Running Head: COPD Admissions and Mortality During COVID-19
Funding Support: This study was supported in part by grants from CapNetz Stiftung (to R-H.H.), Lungenemphysem Register e.V. (to R-H.H.), and Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB 1449 (project ID 431232613 to M.W.), sub-project B02 (to M.W.); and SFB-TR84 (project ID 114933180 to M.W.), sub-projects C06 and C09 (to M.W.) and by the German Federal Ministry of Education and Research (BMBF) in the framework of e:Med CAPSyS (01ZX1604B to M.W.), PROVID (01KI20160A to M.W.), e:Med SYMPATH (01ZX1906A to M.W.), NUM-NAPKON (01KX2021, 01KX2121 to M.W.), Phage4Cure (16GW0141 to M.W.), MAPVAP (01KI2124 to M.W.), PulmVasC (01EP2102B to M.W.) and CAP-TSD (031L0286B to M.W.)
Date of Acceptance: April 20, 2023 │ Published Online Date: May 3, 2023
Abbreviations: CAT=COPD Assessment Test; COPD=chronic obstructive pulmonary disease; COVID-19=coronavirus disease 2019; EBV=endobronchial valve; ELVR=endoscopic lung disease reduction; FEV1=forced expiratory volume in 1 second; GOLD=Global initiative for chronic Obstructive Lung Disease; LE=lung emphysema; mMRC=modified Medical Research Council; SGRQ=St George’s Respiratory Questionnaire
Citation: Pappe E, Hammerich R, Saccomanno J, et al. Impact of coronavirus disease 2019 on hospital admissions, health status, and behavioral changes of patients with COPD. Chronic Obstr Pulm Dis. 2023; 10(3): 211-223. doi: http://doi.org/10.15326/jcopdf.2022.0383
Online Supplemental Material: Read Online Supplemental Material (194KB)
Introduction
The coronavirus disease 2019 (COVID-19) has had a profound impact on all areas and aspects of society and everyday life. Many countries announced radical physical distancing and lockdown measures of the public and private sector during the pandemic, such as closing fitness and rehabilitation facilities,1 in order to protect their citizens from COVID-19. In Germany, the first lockdown due to illness caused by severe acute respiratory syndrome-coronavirus- 2 (SARS-COV-2) was declared on March 22, 2020. The lockdown period was followed by a cycle of easing and tightening of restriction measures over the next 2 years in accordance with the respective decreased and increased COVID-19 case numbers.
Patients with COPD are identified as a high-risk patient group for worse outcomes2-4 of COVID-19. Particularly those patients with advanced stages of COPD as characterized by a forced expiratory volume in 1 second (FEV1) less than 50%, and a high symptom burden based on the Global initiative for chronic Obstructive Lung Disease (GOLD) risk classification, were seen as highly vulnerable.5,6 Extensive social isolation and protective measures were, and still are, highly recommended for this patient group. It was assumed that wearing medical face masks, staying at home, or reducing personal contacts to a minimum might reduce the risk6 of being exposed to SARS-COV-2 and developing COVID-19.
Medical services were also adjusted akin to social restrictions to better manage high case numbers7 of COVID-19. A worldwide decrease in the number of stroke or cardiovascular disease patient admissions was observed during the pandemic.8-12 Moreover, treatment delays were revealed to lead to increased disease severity and mortality, underlining the burden of collateral damage caused by the COVID-19 pandemic and by its various lockdown and containment measures.9,11,12 Interestingly, fewer acute exacerbations of COPD were also observed in hospitals.2 A number of possible reasons for this observed decline in COPD admissions have been discussed, and possible respective causes include reduced transmission of various respiratory viruses or bacteria due to stricter adherence to isolation measures of COPD patients, a general avoidance of seeking health care of this patient group due to fear of SARS-COV-2 infections, as well as possible lower industrial emissions leading to better air quality and thus, decreased symptom severity in these patients.6,13-15 Despite these seemingly overall positive effects of isolation measures for the general population, long time consequences and side effects of COVID-19 lockdowns for patients with severe COPD have barely been investigated thus far. Particularly, anxiety and depression are common comorbidities in patients with severe COPD and are seen as an important contributor to poor quality of life.16 Moreover, social isolation and restricted life-space mobility in severe COPD patients have been found to be associated with more severe exacerbations, progressive dyspnea, worse quality of life, and greater psychological symptoms in the latter.17
Hence, the long period of COVID-19 lockdown, combined with measures of social isolation as well as limited access to health care providers, may have and still may be, exerting a negative influence on the outcome of patients with severe COPD. We, therefore, investigated the impact of the COVID-19 pandemic on hospital admissions, ventilation therapy, and mortality among hospitalized COPD patients. Furthermore, we are questioning the impact on behavioral changes and health status in COPD patients during the lockdown.
Methods
Study Time
The German government instructed the first nationwide lockdown to begin on March 22, 2020 (Figure 1). Public health measures of this lockdown included stay-at-home orders, the closing of schools and of non-essential shops, (e.g., restaurants, warehouses) as well as mandatory facemask wearing. COVID-19 cases increased again rapidly in September 2020. As a consequence, a light lockdown was announced in Germany on October 28, 2020. This showed little or no effect though, causing the second nationwide lockdown to be declared in December 2020. The latter included stricter infection-prevention measures such as limiting private gatherings to 2 households with a maximum of 5 people and lasted until January 2021. Nonetheless, the renewed high numbers of COVID-19 cases due to the spreading of the highly contagious Delta variant led to a third lockdown on April 21, 2021, which again included strict nationwide containment measures. After the introduction of an efficient vaccine and implementation of a successful vaccination program against SARS-COV-2 infection in Germany, the incidence of the disease dropped decidedly and remained low between June and September 2021. This resulted in a general reduction and relaxation of public health measures against COVID-19.
