Running Head: Expanded COPD-Based Algorithm For Case Finding
Funding Support: This study was supported by an unrestricted grant from Novartis, Israel. The sponsor had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript, and the decision to submit the manuscript for publication.
Date of Acceptance: May 17, 2023 │ Published Online Date: May 18, 2023
Abbreviations: BMI=body mass index; COPD=chronic obstructive pulmonary disease; COPDGene=COPD Genetic Epidemiology study; FEV1=forced expiratory volume in 1 second; FEF=forced expiratory flow at 25%-75%; GOLD=Global initiative for chronic Obstructive Lung Disease; PRISm=preserved ratio-impaired spirometry; SD=standard deviation; SPIROMICS=SubPopulations and InteRmediate Outcome Measures In COPD Study; SZMC=Shaare Sedek Medical Center
Citation: Bohadana A, Rokach A, Wild P, et al. Clinical use of an exposure, symptom, and spirometry algorithm to stratify smokers into COPD risk phenotypes: a case-finding study combined with smoking cessation counseling. Chronic Obstr Pulm Dis. 2023; 10(3): 248-258. doi: http://doi.org/10.15326/jcopdf.2022.0368
Online Supplemental Material: Read Online Supplemental Material (169KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex, heterogeneous, chronic lung disease that is associated with enormous morbidity and mortality worldwide, with 3.3 million deaths1 in 2019. Smoking is the major and strongest risk factor for COPD, although not the only one.1,2 The disease is insidious and is usually diagnosed when lung function has significantly deteriorated.2 It is estimated that up to 60% of patients with COPD living in the United States are undiagnosed.3 Therefore, earlier diagnosis of COPD is warranted and screening strategies are needed. While there is insufficient evidence that screening for COPD in asymptomatic individuals improves health outcomes, active case finding – i.e., screening of individuals likely to have COPD due to respiratory symptoms or exposure to noxious particles (e.g., smoking) –is recommended.2,4 The detection of fixed airflow obstruction, characterized by a ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) of less than 0.7, is the current criterion for a positive diagnosis of COPD.2
However, using an FEV1/FVC ratio < 0.7 to diagnose a disease as heterogeneous as COPD may be problematic because several cohort studies have shown that many patients without "classic" COPD have significant impairment, with exacerbation-like respiratory events and associated hospitalizations, lung structural changes, and activity limitations.5-7 In addition, there is evidence that smokers with a normal FEV1/FVC ratio but a low FEV1, preserved ratio-impaired spirometry (PRISm), have poor outcomes, including COPD-like exacerbations, reduced activity, and increased comorbidity and mortality.8
Based on the premise that spirometry alone is not sufficient to characterize the disease burden associated with smoking, the COPD Genetic Epidemiology (COPDGene®) study(a 10-year longitudinal evaluation of more than 10,000 current and former smokers) showed that redefining the diagnosis of COPD through an integrated approach using: (1) environmental exposures (i.e., smoking), (2) symptoms, (3) spirometry, and (4) assessment of structural lung abnormalities using chest computed tomography (CT) criteria, provides a better definition of the disease and an understanding of the likelihood of lung function decline and mortality.9 Subsequently, a clinical algorithm was proposed to encourage primary care physicians and pulmonologists to use the COPDGene approach in current and former smokers.10
In line with the COPDGene study and the Lancet Commission Reportcalling for an expanded definition of COPD,11 this study aimed to implement this new concept in the context of case finding. To this end, we used a clinical algorithm to stratify smokers into COPD risk phenotypes; however, because we were unable to obtain CT scans, our algorithm only considered exposure, symptoms, and spirometry. In addition, in line with the COPDGene concept, and to our knowledge for the first time in case finding, we evaluated the acceptability and effectiveness of combining smoking cessation counseling with the case-finding intervention.
Methods
Study Design, Setting, and Population
We analyzed cross-sectional data from a case-finding study12 conducted at Shaare Zedek Medical Center (SZMC), Jerusalem, from May 2014 to June 2017. Participants were recruited from among the visitors to our medical center through promotional posters placed at strategic locations in the hospital. Individuals who were hospitalized or seeking medical care were not eligible for the study. The study recruited 1001 current or former smokers. From this dataset, we excluded ex-smokers and individuals younger than 30 years, leaving a study population of 864 active smokers aged ≥ 30 years. The Helsinki Ethics Committee of Shaare Zedek Medical Center approved all study procedures [Reg: 16/14 SZMC] and all participants provided written informed consent before any procedure.
