Running Head: Impact of Bronchiectasis on COPD and Alpha-1 as a Risk Factor
Funding Support: SPIROMICS was supported by contracts from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune, Bayer, Bellerophon Therapeutics, Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici S.p.A., Forest Research Institute, Inc., GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH, ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi, Sunovion, Takeda Pharmaceutical Company, and Theravance Biopharma and Mylan. Genetic studies of SERPINA1 were supported by NHLBI grants R01 HL142992 and R01 HL111527 and resequencing services provided through the Resequencing and Genotyping Service by the Northwest Genomics Center at the University of Washington, Department of Genome Sciences, under U.S. Federal Government contract number HHSN268201100037C from the NHLBI.
Date of Acceptance: May 3, 2023 | Published Online Date: May 17, 2023
Abbreviations: BMI=body mass index; BOE=bronchiectasis; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CRP=C-reactive protein; CT=computed tomography; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; HRCT=high-resolution computed tomography; HU=Hounsfield unit; OR=odds ratio; % pred=percentage predicted; post-BD=post-bronchodilator; PRMfSAD=parametric response mapping functional small airways disease; SD=standard deviation; SGRQ=St George’s Respiratory Questionnaire; SNP=single nucleotide polymorphism; SPIROMICS=SubPopulations and InteRmediate Outcome Measures In COPD Study
Citation: Izquierdo M, Marion CR, Genese F, et al; for the SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) investigators. Impact of bronchiectasis on COPD severity and alpha-1 antitrypsin deficiency as a risk factor in individuals with a heavy smoking history. Chronic Obstr Pulm Dis. 2023; 10(3): 199-210. doi: http://doi.org/10.15326/jcopdf.2023.0388
Introduction
Chest computed tomography (CT) scans have become commonplace in both clinical practice and research, resulting in the demonstration by epidemiologic studies of the high prevalence of bronchiectasis in older individuals with chronic obstructive pulmonary disease (COPD) and a history of cigarette smoking.1,2 Estimates of bronchiectasis prevalence in COPD range between 4%–72%, likely related to the different ages and tobacco smoke exposures among cohorts.3 The largest meta-analysis of 6 observational studies totaling 881 participants with COPD noted a mean prevalence of 54.3% with bronchiectasis.1 This frequent association motivated the Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines to acknowledge bronchiectasis as a comorbid factor in COPD.4
Clinical associations of bronchiectasis in COPD include lower lung function1 and more frequent exacerbations.5,6 Among bronchiectasis patients, those with COPD were more likely to be colonized with Pseudomonas species, a risk factor for increased mortality, compared to those without COPD.7-9 The etiologic factors underlying the pathogenesis of bronchiectasis are unclear but are likely an overlap of features related to the development of an infective phenotype overlapping with COPD characterized by the mucus hypersecretion, mucin dysregulation, and impaired mucociliary clearance characteristic of genetic causes of bronchiectasis and chronic bronchitis.
In individuals with a smoking history, alpha-1antitrypsin deficiency is the strongest genetic risk factor for COPD. Alpha-1 antitrypsin deficiency has been implicated in concomitant bronchiectasis development, due to loss of inhibition of neutrophil elastase and the resulting inflammatory and proteolytic destruction of the large airways.10 Besides biologic plausibility, the relationship between alpha-1 antitrypsin deficiency and bronchiectasis is based on case reports and alpha-1 antitrypsin deficiency registry-based epidemiologic studies, irrespective of smoking history.11-14 Bronchiectasis was found in the majority of those in a registry-based study enriched for alpha-1 antitrypsin deficiency, and was associated with CT scan-based emphysema but not with reduced lung function.15 In contrast, in a general population with CT scan-based bronchiectasis, alpha-1 antitrypsin deficiency was rarely found.16 Gene-by-environment interactions with variation at the locus encoding alpha-1 antitrypsin, SERPINA1, likely determine the relationship between alpha-1 antitrypsin deficiency and bronchiectasis development in individuals with smoking histories. However, both the full extent of the clinical implications of bronchiectasis on COPD severity and the impact of SERPINA1 variation on the development of bronchiectasis in those with heavy smoking histories have yet to be determined.17