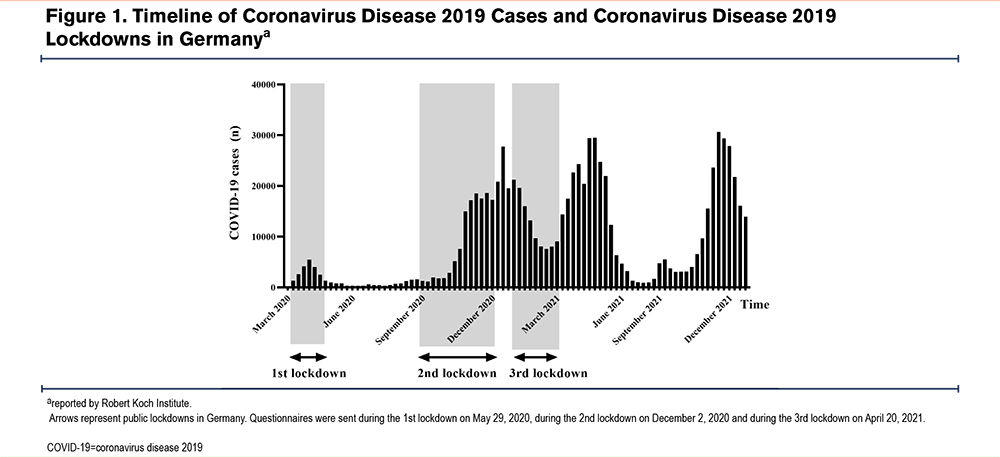
Our observational study was carried out with data from Charité-Universitätsmedizin, Berlin, Germany.
COPD Patients at Charité-Universitätsmedizin Berlin
Numbers of admitted patients with the primary diagnosis of COPD (International Classification of Diseases, version 10 [ICD-10]-GM: J44.0, J44.1) or pneumonia (pneumonia was determined according to the specification of a diagnosis table with a total of 36 ICD codes: German Inpatient Quality Indicators Version 5.3) were acquired by extraction of respective data from the anonymous Charité hospital databases from the pre-pandemic (2012–2019) and pandemic time periods (2020–2021). Pneumonia cases were used as a comparator in order to understand COPD admission rates better in relation to other respiratory diseases. Cases of admissions, as well as cases of ventilation and mortality, were analyzed in absolute numbers and relative to all patients hospitalized at Charité-Universitätsmedizin Berlin from each respective year (ratio COPD or pneumonia patients/ all cases in %). The number of subgroups (gender, age, COPD stages, ventilation, and deaths) was calculated relative to the number of all COPD or pneumonia admissions. The number of COVID-19 pneumonia (ICD-10-GM: U07.1) cases was included in the number of all pneumonia patients. Only patients older than 20 years were included in the study. For COPD, patients with all COPD GOLD stages were included.
Lungenemphysem Register e.V.
We used data from 11 active clinical centers (i.e., hospitals with more than 4 treatments of lung volume reduction per year) from the Lungenemphysem Register e.V., a German independent lung emphysema (LE)-registry. The LE-registry collects clinical data solely from severe emphysema patients (GOLD stage 3 and 4 status) in Germany,18 and is listed within the German Clinical Trials Register (DRKS00021207). Study data were collected and managed using REDCap electronic data capture tools hosted at Charité-Universitätsmedizin Berlin.19,20 Inclusion and exclusion criteria for this register have been described previously.18 For our current study we compared the number of treatments and assessed the proportion of 3-month follow-ups, as well as complications in respective 6-month periods of each of the pandemic (2020–2021) and pre-pandemic (2018–2019) time intervals. The number of included treatments for lung volume reduction was limited to endoscopic lung volume reduction (ELVR) using one-way valves. The ethics committee of the Charité-Universitätsmedizin Berlin approved the study (EA2/149/17). All patients provided informed consent.