Clinical Algorithm
The algorithm we have used is based on the 6-point COPDGene clinical algorithm proposed by Make10 for use by primary care physicians and pulmonary specialists.
1. Ask about smoking. After completing a general demographic and medical history questionnaire, participants completed a self-administered smoking questionnaire. Detailed assessment of smoking history included: the age at which participants began smoking, duration of smoking (i.e., the difference between age at enrollment and age at smoking initiation), the average number of cigarettes smoked per day since smoking initiation, and current cigarette consumption. Smoking burden was also measured in terms of pack years, which is the product of the average number of cigarettes smoked per day since smoking initiation and the duration of smoking in years.
2. Ask about shortness of breath, cough, and phlegm: Respiratory symptoms were assessed using the self-administered questionnaire developed by the Canadian Respiratory Society and recommended for COPD case finding in primary care.13 The questionnaire consists of 5 questions:
1. Do you cough regularly?
2. Do you cough up phlegm regularly?
3. Do even simple chores make you short of breath?
4. Do you wheeze when you exert yourself or at night?
5. Do you often have colds that last longer than those of other people you know?
A positive answer to any question is considered clinically significant.13
3. Perform pre-bronchodilator spirometry: This was performed by a certified technician using an electronic spirometer (Pony Desktop Spirometer, Cosmed Srl, Italy) according to the American Thoracic Society/European Respiratory Society guidelines.14 The spirometer is equipped with software that provides several reference equations, from which we have selected the standardized reference values produced by the Global Lung Function Initiative network.15 Airflow obstruction was diagnosed in participants with an FEV1/FVC ratio2 <0.70. The severity stages defined by Global initiative for chronic Obstructive Lung Disease (GOLD)2 are as follows: Stage 0: FEV1/FVC≥0.7 and FEV1≥80% of predicted value and respiratory symptoms; Stage 1 (mild): FEV1/FVC<0.7 and FEV1≥80% of predicted value; Stage 2 (moderate): FEV1/FVC<0.7 and FEV1<80% but ≥50% of predicted value; Stage 3 (severe): FEV1/FVC<0.7 and FEV1<50% but ≥30% of predicted value; and Stage 4 (very severe): FEV1/FVC<0.7 and FEV1<30% of predicted value.1 PRISm8 was defined as FEV1<80% of predicted value and FEV1/FVC ratio≥0.70.
4. Consider assessment of structural lung disease by chest computed tomography (CT). For practical reasons, including limited resources, we were unable to perform CT scans.
5. Determine the number of positive features identified in steps 1 through 4. According to COPDGene, 2 clinical features indicate possible COPD, 3 clinical features indicate probable COPD, and 4 clinical features indicate definite COPD. However, as mentioned above, because it was not possible to obtain a CT scan, we could only classify smokers as having no COPD, possible COPD, or probable COPD.
6. For patients with possible, probable, and definite COPD according to COPDGene, consider the following:
a. Patient education on the risk of disease progression and mortality: All participants received information about how smoking causes COPD and that continued smoking increases the risk of disease progression, disability, and premature death.
b. Implementation of aggressive and repeated attempts to quit smoking: All participants received smoking cessation counseling from the research team's pulmonologists using their own flow-volume curve. Participants were first informed that: (1) there is no "safe level" of cigarette consumption, (2) reducing smoking as a goal is not beneficial, and (3) only complete cessation has been shown to have positive health effects. They were then given a brief explanation of the strategies and treatments currently available for quitting smoking. These strategies included quitting cold turkey, i.e., on a specific date, and quitting gradually, with the intention of quitting very soon. They were told that it may be easier to quit cold turkey or by gradual reduction with medications such as nicotine replacement therapy and varenicline, administered under medical supervision. They were invited to make an appointment with either our department or a smoking cessation clinic. Regardless of their choice, they were urged to quit smoking as soon as possible: those with normal spirometry to prevent disease and those with obstructed airflow to prevent further damage. All willingly accepted the advice.
c. Additional pharmacological and non-pharmacological management: All participants were advised to make an appointment with their primary care physician for confirmatory spirometry and additional treatment if needed.
7. Follow-up: A letter was sent to each participant with a detailed spirometry interpretation, a reminder to quit smoking, and instructions to make an appointment with their physician to discuss the survey results and to schedule further testing if needed. Three months later, we assessed participants' smoking status, cigarette consumption, and use of COPD medical resources through a telephone survey. Medical follow-up of symptomatic participants was not part of the study.
