Running Head: The Association of Hypertension and COPD
Funding Support: No specific funding was received from the public, commercial, or not-for-profit sectors to carry out the work described in this article.
Date of Acceptance: March 19, 2023 │ Published Online Date: March 27, 2023
Abbreviations: BMI=body mass index; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CVD=cardiovascular disease; ECM=extracellular matrix; HTN=hypertension; IQR=interquartile range; NHANES=National Health and Nutrition Examination Survey
Citation: Liang X, Chou OHI, Cheung BMY. The association between systemic arterial hypertension and chronic obstructive pulmonary disease. Results from the U.S. National Health and Nutrition Examination Survey 1999-2018: a cross-sectional study. Chronic Obstr Pulm Dis. 2023; 10(2): 190-198. doi: http://doi.org/10.15326/jcopdf.2022.0306
Online Supplemental Material: Read Online Supplemental Material (205KB)
Introduction
Cardiovascular diseases (CVD) and chronic obstructive pulmonary disease (COPD) are 2 leading causes of death in the world.1,2 Systemic arterial hypertension (HTN) is an important modifiable risk factor for CVD, including coronary heart disease, stroke, and heart failure.3,4 It contributes to 54% of strokes and 47% of ischemic heart diseases.2 Antihypertensive treatment helps reduce cardiovascular mortality and morbidity,5 such that effective blood pressure control reduces the risks of coronary heart disease by 25%, stroke by 35%, and heart failure by 50%.
COPD is a group of progressive lung conditions that causes incomplete reversible airflow due to airway or alveolar abnormalities.6 It is the third leading cause of death worldwide.1 COPD patients have over a 2-fold increase in CVD hospitalization and mortality compared to those without COPD.7,8 COPD is strongly associated with CVD, including ischemic heart diseases, stroke, and atrial fibrillation.9,10 Several potential mechanisms, such as aging,11 systemic inflammation,12 arterial stiffness,13 and autonomic nerve dysfunction14 were proposed to explain the association. Previous studies examined the relationship between CVD and COPD.7-9 They focused on different targeted populations. One of the prospective studies included people aged 40 years or older,8 while another study included men around 55 years old.9
Recently, the Eighth Joint National Committee highlighted the importance of managing both HTN and COPD to help reduce the burdens of the comorbidities.15 Few have investigated the association between systemic arterial HTN and COPD in the general population. A Korean study revealed that COPD is independently associated with HTN in men aged 40 years and above.16 Another study demonstrated that HTN is more prevalent among severe COPD patients aged 45 years or above but not below.17 Further investigations are needed to investigate the association between COPD and HTN in the general population.
This study aimed to assess the association between HTN and COPD using the data from the U.S. National Health and Nutrition Examination Survey18 (NHANES) 1999–2018.
Patients and Methods
Study Population
NHANES was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. A complex, multistage, probability sampling design was used to select participants representative of the civilian non-institutionalized U.S. population. Informed consent was obtained from all adult participants, and the study was approved by the National Center for Health Statistics’ Ethics Review Board. A total of 52,398 individuals aged 20 years and over participated in the Mobile Examination Center of NHANES 1999-2018. A total of 3725 participants with missing, unreliable, or uncertain important covariables, including socioeconomic factors (education level, family income level, occupation, and health insurance), body mass index, diabetes, smoking records, or asthma, were excluded (Supplementary Figure 1 in the online supplement). We also excluded incomplete, unreliable, or uncertain data for HTN (n=11) and COPD (n=162). Participants who were pregnant or lactating (n=1696) were also excluded. The demographics, examination results (blood pressure, body measures), medical condition questionnaire, laboratory data (glycated hemoglobin, plasma fasting glucose), and prescription medication information (theophylline and inhaled corticosteroids) were retrieved18 from NHANES 1999–2018.