Patient Questionnaire
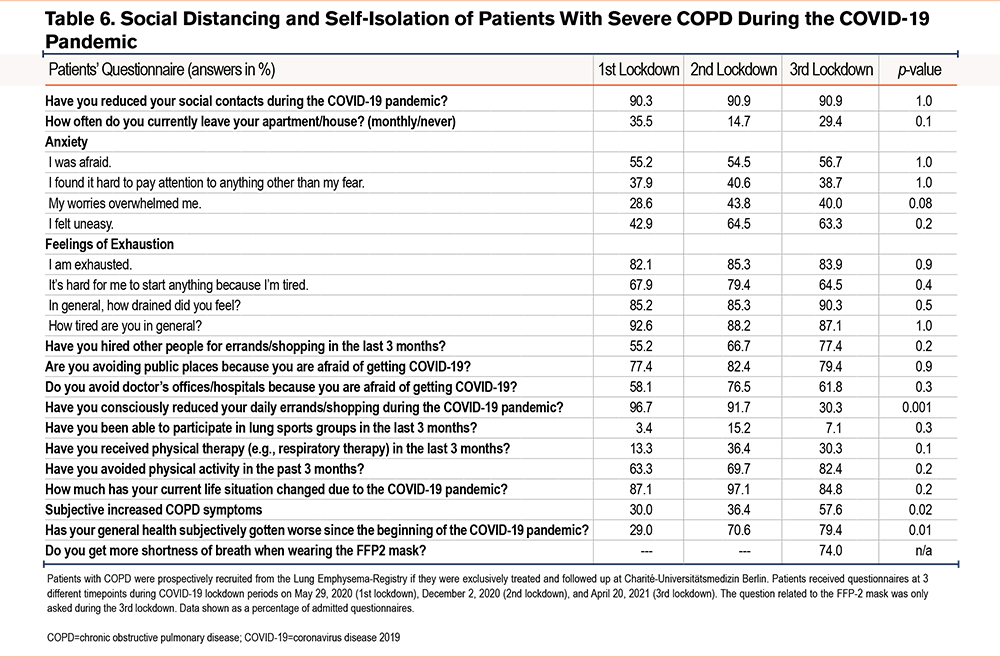
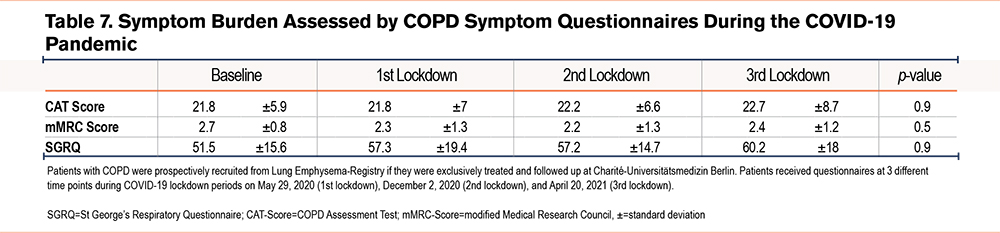
Patients who were treated and who received follow-up treatment at Charité-Universitätsmedizin Berlin were prospectively recruited from the LE-Registry. Respective questionnaires were mailed to 52 patients at 3 different time points during the COVID-19 lockdown periods, e.g., either on May 29, 2020 (1st lockdown), on December 2, 2020 (2nd lockdown), or on April 20, 2021 (3rd lockdown). Data were excluded from 2 out of a total of 52 patients in the study who died as a result of their COPD after the first lockdown. The questionnaire aimed to survey behavioral changes and the health status of patients with severe COPD during COVID-19 lockdowns. Questions related to overall social circumstances and to the extent of a patient’s social contacts, as well as daily activities, anxiety, and feelings of exhaustion and were compared to those of the pre-lockdown time period. COPD symptom questionnaires such as the St George’s Respiratory Questionnaire (SGRQ), the COPD Assessment Test (CAT), and the modified Medical Research Council (mMRC) dyspnea scale were used to assess psychological and physical impact, as well as to collect data on symptom burden of the included emphysema patients during the lockdown. Questions were asked with either a possible binary response (yes/no), a given answer option, or a scalable answer on a 5-point Likert scale. For scalable answers, the differences were calculated between the numbers of the answers “not at all” and the numbers of the answers “a little, moderately, and quite a lot.” For the SGRQ, CAT, and mMRC, pre-pandemic baseline data were taken from the last recorded routine follow-up from the LE-Registry. Baseline was defined as the time after LVR treatment and before the pandemic period.
Statistical Analysis
Absolute numbers of COPD or pneumonia admissions, ventilations, and deaths were analyzed under the assumption that respective numbers follow a Poisson distribution. The percentage of COPD or pneumonia admissions, ventilations, and numbers of deaths among all admitted patients/all COPD or pneumonia patients were estimated and compared using the Binomial test.
The unpaired t-test was used for the comparison of the LE treatments and for the follow- ups between the pandemic and the pre-pandemic period, and the Chi-squared test was employed for the comparison of relative subgroup numbers and for categorical data of baseline characteristics. The Chi-squared test was also used for comparing the frequency of respective patients’ answers in survey-based data. One-way analysis of variance was used for the comparison of metric baseline characteristics and CAT, MMRC, and SGRQ scores.
Since all continuous variables displayed normal distribution as examined with the Shapiro-Wilk test, all parameters are presented as means with standard deviation. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (Version 27.0.0.0, IBM Corp., Armonk, New York) and R-Studios (Version 1.4.1106). Graphics were created using GraphPad (Version 9.2.0 [283]).
Results
COPD and Pneumonia Cases During the Pandemic
The average admission rate per year to Charité-Universitätsmedizin Berlin decreased from 136,224±6881 patients in the pre-pandemic time period to 121,249±7250 patients during the pandemic time interval (p<0.001, data not shown).
Cases of pneumonia were compared to those of COPD in order to determine COPD admission rates relative to other respiratory diseases. There was an absolute and relative increase in admissions of patients with pneumonia during the pandemic time period as compared to pre-pandemic times (both p<0.001, Figure 2, A and B, baseline characteristics see Table 1). On average, 1110±233 of these 2129±35 patients (52.1%) tested positive for SARS-CoV-2 RNA per pandemic year, while only 6 out of 513±33 primarily coded COPD patients (1.2%) had a SARS-CoV-2 infection. However, among COVID-19 pneumonia cases, 174±23 (15.7%) patients had a secondary diagnosis of COPD. Patients with pneumonia during the pandemic time period were more frequently ventilated as compared to pre-pandemic times (both p<0.001, Figure, 2 C and D), with COVID-19 contributing to the majority of ventilation cases (67.3%, data not shown). There was a significant increase in absolute and relative rates of mortality due to pneumonia (both p<0.001, Figure 2, E and F), predominately a consequence of COVID-19 infection, but irrespective of age and gender (Table 2). A total of 43±0 (24.7%) patients with a secondary COPD diagnosis died of COVID-19 pneumonia during the pandemic.