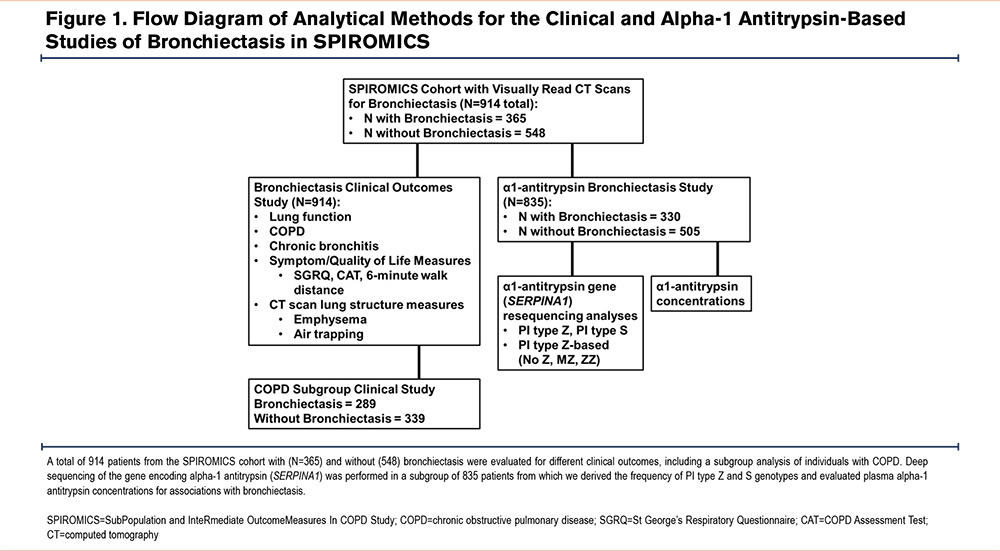
We hypothesized that the presence of bronchiectasis will adversely impact COPD risk and severity and that SERPINA1 variation is a risk factor for bronchiectasis in individuals with a significant smoking history. To test these hypotheses, we evaluated clinical and genetic risk factors for CT scan-based bronchiectasis and its effect on COPD severity in a subgroup of the SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) cohort with visually read CT scan data.
Methods
Study Population
SPIROMICS is a National Heart, Lung, and Blood Institute (NHLBI)-sponsored multicenter observational study of COPD designed to further explore COPD and potential therapeutics. Here, we analyzed 914 current or ex-smoking participants with a ≥20-pack-year smoking history, with or without spirometrically-defined COPD (post-bronchodilator forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio<0.7) based on previously described strata 2–4 (SPIROMICS strata 2 [N=287], 3 [N=410], and 4 [N=217], Figure 1).18 Participants were 40–80 years old at enrollment; all had coached inspiratory (total lung capacity) and coached expiratory (residual volume) baseline thoracic high-resolution CT (HRCT) scans.19 This analysis examines those with radiologist visual reads. All SPIROMICS participants were characterized with post-bronchodilator spirometry and standardized questionnaires. Consent for the clinical and genetic studies has been previously described.20 Protocols and ethics criteria were reviewed and approved by institutional review boards at each participating site.
Statistical Analysis
Definition of Bronchiectasis and Clinical Outcomes
Bronchiectasis was determined using a lack of tapering over 2 cm and airway size greater than adjacent pulmonary artery size as bronchial/arterial ratios alone have the potential to misclassify the presence of bronchiectasis.21 These determinations were made by a single chest radiologist visually identifying radiographic evidence of bronchiectasis on standard SPIROMICS CT scans without the presence of concomitant pulmonary fibrosis or cicatrization in the area of bronchiectasis. Linear regression models for continuous outcomes tested for the association between bronchiectasis and our co-primary lung function measures of post-bronchodilator FEV1 and FVC as a percentage of predicted and the FEV1/FVC ratio. Secondary COPD-related outcomes included quantitative CT scan measures of air trapping based on the percentage of bilateral lung voxels ≤-856 Hounsfield units (HU) at full exhalation, quantitative CT scan measures of emphysema based on the percentage of bilateral lung voxels ≤-950HU at full inspiration, 6-minute walk distance, and the COPD Assessment Test (CAT) score (dichotomized at ≥10) which were evaluated with linear regression models for continuous outcomes and logistic regression for dichotomous outcomes (Figure 1). The regression-based model included age, sex, body mass index (BMI), race, pack-year smoking history, current smoking status, and clinical site with height included for radiographic measures. Analyses were performed in all patients with a ≥20-pack-year smoking history and stratified by the presence and absence of COPD based on GOLD criteria.4 A p-value <0.05 was used to determine significance. Analyses were performed using JMP Pro (version 15.2.1 SAS Institute Inc., North Carolina).
SERPINA1 DNA Resequencing and Genetic Studies
To identify less common and low frequency-to-rare SERPINA1 variants, n=835 participants (n=668 White) underwent deep DNA resequencing of a 16.9kB genomic region containing SERPINA1 with sequencing coverage at an average read depth of 61.8 reads (×; median=60.5×) through the NHLBI Resequencing and Genotyping Service as previously described (Figure 1).20 This same subset had concentrations of alpha-1 antitrypsin and C-reactive protein (CRP) measured on their baseline samples using the RBM-Myriad platform (Austin, Texas).