Definition
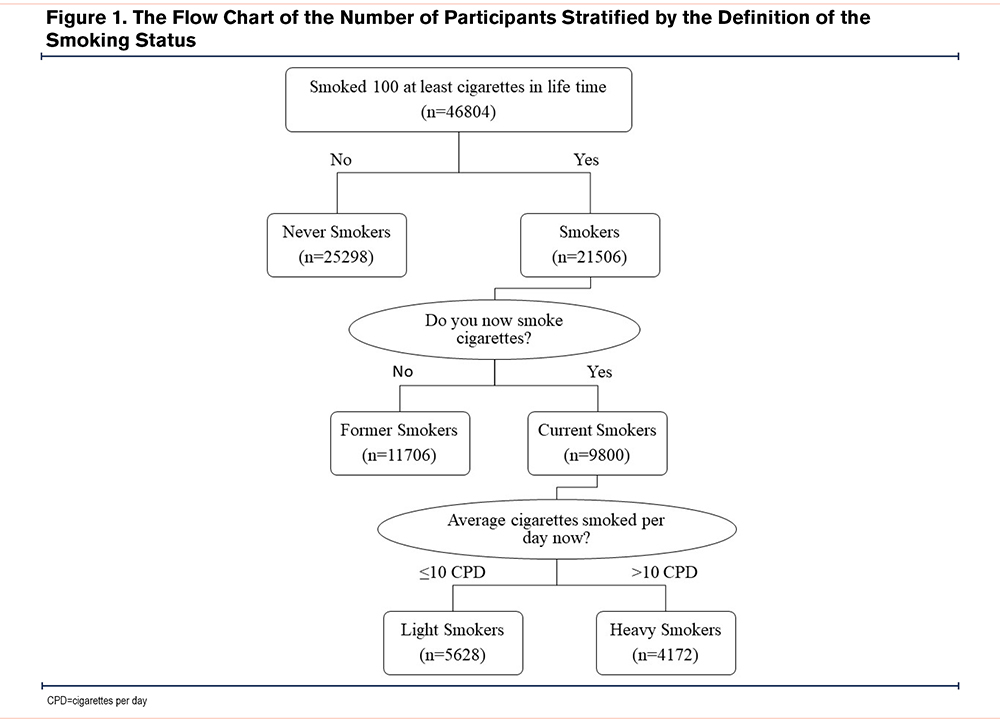
According to the self-reported demographics questionnaire, race/ethnicity was classified into 4 groups, namely non-Hispanic Whites, non-Hispanic Blacks, Mexican Americans, and others (including other Hispanic and multi-racial). Education levels were divided into 3 groups: high school or below, college, and college graduates or above. Household income was classified as below 130%, 131%– 338%, and above 339% based on the ratio of income to poverty. The participants were also categorized as employed or unemployed. Health insurance was categorized into private health insurance, public health insurance only, and no health insurance. Participants were categorized by age into 3 groups, 20–39 years old, 40–59 years old, and ≥60 years old. The “never smoker” status was defined as smoked less than 100 cigarettes in their lifetime. The “former smoker status” was defined as used to smoke 100 cigarettes or more in their lifetime but is no longer smoking now. “Current light smokers” was classified as smoking less than 10 cigarettes per day now, and “current heavy smokers” were people who smoke over 10 cigarettes per day (Figure 1). Diabetes was defined either as a self-reported physician diagnosis of diabetes or prescribed medicine for diabetes or elevated levels of fasting glucose (≥7.0 mmol/L [≥126 mg/dL]) or HbA1c (≥6.5%). Participants were defined as hypertensive if they have self-reported HTN, are taking prescribed antihypertension medications, or their measurement of blood pressure is ≥130/80mmHg according to the American Heart Association/American College of Cardiology 2017 guidelines.3 COPD was defined according to the answers to the clinical questions "Has a doctor ever said you had COPD" or “Have you ever been told you had chronic bronchitis” or “Have you ever been told you had emphysema” in the medical conditions questionnaire as validated in a previous study.19 Different methods of defining COPD were used in NHANES 2007–2012 as sensitivity analysis, including questionnaire-based and spirometry-based definitions. Theophylline and inhaled corticosteroids were defined using the unique generic drug code in NHANES.
Statistical Analysis
Complex sample weights were adopted due to unequal probabilities of selection, nonresponse bias, and oversampling of non-Hispanic Blacks by using primary sampling units and strata. The characteristics of the participants were further stratified by hypertension status and COPD status. The independent t-test was used to compare continuous variables and the Chi-square test was used to compare categorical variables. Multiple logistic regression was used to assess the association between COPD and HTN. The demographics (age, gender, race/ethnicity), socioeconomic factors (education level, family income level, occupation, and health insurance), smoking, diabetes, body mass index, and medication use (inhaled corticosteroids and methylxanthines) were adjusted in the multiple logistic regression. The association between COPD and HTN was further stratified by gender, age, and smoking status. A mediation analysis of medication (theophylline and inhaled corticosteroids) between COPD and hypertension was conducted. Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and P values were reported. All significance tests were 2-tailed and considered significant if P values were less than 0.05. Statistical analysis was performed using STATA (version 15.1).