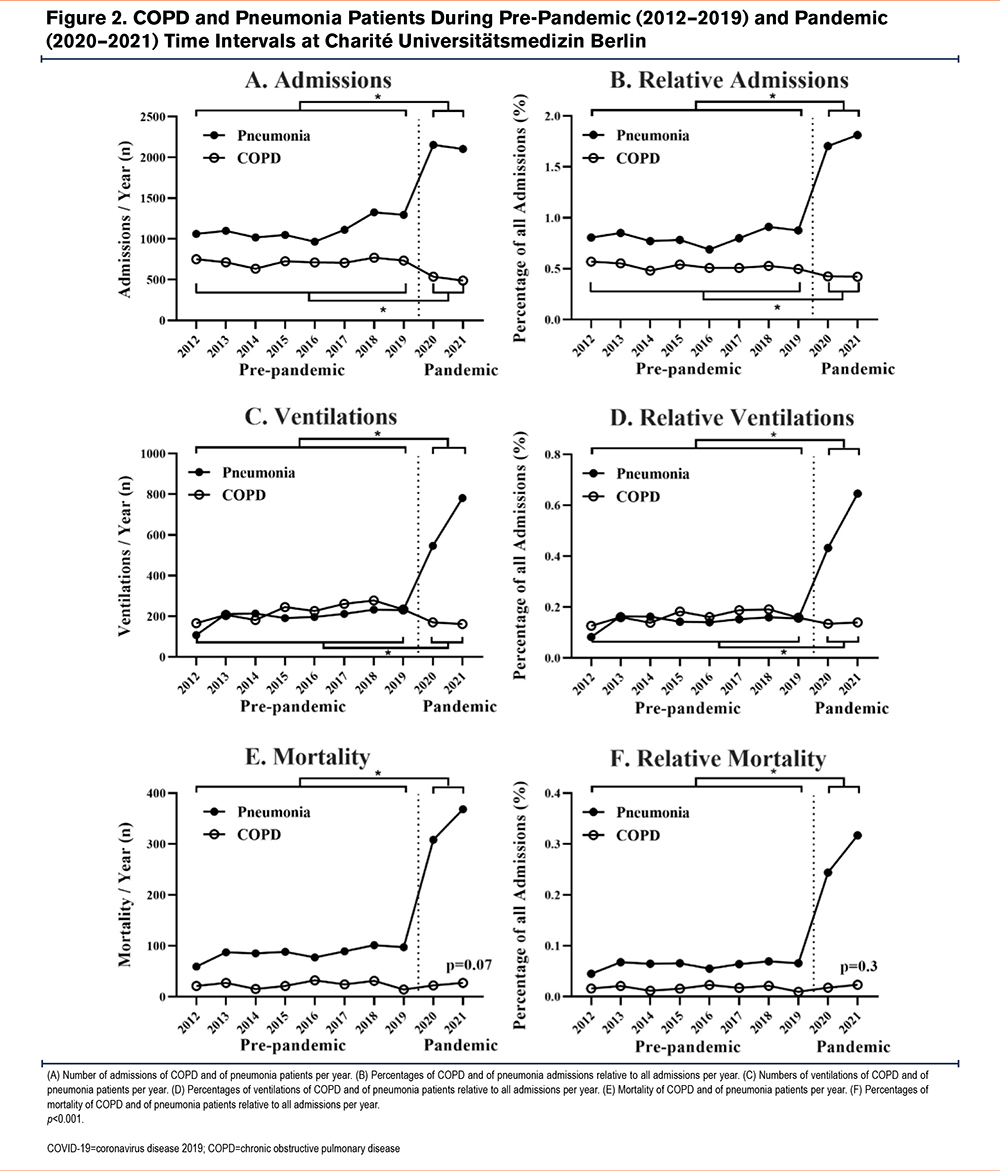
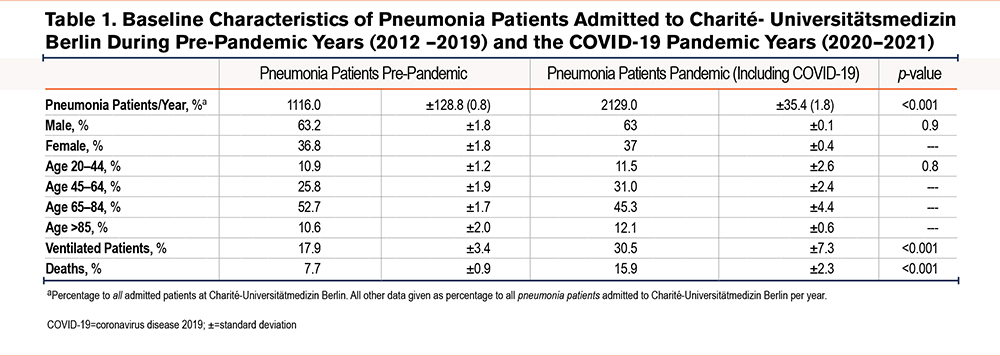
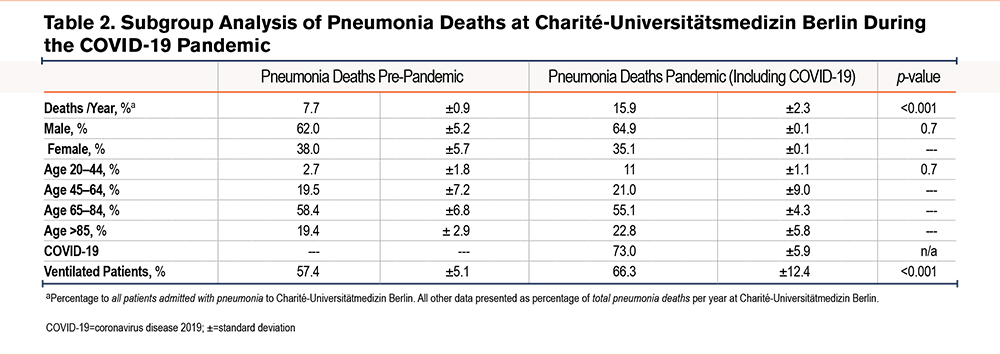
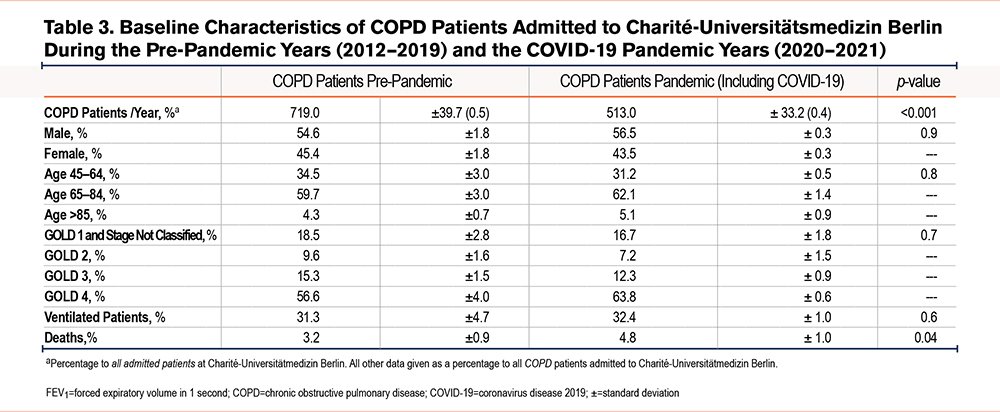
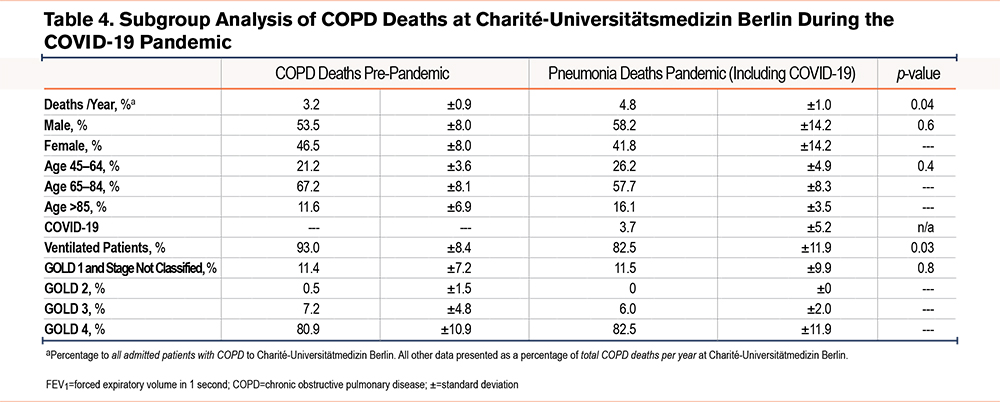
In contrast to pneumonia admissions, there was a decrease in absolute and relative admissions of COPD patients during the pandemic as compared to the pre-pandemic time period (both p<0.001, Figure 2, A and B, baseline characteristics see Table 3). Concomitant with lower admission rates, COPD patients received less absolute and relative mechanical ventilation during the pandemic compared to the pre-pandemic time period (both <0.001, Figure 2, C and D). However, in contrast to lower admission rates of COPD patients, absolute COPD deaths and COPD deaths related to all hospital admissions remained unchanged during the pandemic in comparison to pre-pandemic times (p=0.07, Figure 2E, and p=0.3 Figure 2 F). Next, the death rate of COPD patients was determined for patients hospitalized with COPD. Here, mortality was slightly higher during the pandemic than during pre-pandemic timespans (p=0.04, Table 4). Mortality was highest in older and ventilated patients and in patients with GOLD stage 4 status, although ventilations among all COPD deaths were significantly lower during the pandemic compared to the pre-pandemic time period (Table 4).