Main Outcomes
By combining responses to the symptom questionnaire and spirometry with exposure, participants were classified into mutually exclusive categories or phenotypes. We use the term phenotype because symptoms and airway obstruction are attributes that require treatment (e.g., smoking cessation) to change the outcome (i.e., COPD risk and pulmonary morbidity).16 With exposure (i.e., smoking) considered positive in all participants, 4 phenotypes, per COPDGene, were obtained by combining disease characteristics:
1. Phenotype A. This reference phenotype, no COPD, was characterized by the absence of symptoms and normal spirometry.
2. Phenotype B, characterized by symptoms and normal spirometry indicated possible COPD.
3. Phenotype C, characterized by the absence of symptoms and abnormal spirometry also indicated possible COPD; and
4. Phenotype D, characterized by symptoms plus abnormal spirometry, indicated probable COPD.
Statistical Analysis
Statistical analysis was performed using the Stata (version 16) statistical software (Stata Corp, Texas). Phenotypes were first described in terms of the variables on which they were based, i.e., symptoms and spirometric variables, including GOLD/PRISm categorization. As a null hypothesis of no difference between phenotypes with respect to these variables makes little sense, these differences were not tested. Differences between phenotypes were tested for each of the following independent variables: age, sex, body mass index (BMI), cigarettes per day, age at first cigarette, duration of smoking, and pack years using nonparametric statistics (Kruskal-Wallis rank tests for quantitative variables and Fisher exact tests for qualitative variables). In addition, the trend from the reference phenotype of no COPD to the phenotype of probable COPD was modeled as a function of the same independent variables using stepwise adjusted ordered logistic regression. Finally, the evolution of smoking between baseline and follow-up, characterized by respectively a decreasing, stable, and increasing number of daily cigarettes, as well as the medical treatment of COPD at follow-up, were tested against the phenotypes using the same nonparametric statistics. P values <0.05 are considered significant.