Results
Characteristics of Study Participants
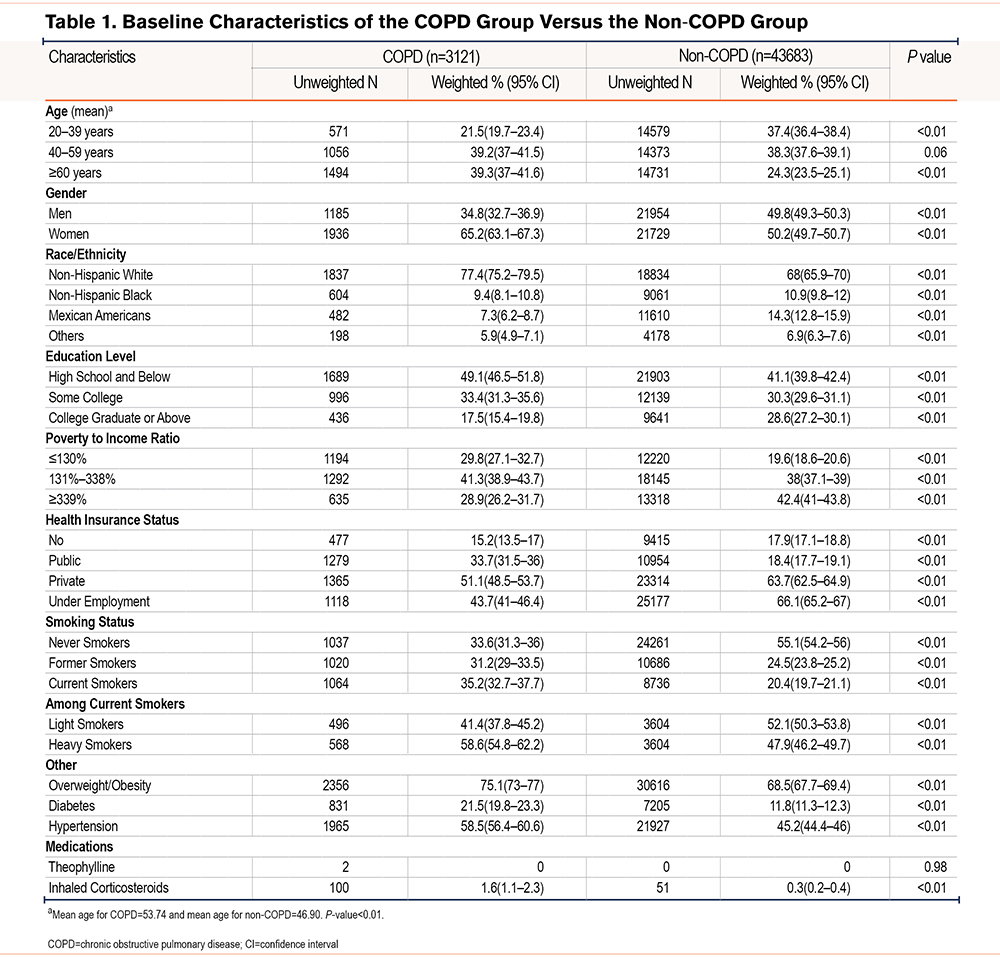
In our study, a total of 46,804 eligible participants (interquartile range [IQR]=26 years; 48.8% [95% CI, 48.3–49.2] male) were included in this analysis. This sample represented 198,587,043 civilian non-institutionalized adults in the United States. The characteristics of the participants stratified by COPD status are shown in Table 1. Compared to the individuals without COPD, participants with COPD were older (mean age 53.74 versus 46.90, P <0.01). HTN was more prevalent among COPD participants than in participants without COPD (58.5% [95% CI, 56.4–60.6] versus 45.2 [95% CI, 44.4–46.0]). The characteristics of participants with or without HTN are shown in Supplementary Table 1 in the online supplement. The prevalence of COPD was much higher among hypertensive individuals than those without HTN (8.6% [95% CI, 8.1–9.2] versus 5.2% [95% CI, 4.8–5.7], P <0.01).