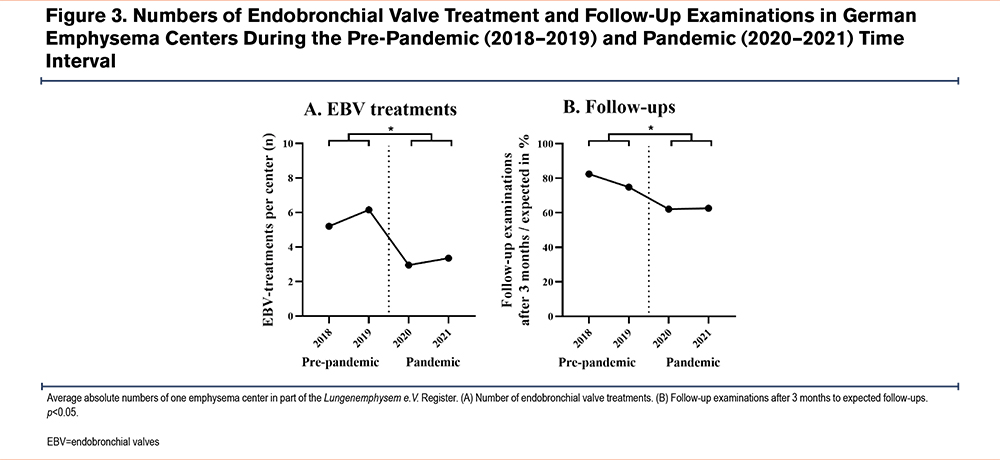
Endoscopic Lung Volume Treatments During the Pandemic
The mean number of ELVR treatments per lung emphysema center was observed to be lower during the pandemic (p<0.02; Figure 3A) as compared to the pre-pandemic timespan. Likewise, there were fewer 3-month follow-up examinations in COPD patients after the intervention when comparing the 2 respective time segments (p<0.016; Figure 3B).
Health Status and Behavioral Changes of COPD Patients During the Pandemic
The average response rate of the questionnaires for the 3 lockdowns was 66.0%. No differences were observed in baseline characteristics among the 52 enrolled COPD patients during all lockdowns (Table 5). All patients were classified as GOLD 3 or GOLD 4 stage, whereas around 40% received long-term oxygen therapy and 13% non-invasive ventilation. The majority of latter patients had been treated with endobronchial valves. A total of 77% of patients were pensioners and 33% obtained ambulatory nursing services. Only 6% of all patients reported having COVID-19, all of which showed only mild disease symptoms. Around 80% of all patients were vaccinated against COVID-19 during the third lockdown.
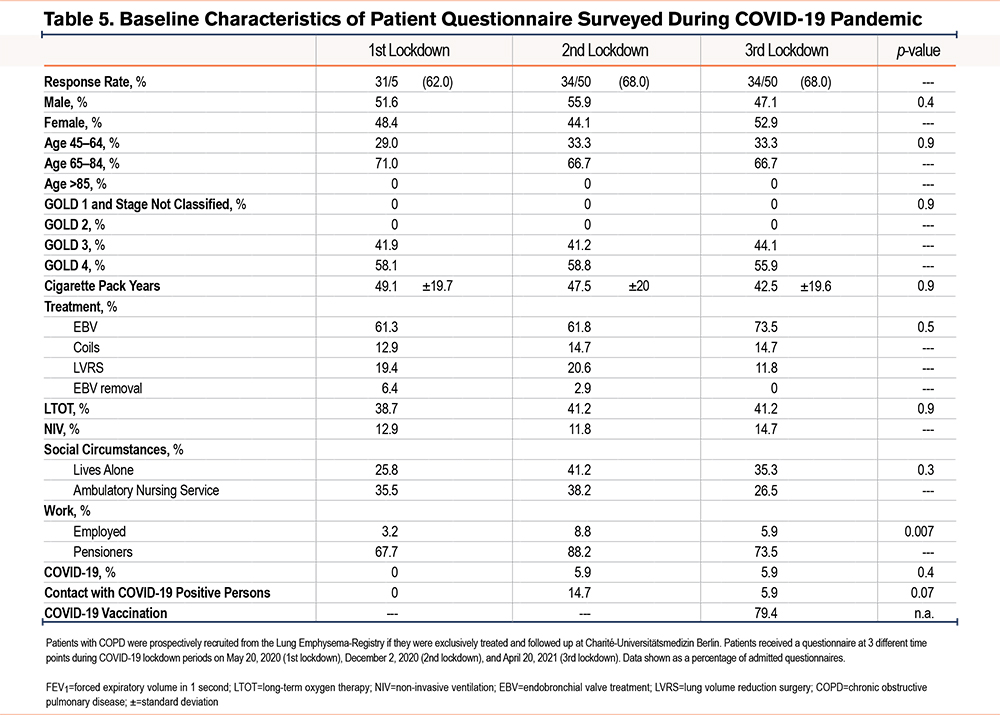
Table 6 gives an overview of patient answers pertaining to their behavioral changes during the lockdown. Over 90% of patients reported their social contacts during all 3 lockdowns had been reduced, and on average 26.5% of patients left their homes less than once a month . An increasing proportion of patients reported feelings of anxiety during lockdown and the greater part of patients had feelings of exhaustion. The majority of patients authorized other people for shopping, avoided public places and doctors’ offices, and reported a reduction of daily activities during the lockdown. Only a small proportion of participants were able to receive physiotherapy or take part in lung exercise groups during the lockdown period. Overall, physical activities decreased between the first and third lockdowns in the COPD patients interviewed. Around 60% of the patients reported a significant, subjective increase in their COPD symptoms as well as a deterioration of their general health the longer the lockdown lasted. This did not, however, result in an increased COPD burden as queried by the COPD symptom questionnaires-- the SGRQ, CAT, or mMRC—since all 3 of the latter reported stable COPD symptoms during the lockdown. (Table 7).