Results
Phenotypes
Definition and Distribution:
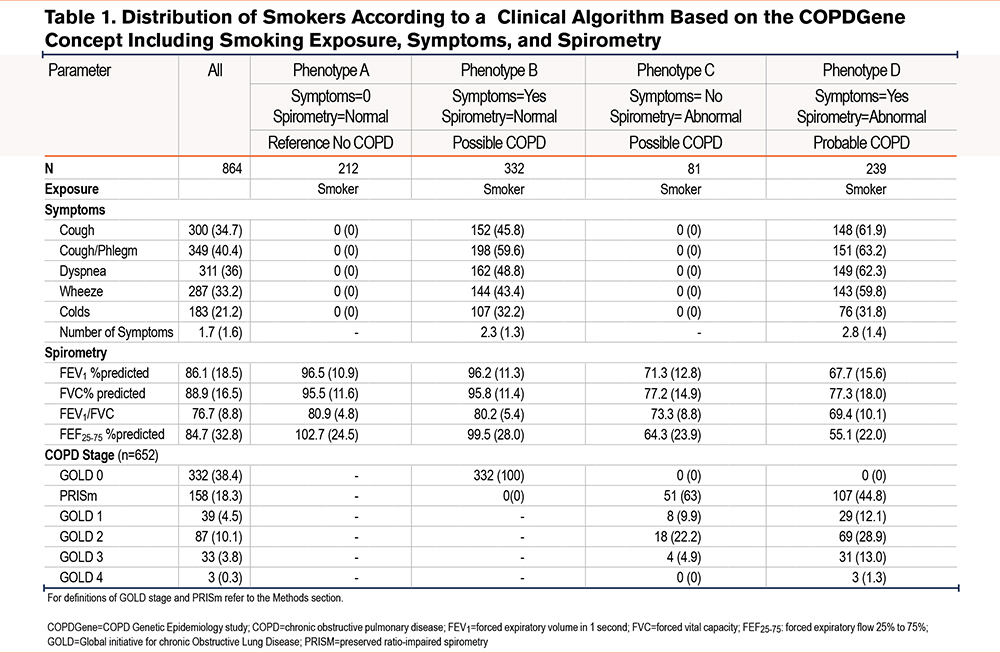
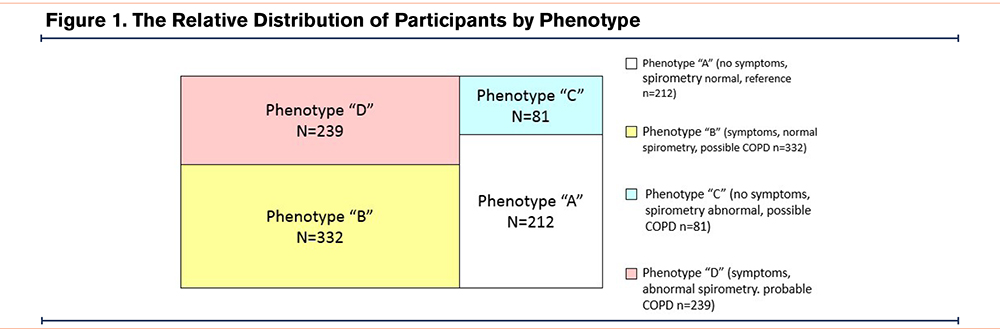
The combination of exposure, symptoms, and spirometry classified the smokers into 4 phenotypes. As shown in Table 1, there were 212 (24.5%) smokers with phenotype A (i.e., no symptoms, normal spirometry); 332 (38.4%) with phenotype B (i.e., symptoms plus normal spirometry); 81 (9.4%) with phenotype C (i.e., no symptoms, abnormal spirometry); and 239 (27.7%) with phenotype D (i.e., symptoms plus abnormal spirometry). The relative distribution by phenotype is shown in Figure 1.
Clinical Characterization:
These are shown for the 4 phenotypes in Table 2. The mean (standard deviation [SD]) age was 50.4 (11.3) years, the mean (SD) BMI was 26.8 (4.7)kg/m2, and the number of males (%) was 640 (74.1%). The clinical characteristics of the phenotypes can be briefly described as follows:
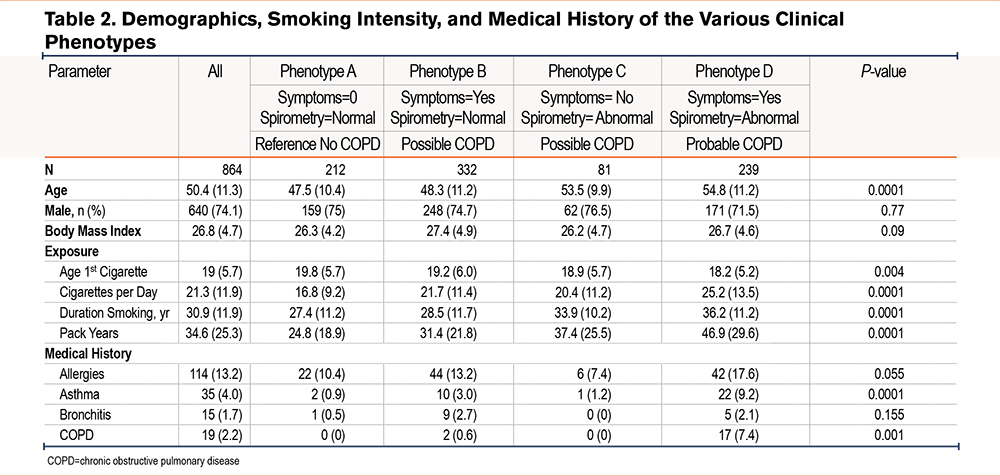
Phenotype A (reference category, n=212, no COPD). This was the youngest and least affected group, with a mean (SD) FEV1 as a percentage of the predicted value of 96.5 (10.9) and a mean (SD) FEV1/FVC as a percentage of the observed value of 80.9 (4.8). Medical history consisted primarily of a history of allergies, reported by approximately 10% of participants.
Phenotype B (symptoms, n=332, possible COPD). Smokers in this phenotype were similar in age to phenotype A but had respiratory symptoms. Their spirometry was similar to that of phenotype A with a mean (SD) FEV1 as a percentage of the predicted value of 96.2 (11.3) and a mean (SD) FEV1/FVC as a percentage of the observed value of 80.2 (5.4). In the medical history, other than allergies, small proportions of asthma, bronchitis, and COPD were reported.
