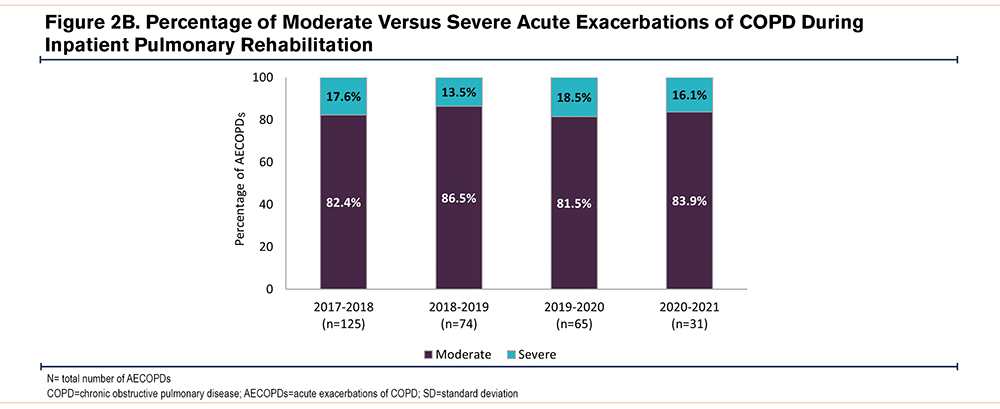
No significant differences were observed in the mean AECOPD rate per patient between the different periods (Figure 2A): 1.51±0.80, 1.35±0.67, 1.51±0.88, 1.41±0.91 in 2017–2018, 2018–2019, 2019–2000, and 2020–2021, respectively (ANOVA F3,199=0.552, p=0.647). Moreover, no significant differences were observed between the relative number of moderate (ANOVA F3,199=0.129, p=0.943) and severe AECOPDs (ANOVA F3,199=0.475, p=0.700) in the different time periods (Figure 2B).
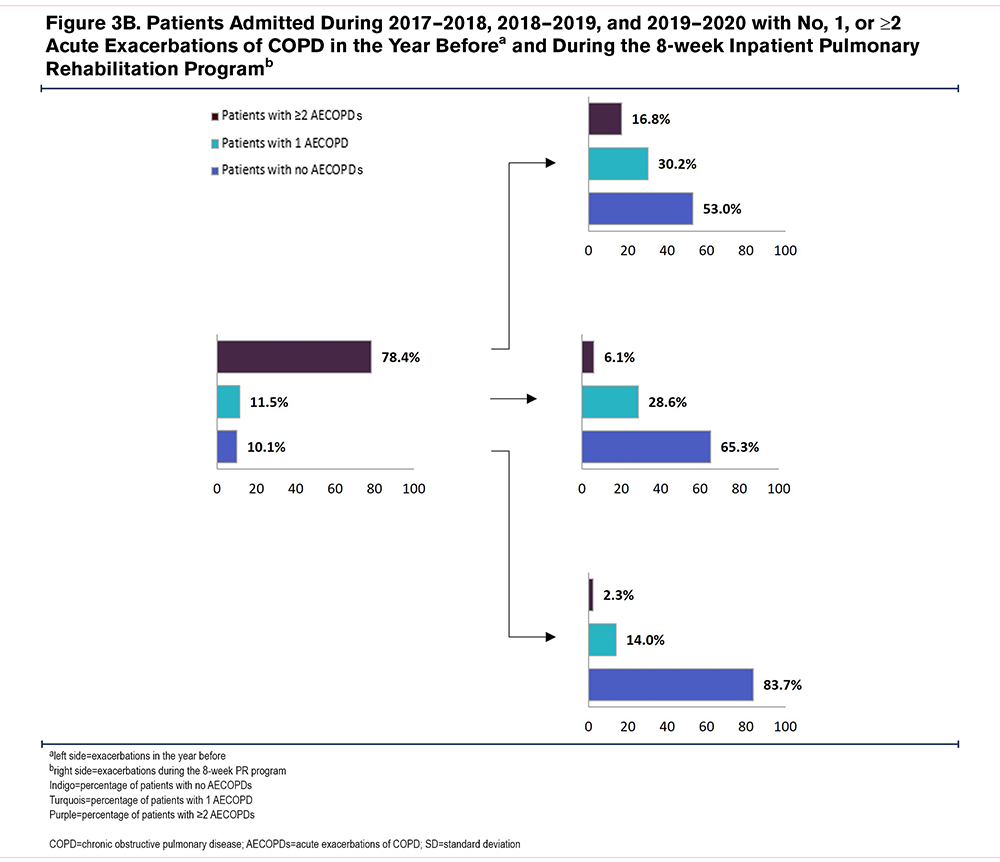
The majority of patients with a history of 2 or more AECOPDs in the year before admission for PR experienced no or 1AECOPD during inpatient PR (Figures 3A and 3B).