As described above, for analyses of COPD-related outcomes we evaluated associations of bronchiectasis with genotype groups, using regression-based allelic association tests and burden tests.20 Groups were defined based on the presence or absence of the PiZ genotype (Glu366Lys, alleles C/T, rs28929474), the PiS genotype, and other non-PiS or PiZ low frequency-to-rare variants (allele frequency<0.05, VR). Thus, these burden-based regression models compared individuals without a PiZ allele (“No Z” genotype), those with only one copy of PiZ without background PiS or other rare variants (“PiMZ” genotype), and PI Z homozygotes (“PiZZ” genotype). In both allelic association and burden models, covariates consisted of age, sex, BMI, pack-year smoking history, 5 principal components of genetic ancestry using EIGENSOFT.22 These models were performed for the multi-racial (White versus other) cohort and stratified by race to identify ancestry-specific effects.
Results
Baseline Characteristics Based on Bronchiectasis
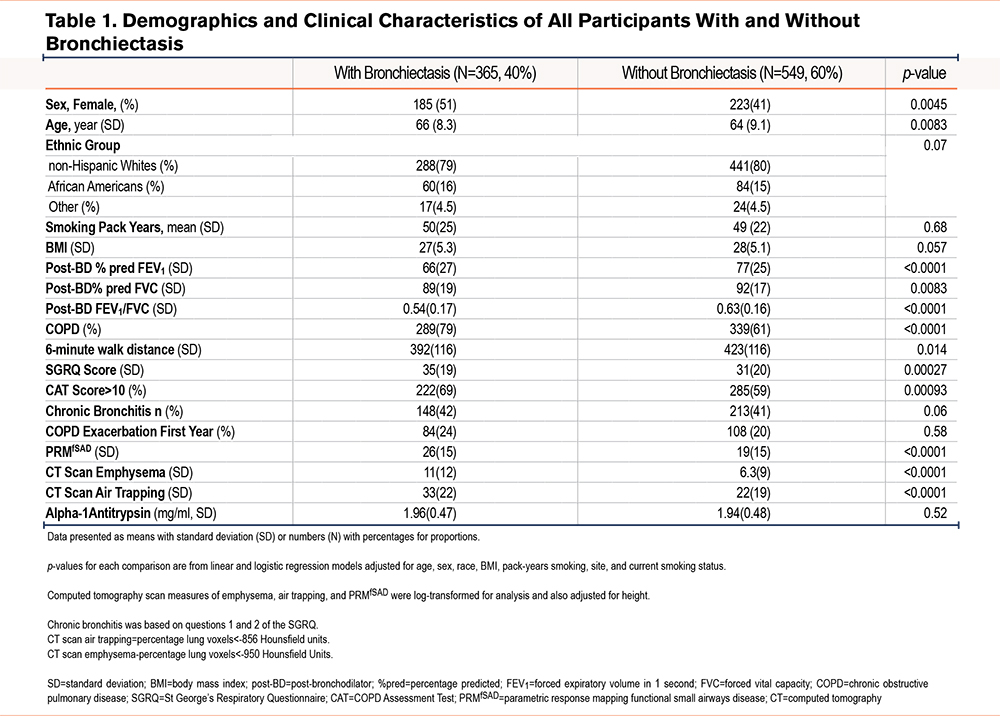
Overall, bronchiectasis was common (40%) among these participants with at least a 20-pack-year smoking history. Bronchiectasis was more frequently found in women than men (45% versus 36%, p=0.0045) (Table 1). Those with bronchiectasis were on average slightly older (mean age=66 [standard deviation (SD)=8.3] versus 64 [9.1] years, p<0.0001) and as a group were more likely to have spirometrically defined COPD (79% versus 61% of participants, p<0.0001). Participants with bronchiectasis had a significantly reduced lung function (FEV1 percentage predicted [% pred]=66% [SD=27] versus 77% [SD=25], p<0.0001 [Figure 2]; FVC % pred=89% [SD=19] versus 92% [SD=17], p=0.0083; FEV1/FVC ratio=0.54 [SD=0.17] versus 0.63 [SD=0.16], p<0.0001 [Table 1]). Their exercise capacity was worse: (6-minute walk distance=392 [SD=116] versus 423 [SD=116], p=0.014), and they had a greater symptom burden, as evidenced by both the CAT score ≥10, (69% versus 59% of participants, odds ratio [OR]=1.75, 95% confidence interval [CI]=1.25–2.45, p=0.00093) and the St George’s Respiratory Questionnaire (SGRQ) which was statistically but not clinically significant (p=0.00027).23 However, they did not have significantly greater smoking histories or prospective exacerbations.