The Association Between Hypertension and COPD
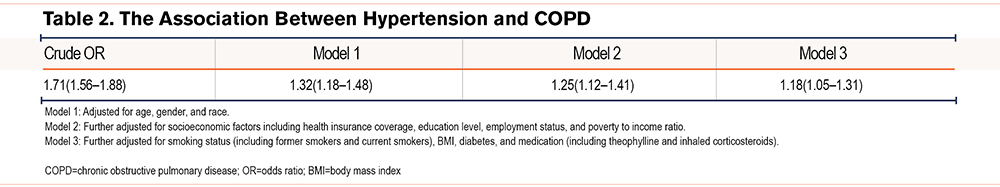
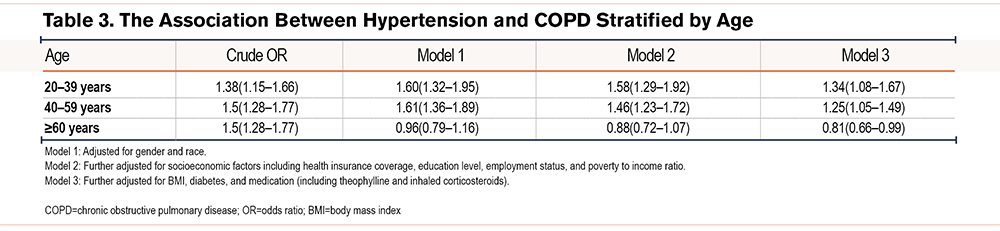
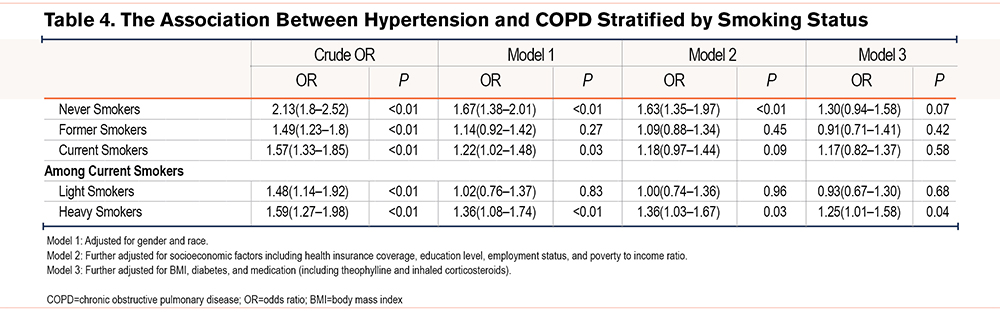
The multiple logistic regression analysis of COPD and HTN before and after adjustments for the covariates is displayed in Table 2. COPD was significantly associated with HTN before adjustments (unadjusted OR=1.71 [95% CI, 1.56–1.88]). After adjustments, the association remained significant (OR=1.18 [95% CI, 1.05–1.31], P<0.01). The association between HTN and COPD was significant among adults aged less than 60 years (P<0.01), and presence of COPD was associated with a decreased odds of HTN in adults > age 60 (OR=0.81, 95% CI [0.66–0.99], P=0.05) (Table 3). Stratified by gender, the association between HTN and COPD was found among women after adjustments (OR=1.14 [95% CI, 1.02–1.22], P<0.01) (Supplementary Table2 in the online supplement). Among current heavy smokers, COPD was positively correlated with HTN after adjustments (1.25, 95% CI [1.01–1.58], P=0.04). (Table 4).The mediation analysis demonstrated that the association between HTN and COPD was not mediated by theophylline and inhaled corticosteroids (Supplementary Figure 2 in the online supplement). The sensitivity analysis demonstrated that the association between HTN and COPD remained significant upon including pregnant or lactating women (Supplementary Table 3 in the online supplement). This association remained consistent with different COPD definitions, including questionnaire-based definitions and spirometry tests. (Supplementary Table 4 in the online supplement).
Discussion
This nationally representative survey of 46,804 participants suggested that HTN was associated with COPD in the general population after adjustments. The association was present in women, those aged less than 60 years, and current heavy smokers.