Discussion
This single-center, longitudinal study at Charité-Universitätsmedizin Berlin analyzed data of COPD patients, during the 2 years of the COVID-19 pandemic, regarding patients’ hospital admissions, ventilation therapy, mortality, and numbers of ELVR therapies received, as well as their overall health status and observed behavioral changes. Analysis of the respective data revealed not only decreased hospital admissions for these patients during the pandemic but also reduced use of ventilation and ELVR therapies among them, while mortality of COPD patients among hospitalized COPD patients was slightly higher compared to the pre-pandemic time period. The survey-based data of patients with severe COPD revealed distinct behavioral changes in this patient group during the lockdown, with their subjective perception being a worsening of COPD symptoms as well as that of their general state of health the longer the lockdowns lasted. Interestingly, data from COPD symptom questionnaires revealed stable COPD symptoms during the pandemic, however.
Lower admissions of COPD patients are in line with other studies.15,21-24 Strict hand hygiene, the wearing of protective masks, and social distancing have been previously known to reduce transmission of COVID-19 and to lower the number of incidences of COPD exacerbations.21,22,24 Two studies reported significantly reduced numbers of patients with respiratory virus infections, which is attributed to lockdown measures.25,26 Patients’ perceptions, as well as their anxiety regarding personal vulnerability and an increased risk of acquiring COVID-19, could have led to their stricter adherence to lockdown measures, especially during the first lockdowns, which also lacked the availability of an effective vaccine. Another study with U.S. veterans was able to demonstrate higher compliance with lockdown measures in patients with severe COPD, as well as their higher vaccination rates when compared to patients with only mild to moderate COPD.27 McAuley H et al observed an increase in community-managed exacerbation events of patients with COPD, as well as an increase in inhaler compliance.15 The reduction of severe exacerbations leading to hospital admissions during the pandemic led to a positive reinforcement of behavioral changes in COPD patients due to the lockdown measures. Because of the high effectiveness and acceptance of COVID-19 preventative interventions in patients with chronic airway diseases,28 novel strategies for the prevention of COPD exacerbations might include some of these interventions.
Interestingly, the mortality of COPD patients at Charité-Universitätsmedizin Berlin was determined to have increased among hospitalized COPD patients. Although absolute numbers did not reveal a statistically significant difference, the number of deaths among hospitalized COPD patients was slightly higher during the pandemic years as compared to those of pre-pandemic times. Causes for this observed increase in COPD mortality among all hospitalized COPD patients during the pandemic remain to be elucidated. It appears likely that reduced hospital admissions also lead to reduced treatment of COPD patients, a phenomenon already observed in other studies. Similarly, mortality of patients with cardiovascular diseases has been observed to have increased during the pandemic years, as well as an increase in poorer cardiac outcomes for acute myocardial infarction during the early phase of the COVID-19 pandemic.11,29,30 The diminished or delayed access to health care is generally considered the main reason for the observed increase in levels of mortality.29,30 The increased mortality of patients with cardiovascular diseases occurred primarily outside of the hospital setting.29,31 Reduced hospital presentations could have been caused by patients’ fears of contracting COVID-19 in the hospital setting, or by their attempt to prevent admittance to potentially overrun or overcrowded clinics,29 especially during the first lockdown phase, which was characterized by a lack of information about COVID-19in general. Therefore, admissions of generally sicker COPD patients could be the cause of increased mortality among hospitalized COPD patients. Nevertheless, data on the mortality of COPD patients during the pandemic are inconsistent and further studies are warranted.22,32,33
The ventilation therapy use among COPD deaths was significantly lower during the pandemic when compared to the pre-pandemic time period. This is an interesting observation and could be due to the fact that COPD patients were admitted to the clinic at an already severely exacerbated state of their disease, too advanced to administer mechanical ventilation. Another potential reason for the reduced ventilation use observed in COPD patients could theoretically have been a limited number of available ventilators caused by the respective needs of the very high numbers of severely ill COVID-19 patients during the pandemic. However, this was not the case at the intensive care unit at Charité-Universitätsmedizin Berlin and in Germany in general, because respective limits of the capacity of ventilators were never reached.34
Most of the non-urgent, ambulant interventions were canceled during the lockdown as mirrored by the significant reduction in ELVR treatments performed at centers that are part of the German lung emphysema registry. Further consequences of canceled or delayed medical interventions of COPD patients during the pandemic are anticipated to be disadvantageous for patients’ state of health in the next years but remain to be seen.