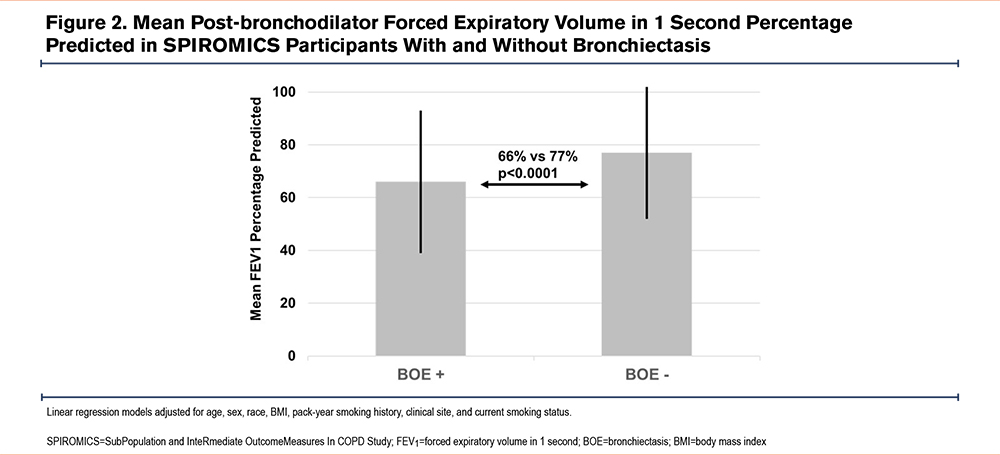
Individuals with bronchiectasis also had greater quantitative CT-based evidence of other lung anatomic abnormalities, including greater air trapping (%voxels ≤-856HU=33% [SD=22] versus 22% [SD=19], p<0.0001), emphysema (%voxels ≤-950HU=11% [SD=12] versus 6.3% [SD=9], p<0.0001), and functional small airways disease (parametric response mapping functional small airways disease [PRMfSAD]=26 [SD=15] versus 19 [SD=15], p<0.0001, Table 1). Of note, PRMfSAD represents regions of air trapping on expiration not associated with regional emphysema on inspiration.
COPD-Stratified Analysis:
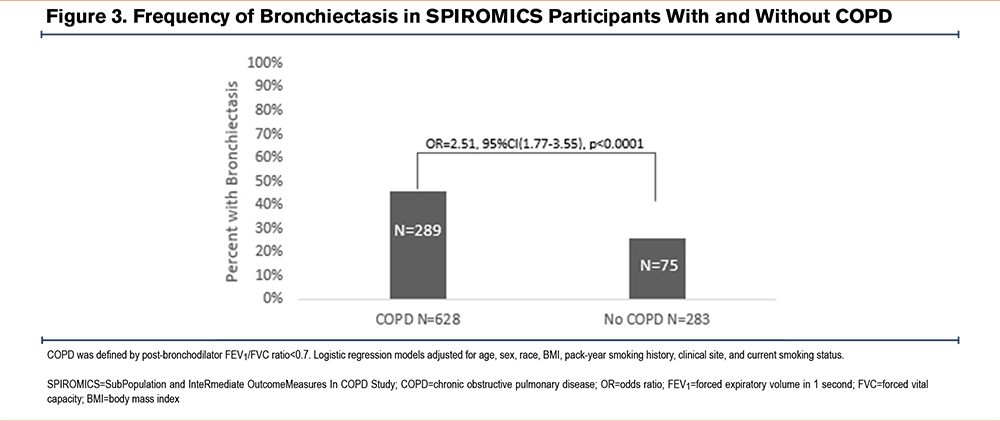
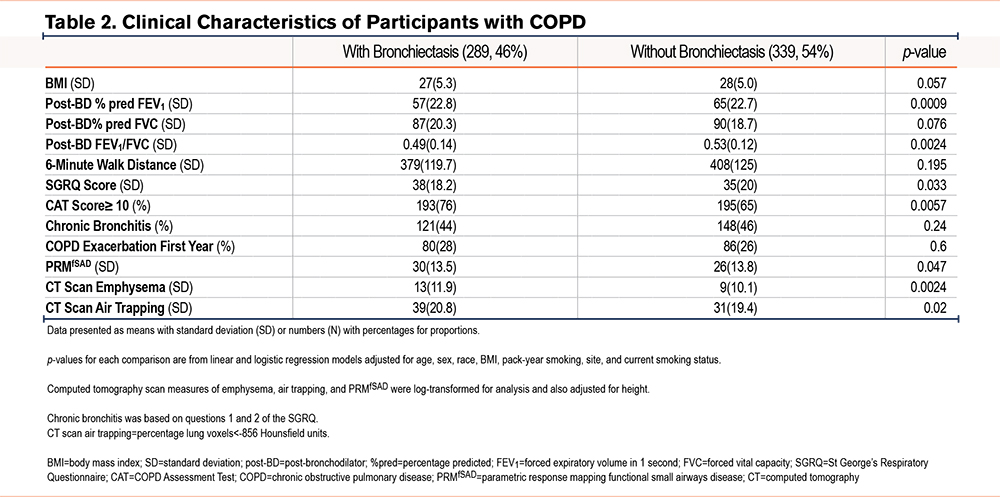
Among all those with bronchiectasis, a spirometrically defined diagnosis of COPD was more commonly appreciated (46% versus 26%, p<0.0001, Figure 3). In the COPD subgroup, the presence of bronchiectasis was associated with worse lung function (FEV1=57% [SD=22.8] versus 65% [SD=22.7] predicted, p=0.0009; FVC=87% [SD=20.3] versus90% [SD=18.7] predicted, p=0.076; FEV1/FVC=0.49 [SD=0.14] versus 0.53 [SD=0.12], p=0.0024) than in its absence. COPD participants with bronchiectasis showed higher CT-based air trapping (39% [SD=20.8] versus 31% [SD=19.4], p=0.02). They also experienced worse quality of life, based on higher SGRQ scores (38 [SD=18.2] versus 35 [SD=20], p=0.033 and greater symptom burden (CAT score≥10: 76% versus 65% of participants, p=0.0057) compared to COPD participants without bronchiectasis (Table 2).
