Running Head: Cardiac Autonomic Function and COPD Health
Funding Support: CLEAN AIR and the CLEAN Air Heart Ancillary Study were supported by the National Institutes of Health/National Institute of Environmental Health Sciences grants: R01ES022607 and R21ES025840, as well as a Johns Hopkins University Catalyst Award. SR was supported by NHLBI: K12HL143957 and K23HL164151.
Date of Acceptance: May 25, 2023 │ Published Online Date: June 2, 2023.
Abbreviations: BMI=body mass index; bpm=beats per minute; CAT=COPD Assessment Test, CI=confidence interval; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; GOLD=Global initiative for chronic Obstructive Lung Disease; HF=high frequency; HRV=heart rate variability; ICS=inhaled corticosteroid; IRR=incidence rate ratio; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; LF=low frequency; OR=odds ratio; %pred=percentage predicted; RMSSD=root-mean square of successive differences; SDNN=standard deviation of normal to normal intervals; SGRQ=St George’s Respiratory Questionnaire
Citation: Raju S, Woo H, Fawzy A, et al. Decreased cardiac autonomic function is associated with higher exacerbation risk and symptom burden in chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2023; 10(3): 328-334. doi: http://doi.org/10.15326/jcopdf.2023.0410
Introduction
Chronic obstructive pulmonary disease remains a significant contributor to morbidity and mortality worldwide.1 Current measures of severity, including lung function, do not fully explain symptom burden, and there is a growing need to identify novel predictors of exacerbation risk and morbidity in COPD.2,3 Cardiovascular disease remains a significant cause of mortality in COPD and the extent to which subclinical cardiovascular disease contributes to respiratory morbidity in COPD is less well defined.
Cardiac autonomic dysfunction is hypothesized as a pathway driving both respiratory and cardiovascular morbidity in COPD.4-6 Autonomic dysfunction has been associated with reduced resilience, and mucus hypersecretion, and may confer susceptibility to exacerbations. Heart rate variability (HRV) is a marker of cardiac autonomic function that is predictive of cardiovascular health and has promise as a biomarker for multimorbidity in COPD. Early studies demonstrated that HRV is lower in individuals with COPD.7 Recent studies suggest that lower HRV is also associated with exacerbation risk in COPD, however, these studies have been exploratory and relied on brief HRV estimates from electrocardiogram data.8 HRV is more accurately calculated from longer measurements, including from Holter monitors. Commercial wearable devices can now provide long-term data on HR, making HRV a promising non-invasive, and clinically applicable, biomarker of cardiovascular and pulmonary health.9
The CLEAN AIR Heart study,10 designed to investigate the association between indoor pollution and intermediate cardiovascular endpoints in COPD, demonstrated that household air pollution contributes to impaired cardiac autonomic function in COPD. The CLEAN AIR Heart infrastructure provided an ideal opportunity to investigate the association between HRV and markers of COPD morbidity.
Methods
CLEAN AIR Heart methods have previously been described.10,11 Briefly, former smokers with moderate-severe COPD, enrolled in a randomized controlled trial of a portable air cleaner intervention, underwent repeated clinical assessments at baseline, 1-week, and 3- and 6- months follow-up. Select participants completed 24-hour Holter monitoring during each 1-week assessment period to assess HRV. Participants were recruited at a single center in the Baltimore area, and primarily resided in an urban environment. Participants provided written informed consent and the Johns Hopkins Medical Institutional Review Board approved the protocol (NA_00085617).
Holter data was processed to calculate HRV using GE Marquette 12-SL (GE Healthcare) at a central reading center. Primary HRV measures of interest were the standard deviation of normal to normal intervals (SDNN), estimating overall HRV, and the root-mean square of successive differences (RMSSD), a reflection of parasympathetic activity, given prior associations with cardiovascular and respiratory diseases.4,12 Additional HRV measurements collected included low frequency (LF) and high frequency power (HF). Both RMSSD and HF power correlate with parasympathetic activity.
Clinical assessments at each study period, included reporting of exacerbations, the St George’s Respiratory Questionnaire (SGRQ) and the COPD Assessment Test (CAT) evaluating respiratory quality of life and health status. Chronic bronchitis was assessed through SGRQ and CAT criteria.13,14 Severe exacerbations were adjudicated by 2 trained pulmonologists and defined as requiring an emergency department visit or hospitalization. Baseline cardiovascular comorbidities associated with impaired HRV, including history of hypertension, heart attack, stroke, heart failure, and diabetes, were assessed utilizing self-report and medication inventory.5,15 A subset of participants (n=81) had transthoracic echocardiograms, measuring ejection fraction to objectively assess for heart failure.