Phenotype C (abnormal spirometry; n=81; possible COPD). Smokers with this phenotype were older than those with phenotypes A and B, had no symptoms, but their spirometry was abnormal. Their mean (SD) FEV1 as a percentage of the predicted value was 71.3 (12.8) and their mean (SD) FEV1/FVC as a percentage of the observed value was 73.3 (8.8). A total of 51 participants (63%) had PRISm spirometry, while 30 (37%) had GOLD 1–3 spirometry. The medical history was similar to that of phenotype A with allergies being the most common complaint.
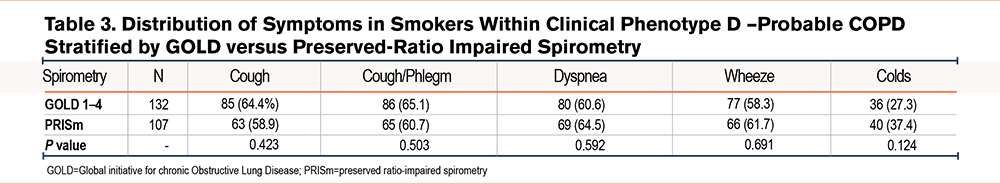
Phenotype D (symptoms plus abnormal spirometry; n=239; probable COPD). This phenotype was clearly the most severe. Smokers with this phenotype were significantly older than those with phenotypes A and B. They had a mean (SD) FEV1 as a percentage of the predicted value of 67.7 (15.6) and a mean (SD) FEV1/FVC as a percentage of the observed value of 55.1 (22). A large proportion of participants (44.8%; n=107) had PRISm spirometry, whereas 55.2% (n=132) were classified as GOLD 1–4. To test the relative contribution of PRISm versus GOLD spirometry to the clinical burden of this phenotype, we compared the prevalence of symptoms between them. As shown in Table 3, there were no differences between the 2 groups regardless of symptoms. In the medical history, a significant proportion of participants had a history of allergy, asthma, bronchitis, and COPD (Table 2).
Smoking Patterns:
Smoking patterns differed significantly between phenotypes for all cigarette exposure parameters. When testing the trendfrom the referent phenotype A to the probable COPD phenotype D according to smoking variables (cigarettes per day, age at first cigarette, duration of smoking, pack years), age, sex, and BMI, only cigarettes per day and duration of smoking were statistically significant predictors (p<0.0001). After adjustment for these 2 variables, none of the other variables were statistically significant (see online supplement).
Follow-up Survey
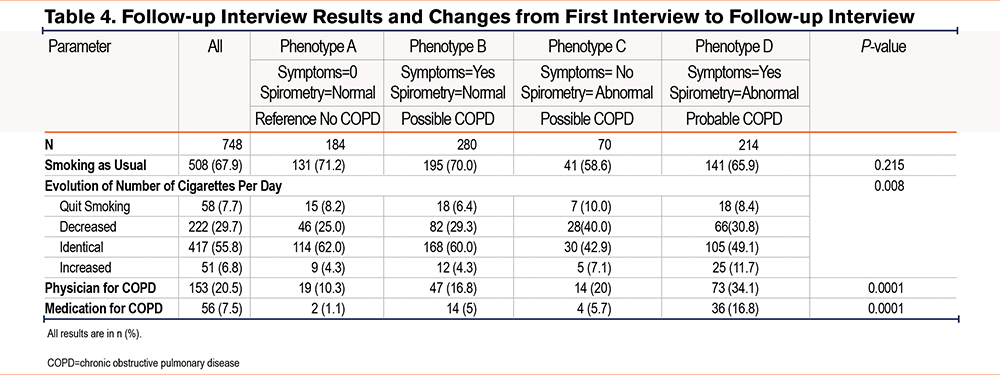
After 3 months (Table 4), 748 (86.6%) participants could be reached. Of these, 508 (67.9%) reported smoking as usual, which did not depend significantly on the phenotype. In detail, the evolution of the number of cigarettes smoked was significantly different according to the phenotype (p=0.008). However, the number of participants who reported quitting smoking was comparable across phenotypes. Of interest is the high number of participants who increased their smoking in phenotype D. Regarding medical follow-up, 153 (20.5%) participants reported being followed by a physician for COPD. Of these, 56 (7.5%) were receiving treatment for COPD. Both parameters were strongly related to the phenotypes (p=0.0001) with more follow-up and medication in phenotype D. Of note, according to current recommendations, participants with phenotypes A (n=2), B (n=14) and C (n=4), and 7 participants with phenotype D (with PRISm spirometry) (total=27) were receiving non-evidence-based treatment for COPD.