Alpha-1 Antitrypsin, SERPINA1, and the PiZ Variant
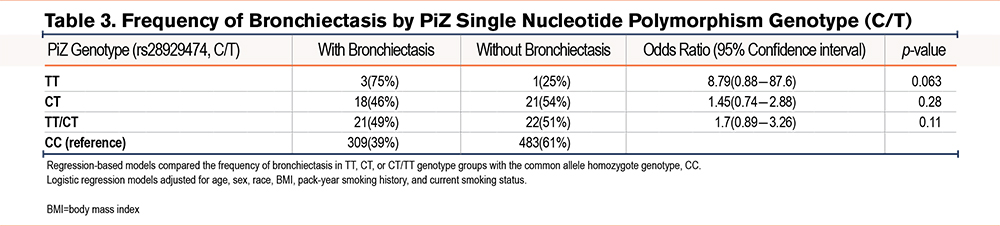
In the subgroup of n=835 participants (n=668 whites) with plasma measurements, concentrations of alpha-1 antitrypsin and CRP were not significantly associated with the presence of bronchiectasis (Table 1). With respect to the PiZ variant (rs28929474, alleles C/T, Glu366Lys), bronchiectasis was found in 3 (75%) of 4 minor allele homozygotes for rs28929474, 18 (46%) of 39 CT heterozygotes and 309 (39%) of 792 CC homozygotes. In a dominant model, (where the assumption is made that having one or more alleles of interest will cause the phenotype being investigated) the combined TT homozygote and CT heterozygote groups had a numerically higher frequency of bronchiectasis compared to CC homozygotes (OR=1.7, 95%CI=0.89–3.26, p=0.11, Table 3). Genotype associations between CC homozygotes versus TT homozygotes (OR 8.79, 95%CI=0.88–87.6, p=0.063) and CC homozygotes versus CT heterozygotes (OR=1.45, 95%CI=0.74–2.88, p=0.28, Table 3) were not significant.
The PiS single nucleotide polymorphism (SNP) was not significantly associated with increased bronchiectasis (21 of 57 [37%] versus 309 of 778 [40%] for PiS heterozygotes versus common allele homozygotes (OR=0.85, 95%CI=0.47–1.5, p=0.58). The cumulative burden of non-S or Z variation was not significantly associated with the increased presence of bronchiectasis but showed an opposite association (5 of 29 [17%] with at least one rare variant versus 283 of 707 [40%] without variants, OR=0.31, 95%CI=0.11–0.85, p=0.013). Accordingly, we focused our burden-based studies on the presence or absence of PiZ.
Burden-based regression models limited to White individuals demonstrated bronchiectasis in 18 of 34 (53%) PiMZ heterozygotes without background rare SERPINA1 variation and 3 of 4 (75%) PiZZ homozygotes, compared to 220 of 554 (40%) without a PiZ allele. A dominant model showed near-significant associations when comparing the combined PiMZ and PiZZ genotype groups to those without a PiZ allele (OR=1.98, 95%CI=0.996–3.9, p=0.051, Figure 4a). These findings were driven by PiZZ homozygotes, who showed near significant associations versus those without a PiZ allele (OR=8.02, 95%CI=0.79–80.73, p=0.052). The association for PiMZ heterozygotes was weaker but in the same direction and not significant (OR=1.72, 95%CI=0.84–3.55, p=0.13, Figure 4a).