Systemic Arterial Hypertension Is Associated With COPD
Our findings demonstrated that COPD is associated with systemic arterial HTN. The results are consonant with the previous studies,16,17,20 which reported the association in specific populations. HTN is among the most frequent comorbidities in COPD patients.21 Multiple pathways have been proposed to explain the association.22-24 Firstly, COPD-induced autonomic dysfunction may contribute to increased blood pressure. Due to airway obstruction and airway inflammation, COPD patients repeatedly suffer from hypoxemia, hypercapnia, and increased intrathoracic pressure, resulting in decreased baroreceptor sensitivity and excessive activation of sympathetic nerves.14 Secondly, COPD patients have increased arterial stiffness, reducing wave reflection time and elevation of blood pressure.25 Arterial stiffness is a consequence of the remodeling process, which involves elastin fragmentation and collagen replacement in the extracellular matrix (ECM). Arterial stiffness is aggravated among patients expressing the emphysema phenotype COPD; they are more susceptible to lung, skin, and arterial extracellular matrix remodelling.26 COPD-related hypoxia may trigger systemic inflammation, accelerating the process of arterial stiffness. Last but not least, the administration of inhaled or systemic corticosteroids for treatment among COPD patients also contributes to the development of HTN.27 There are also several overlapping mechanisms between the 2 conditions. One of which was the changes in the ECM. Altered expression of ECM proteins, such as elastin, collagen, and proteoglycans, contributes to the narrowing of airways and parenchyma.28 The collagen and elastin expression are also altered by the vascular remodeling in hypertension. This resulted in the overproduction of collagen and scarcity in the amount of elastin, which reduces vascular compliance and increases blood pressure.29
Compared to the results in the Korean study,16 which investigated the association in men, our results demonstrated COPD was not associated with hypertension among men after adjustments (Supplementary Table 2 in the online supplement). The discrepancies can be explained by the differences in the targeted population and the disease definition. In the Korean study,16 COPD was defined by the combination of spirometry and smoking history. Participants aged less than 40 years, mild COPD patients, and non-smokers were excluded.
Similarly, a study revealed that severe respiratory dysfunction is associated with a higher risk of comorbid HTN among patients aged over 45 years. Individuals with Global initiative for chronic Obstructive Lung Disease (GOLD)6 stage 3 or 4 COPD had a higher prevalence of HTN upon adjustments (OR=1.6, [95% CI 1.3–1.9]).17 However, individuals “at-risk” of COPD (GOLD stage 0), with respiratory symptoms and risk factors but normal lung function, are also at risk of HTN (OR=1.2, [95% CI 1.1–1.3]). However, adults aged less than 45 years old were not included in this study; other risk factors that would attenuate the association, such as socioeconomic factors and medication use, were also not well addressed in this study.
A robust association between HTN and COPD was found among adults younger than 60, highlighting the need for smoking cessation starting early. Smoking is a well-established risk factor for HTN and COPD. Recently, there has been a substantial increase in the proportion of smokers who started smoking cigarettes in early adulthood (age ≥18 years) and developed habitual cigarette smoking.30
We also found an association between HTN and COPD among current heavy smokers. This suggests the need for tobacco restriction among hypertensive patients. Smoking increases systemic arterial stiffness and has detrimental effects on endothelial functioning.31,32 This subsequently inflicts vascular wall damage and hypertrophy.31 The above pathway results in organ damage, especially in the lungs.26 Recently, endothelial dysfunction was proposed to be correlated with pulmonary lesions in COPD due to vascular inflammation and anti-oxidant suppression.33 However, the endothelial damage introduced by smoking could potentially be reversible as current smokers have worse endothelial function than former smokers.34
This study has some limitations that should be appreciated. Firstly, the NHANES is a cross-sectional survey, such that it does not provide longitudinal follow-up data. Given its retrospective nature, the results only demonstrate correlation but not causation. Future studies are needed to demonstrate the causal relationship. Secondly, NHANES uses a recall questionnaire for some variables, which are prone to recall and response bias. The diagnosis of COPD relies on the questionnaire survey without including standard investigations such as spirometry, as spirometry was only performed in selected years (2007–2012) in NHANES. However, we conducted a sensitivity analysis using different definitions of COPD; the association between HTN and COPD remained significant across the various definitions of COPD. As a previous study35 suggested that questionnaire-based definitions demonstrated an overall accuracy of 89%–90%, the diagnosis of COPD using questionnaire-based definitions may still be a viable alternative. Lastly, due to the lack of forced expiratory volume in 1 data, further risk classification analysis of COPD patients was not available.
Conclusions
In conclusion, our results from this cross-sectional study demonstrated a significant association between hypertension and COPD, especially among adults aged less than 60 years and current heavy smokers. Future prospective studies are needed to examine the relationship between HTN and COPD.
Acknowledgements
Author Contributions: XL is responsible for conceptualization, methodology, data analysis, and drafting of the manuscript. OHIC is responsible for conceptualization and revision of the manuscript. BMC is responsible for methodology, supervision, and revision of the manuscript. All authors have read and approved the submission of the manuscript.
Data Sharing: The data that support the findings of this study are available in NHANES 1999–2018. These data were derived from the following sources available in the public domain https://www.cdc.gov/nchs/nhanes/index.htm
Declaration of Interest
All authors declare no conflicts of interest.