Discussion
The need to detect undiagnosed COPD is widely recognized, although the best strategy to achieve this goal remains unclear. Current case-finding strategies call for screening for COPD only in symptomatic smokers,2,4 with the goal of identifying individuals with an FEV1/FVC ratio below 0.7. Although simple, this approach to disease detection is limited for several reasons. First, it only identifies individuals with moderate to severe disease for which there is no curative treatment. Second, it ignores smokers with symptoms and normal spirometry and the potential importance of PRISm spirometry. Finally, it implicitly considers smokers who do not meet the spirometric criterion for COPD as COPD-free despite the presence of clinical and functional morbidity that warrants investigation. In fact, simply telling these smokers that they do not have COPD could be perceived as a license to smoke and could delay any decision to quit. In contrast, this study showed that the use of an algorithm based on the expanded definition of COPD significantly increased the population of smokers to be considered for a COPD diagnosis (+56.7%), allowing the inclusion of smokers classified according to the progressive number of disease manifestations. In addition, consistent with the concept that the best way to prevent COPD is to never start smoking or, for smokers, to quit, this study showed that incorporating brief smoking cessation counseling into the case-finding intervention was feasible and resulted in a low but clinically meaningful smoking cessation rate at 3 months.
Among the phenotypes, phenotype A was the least affected. However, this was only true within the limits of the clinical algorithm. For example, the use of more sensitive tests to detect obstruction might have changed the categorization of some individuals.17 On the other hand, the use of additional physiological tests might have indicated a risk of COPD even in smokers with normal spirometry.18 In any case, smokers with this phenotype should not be congratulated for not having COPD detected by spirometry but should be encouraged to quit smoking.
The B phenotype, indicating possible COPD, is similar to what was previously called GOLD stage 0, which, after being removed from GOLD in 2006, was found to be predictive of negative outcomes, including impaired health-related quality of life.19,20 More recently, smokers with this phenotype have been found to be at increased risk of exacerbations and activity limitation, as well as loss of lung function over time and increased all-cause mortality.5-7 In the SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) study, Woodruff and colleagues6 demonstrated that smokers and ex-smokers who develop symptoms with preserved lung function have exacerbations, activity limitation, and evidence of airway disease. These authors argued that because the best lung function of their smokers was unknown, it was not possible to compare lung function at enrollment with lung function at an earlier time; therefore, they could not rule out the possibility that obstruction had developed in these individuals even though they did not meet criteria for obstruction at enrollment. Similarly, because of the cross-sectional nature of our study, we cannot exclude the possibility that smokers with the B phenotype belong to the so-called supra-normal lung trajectory, which is characterized by high lung function values in early adulthood that can be significantly damaged by smoking before this is reflected in spirometry.21 Conceptually, smokers with this initial lung function excess could reach mid to late adulthood with pseudonormal spirometry, i.e., values that appear normal despite the presence of symptoms. Notably, 5% of smokers with phenotype B were being treated for COPD without evidence, even though they did not meet the current criteria for COPD (Table 4). Although the appropriateness of this treatment is debatable, it seems no more paradoxical than treating COPD itself, a disease whose diagnosis is based on lack of response to bronchodilators, but whose treatment of choice remains bronchodilators.
Both the C and D phenotypes had abnormal lung function and differed only in the presence or absence of symptoms. Interestingly, the inclusion of PRISm spirometry contributed significantly to the classification of smokers into these 2 phenotypes: PRISm smokers accounted for 63% of the possible COPD category and 44.8% of the probable COPD category (Table 1). This finding is clinically relevant because PRISm spirometry has been associated with breathlessness, increased risk of death, and more comorbidities, including adverse cardiopulmonary outcomes.8,22,23 Although the importance of identifying smokers with PRISm spirometry seems well established, it should be noted that other diseases (e.g., interstitial lung disease) may cause PRISm spirometry. Furthermore, there is evidence that individuals with PRISm spirometry are a rather unstable group, with frequent significant transitions to obstruction and normal spirometry over time.23,24 This is not the case for smokers with GOLD stage 3–4 airflow obstruction, as the pathological changes underlying obstruction of this severity are most likely to be permanent.