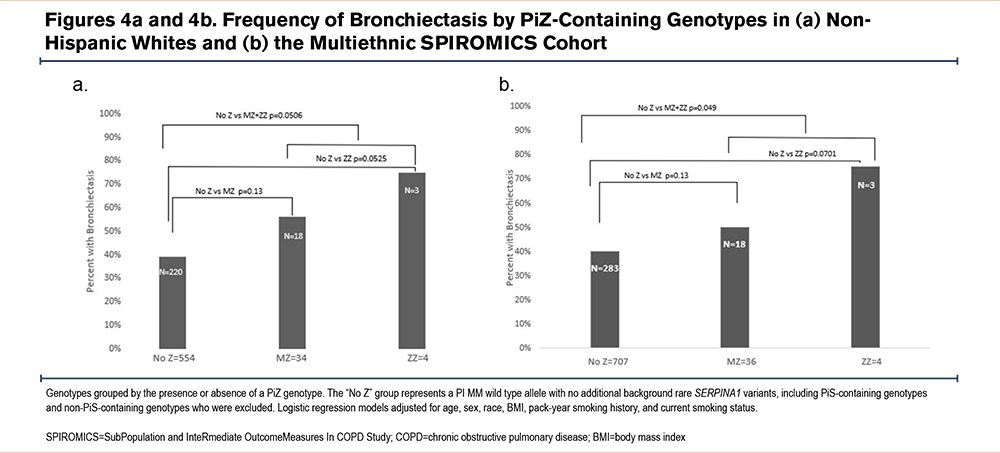
These PiZ-based associations with bronchiectasis were similar for the combined racial groups, where bronchiectasis was detected in 18 of 36 (50%) PiMZ heterozygotes (OR=1.71, 95%CI=0.84–3.48, p=0.13) and 3 of 4 (75%) PiZZ homozygotes (OR=8.38, 95%CI=0.84–83.8, p=0.070) compared to 283 of 707 (40%) without a PiZ allele (Figure 4b). When all racial groups were combined, the dominant model showed significant associations for bronchiectasis in the combined PiMZ and PiZZ genotype groups compared to those without PiZ alleles (OR=1.97, 95%CI=1.00–3.90, p=0.049, Figure 4b).
Discussion
Results of this large (N=914) multi-center cohort study define 2 interrelated features of bronchiectasis in individuals with heavy smoking histories. First, we show that bronchiectasis detected by CT visual reads was common (40%) in such individuals and that they had significantly worse lung function and a greater symptom burden, especially among those with airflow obstruction than those without bronchiectasis. Second, we demonstrate that the PiZ locus is a genetic risk factor for comorbid bronchiectasis in COPD and that the cumulative frequency of PiZZ and PiMZ genotypes in patients with COPD is not uncommon as shown in ours and other studies.20,24 The ability to detect these novel associations was facilitated by the design of the SPIROMICS cohort, which required a heavier smoking history than many studies to date, and which spans COPD severities. This is the largest and most diverse cohort of individuals with heavy smoking histories characterized for CT scan evidence of bronchiectasis. An important strength is its prospective collection of physiologic, clinical, and quantitative CT outcomes plus both proteomic and genetic data specific to alpha-1 antitrypsin biology. Our results have important implications for the care of those with significant smoking histories regardless of the presence of airflow obstruction and should be investigated in COPD resulting from other injurious inhalational exposures.
Our findings extend multiple studies showing a correlation between COPD and bronchiectasis.1,2 The 46% frequency of bronchiectasis we observed among SPIROMICS participants with COPD approximates the results of a large meta-analysis, in which its prevalence was 54% among all with COPD and 57% in moderate-to-severe disease.1 In a bronchiectasis registry, the prevalence of COPD was found to be approximately 20% which is considerably lower than our findings, but the SPIROMICS cohort was enriched for a heavier smoking history and COPD of varying severities.18,25 Identifying comorbid bronchiectasis is crucial, as it is associated with worse outcomes, such as more frequent exacerbations, greater airway colonization by potentially pathogenic microbes, and in those with moderate-to-severe COPD, higher 2-year mortality.3,8 Those same studies and one from Spain26 noted that those with bronchiectasis had higher rates of colonization with Pseudomonas aeruginosa, which has been associated in COPD with longer hospitalization and greater rates of readmission and death.7,27 It is likely that the recurring chronic bacterial infection common in bronchiectasis accelerates COPD progression.28 Hence, CT detection of bronchiectasis identifies a high-risk subset of COPD patients who could be targeted for interventions to improve outcomes and survival, an important topic for future research. Similarly, a key future direction for the SPIROMICS cohort29 will be to investigate the lower respiratory tract bacterial microbiome in relationship to anatomic bronchiectasis, using existing samples.