This study confirmed the vast increase in pneumonia cases during the pandemic and attributed to COVID-19. Charité-Universitätsmedizin Berlin was commissioned as a tertiary COVID-19 center during the pandemic, making it primarily responsible for the management of severe COVID-19 patients in Berlin during this time. Being responsible for the treatment of very ill COVID-19 patients explains the greatly higher rates of ventilation administered during the time of the pandemic, as well as the increased mortality rate of pneumonia patients during this time as compared to pre-pandemic time. Interestingly, mortality rates were noticeably higher in the group of patients with COVID-19 pneumonia and secondary COPD diagnosis, suggesting COPD as a risk factor for COVID-19 death. Concomitantly, a meta-analysis consisting of 22 studies and 13,184 COVID-19 patients reported a higher risk for severe outcomes in patients with underlying respiratory disease, specifically with COPD.35
The survey-based data demonstrates that patients with severe COPD reported a reduction of social contacts, social isolation, and avoidance of public areas and medical institutions during the COVID-19 pandemic when compared to pre-pandemic times. Similar observations were made during the first lockdown in the United Kingdom, revealing distinct behavioral changes in this patient group and strict adherence to lockdown measures. Respective patients reported avoiding physical activity since the beginning of the COVID-19 pandemic15,23 knowing that their predisposition placed them at higher risk of exacerbation of the disease and of contracting it.23,36
Consistent with this observation, around 50% of the COPD patients in the above study reported subjective worsening of their COPD the longer the lockdowns lasted. Previous studies have also demonstrated the first lockdown had a negative impact on the mental health of patients with COPD, increasing their anxiety about their general health status.15,23 In line with studies from McAuley H et al15 and Klooster K et al,37 the majority of patients reported feelings of anxiety and exhaustion during the lockdown. These observations might display a punishment for this underserved patient group due to the general aggravation of symptoms during the pandemic. Patients with COPD require optimal therapy including regular follow-ups, support, and management, and these treatments involve a number of health care professionals and treatments, such as general practitioners, pulmonologists, physiotherapists, rehabilitation, smoking cessation interventions, and psychologists.38 Interestingly, the subjective deterioration of health surveyed in COPD patient questionnaires was not reflected in the COPD symptom questionnaires (CAT, mMRC, SGRQ). This interesting inconsistency between subjectively perceived and clinically measured symptom severity in COPD patients might argue for the feasibility of adequate COPD care at home. Given their vulnerability, at-home care including telehealth-based programs can limit their time in public and can reduce the risk for infection with SARS-COV-2. However, there are also arguments against it. Our study shows that the lockdown led to a reduction of social contacts, loneliness, anxiety, and an interruption of regular therapies such as lung exercise groups in patients with severe COPD, which might not be covered at all with an intensive care program at home.
Limitations of this study are the retrospective nature of the analysis of the admission rates of COPD and pneumonia at Charité-Universitätsmedizin Berlin. Only cases encoded with COPD or pneumonia based on the diagnosis-related group system were included. These calculations may have variable accuracy, and total numbers for ventilation usage and mortality for COPD and pneumonia could differ when transferred to other hospitals, due to the different coding standards. Further potential limitations of the study are the small number of patients participating in the questionnaire, and the fact that not all COPD stages were considered in the comparison of treatments and follow-ups and received questionnaires. However, the hospital admissions data included patients with all stages of COPD.
In summary, this study shows a reduction of COPD patient admissions and elective COPD treatment procedures during the COVID-19 pandemic which were found to be associated with increased mortality among hospitalized COPD patients. In accordance, patients with severe COPD reported subjective deteriorations of their health possibly as a result of strict compliance with lockdown measures. There is a need for an increased focus on care and disease control for patients with COPD in the future, as well as for better preventive actions to avert long time serious collateral damage caused by the COVID-19 pandemic and the lockdown measures associated with it.
Acknowledgments
Author contributions: Study conceptualization was provided by EP and R-HH. Data curation was provided by EP, RH, JS, TS, AP, BS, CG, SE, AH, SE, FS, and R-HH. EP, RH, TS, AP, and R-HH provided a formal analysis of the data. Funding acquisition was provided by R-HH and MW. EP, RH, TS, and R-HH were responsible for the investigation and methodology. EP, TS, and R-HH were responsible for visualization. R-HH and MW were responsible for the supervision of the study and writing the manuscript’s original draft was done by EP and R-HH. Additional writing, review, and editing were provided by RH, JS, TS, AP, BS, CG, SE, AH, SE, FS, and MW. All authors read and approved the final version of the manuscript.
We thank all patients for their participation. We thank Leonore Erdmann for her excellent assistance in data collection. We are grateful to Katharina Ahrens, PhD, for her helpful comments and suggestions and for the critical revision of the manuscript. We thank Grit Barten-Neiner from CapNetz Stiftung, and Enrico Schneemann and Franziska Liebchen from Lungenemphysem Register e.V. for their excellent work in data management.
Declaration of Interests
Authors EP, TS, AP, BS CG, FS, and SEg have nothing to disclose. RH is a member of the scientific advisory board of Initiative Qualitätsmedizin and the German Inpatient Quality Indicators (V5.2 and V5.4), and reports personal fees from Ärztekammer Berlin, Deutsche Rentenversicherung, and AstraZeneca. JS is member of Lungenemphysem Register e.V. SEi reports personal fees from MSD, GSK, AstraZeneca, PulmonX, Bess, Sanofi Genzyme, and Boehringer Ingelheim, and is a member of the scientific advisory board of Acceleron and SOB. AH is a member of the Lungenemphysem Register e.V. MW received funding for research from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Deutsche Gesellschaft für Pneumologie, the European Respiratory Society, the Marie Curie Foundation, Else Kröner Fresenius Stiftung, Capnetz Stiftung, the International Max Planck Research School, Actelion, Bayer Health Care, Biotest, and Boehringer Ingelheim, and received funding for lectures and advisory from Noxxon, Pantherna, Vaxxilon, Aptarion, GSK, Sinoxa, Biotest, AstraZeneca, Berlin Chemie, Chiesi, Novartis, Teva, Actelion, Boehringer Ingelheim, Biotest, Bayer Health Care. R-HH is head of the Lungenemphysem Register e.V.