The association between smoking and accelerated FEV1 decline is well established.25-28 In the Lung Health Study, this association was found by expressing the smoking burden as an increased number of cigarettes per day.29 In the COPDGene cohort, smoking duration alone was even more strongly associated with estimates of the disease component of COPD than cigarettes smoked per day.30 Interestingly, in this study, the number of cigarettes per day and duration of smoking was associated with a trend from baseline A phenotype to probable COPD D phenotype that was independent of age (and other clinical parameters as shown in the online supplement). This means that: (1) the more an individual smokes, the greater the likelihood of a more severe phenotype and, more importantly, (2) the greater tobacco exposure of phenotype D smokers is not due to the fact that they are older and therefore, have smoked more than participants with other phenotypes. This effect is not dissimilar to the dose-dependent relationship observed between smoking and changes in lung function.31
Three months after the intervention, 7.7% of participants reported that they had quit smoking. Although low in absolute terms, this quit rate is clinically important because it compares favorably with the 1%-3% observed after primary care counseling32 and, if applied to the general population of smokers, could represent a large number of successful quit attempts. Second, health care utilization, as expressed by physician follow-up and use of COPD medications, increased steadily across all phenotypes from phenotype A to phenotype D (Table 4), paralleling the clinical trend of the phenotypes (Table 2).
One of the strengths of this study was the ability to combine COPD case finding with smoking cessation counseling. Our strategy was not to inform participants about smoking cessation advice, as smokers generally do not like to be patronized and may have refused advice altogether. Strictly speaking, the effectiveness of smoking cessation advice would have required a controlled trial, which is too complex to combine with case finding. However, our use of an uncontrolled design was acceptable because: (1) the therapeutic effect of counseling is well established,33 (2) counseling is not harmful, and (3) it would have been unethical not to offer counseling to all smokers since smoking cessation is the only treatment likely to alter the natural history of COPD and improve mortality.34 In terms of limitations, the most important was our inability to obtain chest CT scans, the fourth component of the COPDGene approach, which would have classified participants as having definite COPD. Although not always readily available in case-finding interventions in large populations, imaging assessment is important and, in our opinion, has a definite place in the risk assessment of COPD in smokers.5,9,35 Finally, it should be emphasized that our algorithm differs from the COPDGene algorithm by using the Canadian Respiratory Symptoms instead of the modified Medical Research Council dyspnea scale questionnaire to assess symptoms and by the aforementioned lack of imaging assessment. Thus, our clinical algorithm can select smokers with a high likelihood of COPD but is not diagnostic in itself. The diagnosis of COPD should always be confirmed by careful individual clinical assessment after screening.
In conclusion, this study demonstrated that a clinical algorithm incorporating elements of the expanded definition of COPD classified a large sample of smokers into COPD risk phenotypes characterized by increased clinical manifestations mediated by smoking intensity. It is hoped that widespread implementation of the concept of the expanded definition of COPD will stimulate research leading to appropriate treatments for smokers with B and C phenotypes, many of whom receive COPD treatment without an evidence base. Finally, the classification of smokers according to this concept provides a rational basis for preventive measures such as smoking cessation counseling, which has been successfully combined with the COPD case-finding intervention. Prospective studies with large samples of smokers and at-risk individuals are needed to confirm these data.
Acknowledgments
Author contributions: AB, AR, PW, and GI had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AB is the principal investigator of the project, designed the study, wrote the protocol, recruited participants (with assistance), performed the formal analysis, supervised the project, and drafted the manuscript with assistance (PW). AR assisted in participant recruitment, supervised the project, assisted in participant follow-up, assisted in data interpretation, and commented on the manuscript. PW was responsible for formal and statistical analysis and assisted in drafting the manuscript. OK, CCS, and HA assisted with participant recruitment and commented on the manuscript. GI assisted in study design and participant recruitment, oversaw the project, obtained funding, assisted in data interpretation, and commented on the manuscript. All authors approved the final version of the manuscript.
Data sharing: All participant-level data relevant to the study are included in the article. Study data are available from the corresponding author upon reasonable request, after the removal of all personal identifiers, and after approval by the SZMC Ethics Committee.
The authors thank Steve Kraman, MD, for his careful reading and comments on an earlier version of the manuscript. They also thank Ms. Bracha Levy, Ms. Yael Batan, and Mr. Mathieu Dziurla (INRS, Nancy, France) for technical assistance, Drs. Nissim Arish, Ayal Romem, George Kalak, and David Kupferberg for assistance with recruitment, and the smokers for their participation.
Declaration of Interest
The authors have no conflict of interest to declare.