There are 2 plausible reasons that alpha-1 antitrypsin concentrations were not significantly associated with bronchiectasis in our cohort. First, radiographic evidence of bronchiectasis has been highly variable (27%–95%) in cohorts ascertained for alpha-1 antitrypsin deficiency.14,15,30 This broad range likely reflects small sample sizes and the inclusion of younger individuals with less significant smoking histories. For SERPINA1 genotypes previously strongly associated with alpha-1 antitrypsin deficiency,20 bronchiectasis frequency in SPIROMICS was within this range: 75% of PiZZ homozygotes and 51% of PiMZ heterozygotes. Conversely, a previous large screening cohort study ascertained for bronchiectasis alone rarely found individuals with alpha-1 antitrypsin deficiency; however, it was based on alpha-1 antitrypsin concentrations, not SERPINA1 or PiZ variation, and did not consider age or smoking history.11,12,16 These important risk factors interact with pathogenic SERPINA1 variation to cause bronchiectasis, as we and others have demonstrated.
Second, alpha-1 antitrypsin is an acute phase reactant, upregulated by systemic inflammation as in both bronchiectasis and COPD, which might mask relative deficiency. Using SERPINA1 genetic markers to identify deficiency-associated genotypes could circumvent this issue. We demonstrated that having at least one PiZ allele was associated with bronchiectasis. Interestingly, the PiS locus was not significantly associated with bronchiectasis in our study which is a finding that merits exploration in future studies. We were underpowered to assess non-PI S or Z variants (VR). In non-White racial groups where PiZ is much less frequent, we cannot exclude pathogenic compound heterozygote genotypes or ancestry-specific variation as risk factors for bronchiectasis.20 By suggesting that bronchiectasis is more frequent in those with PiZ genotypes, our findings support considering SERPINA1 risk variation, rather than evidence for alpha-1 antitrypsin concentrations below the protective threshold, as a risk factor when a significant smoking history exists.
Limitations of our study include the non-population-based design of SPIROMICS, which preclude extrapolation to the general population. The associations we show are solely cross-sectional from the baseline visit. Moreover, SPIROMICS evaluated the history of environmental respiratory exposures, and in a later clinical visit collected measures of social determinants of health (including area deprivation index). Hence, evaluating longitudinal outcomes and additional factors for contribution to bronchiectasis development will be key future directions. Additional limitations include the fact that bronchiectasis was determined by a single radiologist rather than multiple as has been done in previous studies.13,15 Furthermore, clinical and radiographic measures of severity were not taken into account, both of which necessitate exploration in future studies. Chronic bronchitis based on questions 1 and 2 of the SGRQ as surrogate for sputum production showed no significant differences between those with and without bronchiectasis, however, this has its limitations as a marker of severity and merits further exploration.31
This study highlights early recognition of radiographic bronchiectasis as a means to identify a group at risk for adverse outcomes among those currently or previously exposed directly to tobacco products. Our results should also motivate investigating, in this group, therapies not traditionally recommended in patients with emphysema or COPD without frequent exacerbations. Specific examples could include long-term efforts to improve airway clearance and reduce microbial burden. By demonstrating that the PiZ locus is a risk factor for bronchiectasis in this group, our findings support alpha-1 antitrypsin guideline recommendations to screen for alpha-1 antitrypsin deficiency in an appropriate subgroup but provide evidence that screening might be most appropriate for those with a significant smoking history and at risk SERPINA1 genotypes.
Acknowledgments
Author Contributions: Manuel E. Izquierdo: Writing-original draft; writing-review and editing; formal analysis. Chad R. Marion: Conceptualization; methodology; writing—review & editing; writing—original draft. Frank Genese: Conceptualization; formal analysis. John D. Newell: Investigation; resources. Wanda K. O'Neal: Investigation; resources. Xingnan Li: Investigation; resources. Gregory A. Hawkins: Investigation; resources. Igor Barjaktarevic: Investigation; resources. R. Graham Barr: Investigation; resources. Christopher B. Cooper: Investigation; resources. David Couper: Investigation; resources. Jeffrey Curtis: Investigation; resources. Meilan K. Han: Investigation; resources. Nadia N. Hansel: Investigation; resources. Richard E. Kanner: Investigation; resources. Fernando J. Martinez: Investigation; resources. Robert Paine: Investigation; resources. Vickram Tejwani: Investigation; formal analysis. Prescott Woodruff: Investigation; resources. Joe Zein: resources; formal analysis. Eric A. Hoffman: Investigation; resources. Stephen P. Peters: Investigation; resources. Deborah A. Meyers: Supervision; resources; formal analysis; methodology; writing—review & editing; writing—original draft; investigation; conceptualization. Eugene R. Bleecker: Supervision; resources; formal analysis; methodology; writing—review & editing; writing—original draft; investigation; conceptualization Victor E. Ortega: Conceptualization; investigation; funding acquisition; writing—original draft; methodology; validation; visualization; writing—review & editing; formal analysis; project administration; software; supervision; resources. All authors reviewed and approved the final version of this manuscript for submission and publication.