Discussion
This single-center, real-life, retrospective study showed that COVID-19–related IPC measures did not significantly reduce the occurrence of AECOPDs in patients with COPD admitted for inpatient PR. This could not be explained by differences in AECOPD severity, drop-out, or mortality between the COVID-19–related IPC measures period (2020–2021) and previous years (2017–2020). These results lend credence to the hypothesis that the reduction in AECOPD hospitalizations during the COVID-19 pandemic previously observed might have been driven, at least in part, by altered health care-seeking behavior of patients rather than the installation of IPC measures.
The worldwide reduction in the number of hospital-admitted AECOPDs during the COVID-19 pandemic1-8 was not observed in the present study. However, in contrast to previous studies, the current study was not restricted to AECOPD-related hospital admissions. Indeed, both moderate AECOPDs (events necessitating treatment with systemic glucocorticoids, antibiotics, or both) and severe AECOPDs (events necessitating treatment with [enhanced] oxygen therapy, intensification of long-term home NIV, or escalation to hospital care facilities >24 hours) were studied. It was hypothesized that, regardless of the severity, the underlying mechanism of AECOPD starts with presentation to a health care provider. Patients included in this study received daily nursing during the 8-week PR program. As such, medical care was constantly available. The unique, real-life inpatient setting of this study, therefore, precluded the potential effect of health care avoidance. Moreover, the large time window and the comparability of patient characteristics between the different time periods resulted in a relatively homogenous study cohort enabling us to study AECOPD rates during the COVID-19–related IPC measures period and previous years. Also, the severity of disease and the high proportion of frequently exacerbating patients makes this setting particularly suitable to address the aims of this study.
Whilst IPC measures were effective in contributing to a decrease in COVID-19 cases in the community, and whilst we do not argue the effectiveness of these measures, the current findings suggest that health care avoidance of patients rather than COVID-19–related IPC measures has been driving the previously observed reduction in AECOPDs during the COVID-19 pandemic. An argument against health care avoidance in previous studies has been the absence of increased mortality rates in patients with COPD during the pandemic.27-30 Whilst hospital-admitted AECOPDs exert a direct and independent negative effect on survival, the risk of mortality increases with the frequency of severe AECOPDs.31 Hence, it may be too soon to conclude that the COVID-19 pandemic has had a protective effect on the occurrence of (severe) AECOPDs. Moreover, this argument includes the assumption that hospital admission is purely defined by the severity of physiological distress, and that mortality is equally correlated with the severity of presentation. It can be argued that reasons for hospitalization are highly variable throughout different health care systems and that mortality due to an AECOPD may not purely be linked to the severity of the initial presentation. Thus, the absence of increased mortality does not necessarily mean that the number of severe AECOPDs has declined. What’s more, the confounding effects of altered health care-seeking behavior by patients, such as increased at-home use of rescue medications,5,15 cannot be precluded and may have decreased the number of hospital admissions, but not the number of AECOPDs per se.
To date, only a few studies investigated the occurrence of moderate AECOPDs during the COVID-19 pandemic. Two studies demonstrated a significant reduction in the number of moderate AECOPDs during 2020.32,33 These studies were, however, conducted in an outpatient setting including patients with a less severe disease status,32 or were based on national medication prescription data33 which hampers the comparison of results. The current study predominantly included patients with moderate to very severe COPD. Susceptibility to AECOPDs is related to disease severity,34 and may, therefore, explain the current lack of reduction in the occurrence of AECOPDs. Indeed, despite the overall reduced number of AECOPD-related hospital admissions, the proportion of patients being admitted to the hospital for an AECOPD with GOLD grade 4 was shown to be higher during the COVID-19 pandemic compared to previous years.6 That said, COVID-19–related IPC measures were shown to reduce the risk of an AECOPD-related hospital admission even when adjusted for the level of airflow obstruction and a history of severe AECOPDs.8 In this view, we noted a significant decline in the number of AECOPDs since the national influenza epidemic of 2017–2018 and the establishment of the no handshaking policy in Ciro since 2018 (Chi-square, p<0.001). This suggests that some protective IPC measures might have been in place before COVID-19, providing an additional explanation as to why the current study could not show a significant reduction in the number of AECOPDs during the COVID-19 pandemic compared to previous years.
There are some limitations to the current study. First, its single-center study design, and limited sample size should be considered when interpreting the results. Moreover, although the potential effect of health care avoidance was precluded, the current cohort was unavoidably limited to a group of patients who did not avoid inpatient PR during the COVID-19 pandemic. In addition, the microbiological origin of the AECOPDs was not systematically recorded in the electronic medical records and could, therefore, not be explored in the present study. Previous studies showed that the COVID-19–related reduction in hospital admissions for COPD correlated with a reduced community viral burden.35 In this view, the national influenza rates were substantially reduced in the Netherlands during 2020–2021 compared to previous years.26 This reduction was, however, not reflected in the AECOPD rates of the present study. This suggests that the AECOPDs seen in the current study might not have had a predominate (viral) infectious origin,36,37 and that endogenous triggers (e.g., bacterial burden11 and eosinophilic inflammation36) drive these AECOPDs during PR. Data regarding vaccination status (against influenza, pneumococci, tetanus, diphtheria and pertussis, herpes zoster, and later, also SARS-CoV-2), comorbidities, as well as mild AECOPDs (defined by the use of additional inhalation therapy), were not systematically recorded in the electronic medical records. As a result, the relationship between vaccination status, comorbidities, and changes in health care-seeking behavior such as additional use of inhalation therapy during the pandemic and the AECOPD rate could, unfortunately, not be assessed in the current study. Finally, other respiratory diseases in medical history were not considered exclusion criteria. However, all included patients had a diagnosis of COPD established by a chest physician and confirmed by spirometry. Moreover, the median age of 66.6 years (60.3–71.9) and median number of pack years of 42.0 (30.0–55.0) of the current study population are inherent to a typical COPD population.24
Taken together, this study showed that COVID-19–related IPC measures did not significantly reduce the occurrence of AECOPDs in patients with COPD admitted for inpatient PR. These findings suggest that avoidance of health care may be an important factor in the observed reduction of AECOPD-related hospitalizations during the pandemic. While the post-pandemic maintenance of COVID-19–related IPC measures to reduce the risk of AECOPDs in patients with COPD is increasingly advised,27,38 some caution may need to be taken as many questions remain. Indeed, further research is needed to explore the value of the strict COVID-19–related IPC measures for the prevention of AECOPDs. Specifically, there is a need to assess whether, which, when, and to what extent IPC measures can prevent (the different types of) AECOPDs. Moreover, patients with varying degrees of COPD disease severity should be included to unravel which patients could benefit from such measures. As such, the current findings may indicate that the impact of (COVID-19–related) IPC measures may be more pronounced in patients with a less severe overall clinical presentation.35
Acknowledgments
Author contributions: KWS, SHW, SOS, and FMEF are responsible for the conception and design of the study. KWS and FDJ contributed to the acquisition of data. KWS is responsible for the data analysis and interpretation, and for writing the first draft of the article. All authors contributed significantly to the intellectual content of the article and gave approval for publication of the final version.
The authors are thankful for all patients, physicians, researchers, and staff involved, and to have had the opportunity to conduct this retrospective research.
Data sharing statements: Access to the pseudonymized data is available upon reasonable request, only after approval by the local Medical Ethical Committee and Ciro’s Board of Directors. Data sharing requests may be directed to the senior author.
Declaration of Interest
KWS, SHW, FDJ, NPHVL, and BH declare no conflicts of interest. RP received personal fees from Health Investments, MEDtalks, and Mark Two Academy outside the submitted work. DJAJ reports grants from the Netherlands Organisation for Health Research and Development (ZonMw), Stichting Astmabestrijding, and the Netherlands Respiratory Society, fees from Boehringer Ingelheim, Chiesi, and Abbott outside the submitted work. SOS has received grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Chiesi outside the submitted work. MAS has received grants from the Netherlands Lung Foundation, Stichting Asthma Bestrijding, AstraZeneca, Boehringer Ingeheim, TEVA, and Chiesi outside the submitted work. FMEF has received grants and personal fees from AstraZeneca, Chiesi, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and MSD outside the submitted work. All authors declare no conflicts of interest in relation to the present study.