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible.
Data sharing: More information about the study and how to access SPIROMICS data is available at www.spiromics.org.
The authors would like to acknowledge the University of North Carolina at Chapel Hill BioSpecimen Processing Facility for sample processing, storage, and sample disbursements (http://bsp.web.unc.edu/). We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Patricia Basta, PhD; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell Jr, MD; Robert Paine, III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN;
Declaration of Interest
Drs. Izquierdo, Marion, Genese, O’Neal, Li, Hawkins, Kanner, Tejwani, Zein, and Meyers have nothing to disclose. Dr. Newell reports grants from the National Institutes of Health (NIH), serving as a medical advisor with a consulting income, patents, and stock options with VIDA, and book royalties from Elsevier. Dr. Barjaktarevic reports personal fees from AstraZeneca, Boehringer Ingelheim, Grifols, Verona Pharma, and GSK; grants from AMGEN and grants and personal fees from GE Healthcare and Mylan/Theravance, outside the submitted work. Dr. Barr reports grants from the NIH, the Foundation for the NIH, and the COPD Foundation, during the conduct of the study and grants from the Alpha-1 Foundation, and personal fees from UpToDate, outside the submitted work. Dr. Cooper reports grants from the NIH/NHLBI and the Foundation for the NIH, during the conduct of the study, and personal fees from PulmonX, NUVAIRA, and MGC Diagnostics, and other from GSK outside the submitted work. Dr. Couper reports grants from the NHLBI and the COPD Foundation, during the conduct of the study. Dr. Curtis reports consulting fees paid to his institution from AstraZeneca PLC, Novartis AG, and CSL Behring LLC, outside this work; and personal travel support from AstraZeneca PLC, outside this work. Dr. Han reports personal fees from GSK, BI, AZ, Merck, and Mylan, and non-financial support from Novartis and Sunovion, outside the submitted work. Dr. Hansel reports grants and personal fees from AstraZeneca and GSK, grants from Boehringer Ingelheim, the NIH, and the COPD Foundation, and personal fees from Mylan, outside the submitted work. Dr. Martinez reports grants from the Department of Defense, during the conduct of the study; personal fees, non-financial support and other from AstraZeneca, Boehringer Ingelheim, Genentech, GSK, Inova Fairfax Health System, Miller Communications, the National Association for Continuing Education, Novartis, ProterrixBio, Pearl Pharmaceuticals, PeerView Communications, Prime Communications, Puerto Rican Respiratory Society, Chiesi, Sunovion, Theravance, Potomac, University of Alabama-Birmingham, Physicians Education Resource, the Canadian Respiratory Network, Teva, and Dartmouth University; personal fees from Columbia University, MD Magazine, Methodist Hospital Brooklyn, New York University, UpToDate, WebMD/MedScape, the American Thoracic Society, Rockpointe, Rare Disease Healthcare Communications, the France Foundation, and Physicians Education Resource; other from Afferent/Merck,Biogen, Veracyte, Prometic, Bayer, Bridge Biotherapeutics, ProMedior, and Gala; non-financial support from Gilead, Nitto, and Zambon; personal fees and other from Patara/Respivant and grants from the NIH outside the submitted work. Dr. Paine reports grants from the NHLBI, and the COPD Foundation, during the conduct of the study; and grants from the Department of Veterans Affairs, outside the submitted work. Dr. Woodruff is a consultant for AstraZeneca, Theravance, Glenmark Pharmaceuticals, Sanofi, and Regeneron and has received funding from Genetech and the COPD Foundation. Dr. Hoffman is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. Dr. Peters reports grants from the NIH and the NHLBI during the conduct of the study; personal fees from Array Biopharma, Integrity CE, AstraZeneca, Aerocrine, Boehringer-Ingelheim, Experts in Asthma, Gilead, GSK, Merck, Novartis, Ono Pharmaceuticals, Pfizer, PPD Development, Quintiles, Sunovion, Saatchi and Saatichi, Targacept, TEVA, Theron, AstraZeneca, Sanofi, and Regeneron; and grants from the NIH and the NHLBI, outside the submitted work. Dr. Bleeker reports other from an NIH grant, clinical trials through his employers, Wake Forest School of Medicine and the University of Arizona for AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Johnson and Johnson (Janssen), Novartis, Regeneron, and Sanofi Genzyme; personal fees serving as a paid consultant for AztraZeneca, MedImmune, Boehringer Ingelheim, GSK, Novartis, Regeneron, and Sanofi Genzyme outside the submitted work. Dr. Ortega reports grants from the NIH, personal fees from CSL Behring, and personal fees from Regeneron and Sanofi for its Independent Data and Monitoring Committee participation outside the submitted work.