The association between HRV and exacerbation risk was assessed through zero-inflated negative binomial models, depicting the relationship between baseline HRV and exacerbation risk during the 6-month follow-up period. Associations between HRV indices and respiratory symptom measures were analyzed using mixed-effects models, with random intercepts accounting for repeated measures of HRV and outcomes. Linearity testing was performed for natural scale units, and log transformed units, using cubic spline with 3 knots approach. Associations are presented in natural units as per 10ms decrease in SDNN or RMSSD or 5ms decrease in HF or LF, based on linearity testing and between person variability estimates. All analyses were adjusted for covariates, which were selected based on prior literature and predetermined criteria. These included randomization group, age, sex, race, pack years, forced expiratory volume in 1 second (FEV1) percentage predicted (%pred), controller medication use, cardiovascular comorbidities, and beta-blocker or non-dihydropyridine calcium channel blocker use.10 A sensitivity analysis adjusted for ejection fraction when available. Interaction models analyzed effect modification of the association of HRV with COPD outcomes by the active intervention and placebo groups.
Results
A total of 85 participants contributed 305 Holter measurements coupled with health outcomes (Table 1). The mean age was 65.6 (standard deviation [SD] 8.6), mean FEV1 was 55.2 (18.0) %pred, and mean pack years was 51.8 (SD 32.6). As previously reported, demographics and baseline HRV estimates were comparable across randomization groups.11 During the 6-month follow-up period the mean number of exacerbations per participant was 1.33 (SD 2.29) for any exacerbation and 0.37 (SD 0.80) for severe exacerbations.
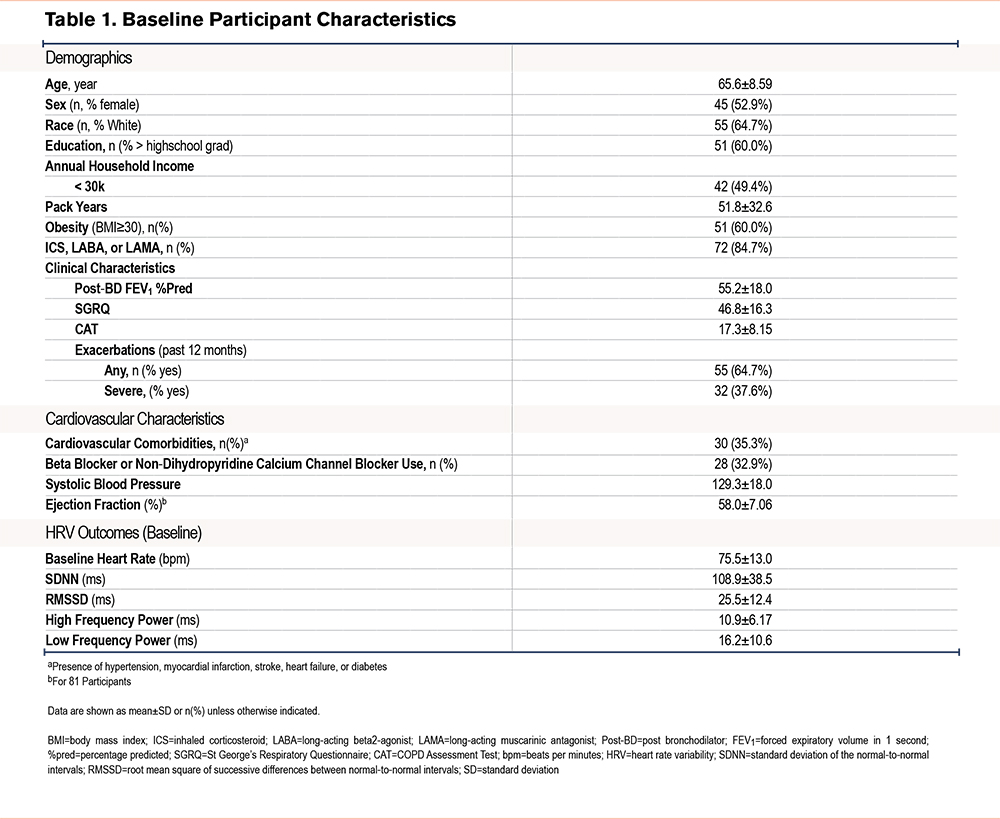
In adjusted models there was a significant association between HRV and exacerbation risk. Decreases in SDNN, LF, and HF power were associated with greater risk for future COPD exacerbations (Table 2). This association was primarily driven by severe exacerbations, with a decrease in all measures of HRV including SDNN (incidence rate ratio [IRR] 1.40; 95% confidence interval [CI] 1.13 to 1.74) and RMSSD (IRR 1.60; 95% CI 1.07 to 2.37) associated with severe exacerbations. In sensitivity analyses, adjusting for ejection fraction, SDNN (1.36; 95% CI 1.09 to 1.71), HF, and LF were associated with greater severe exacerbation risk, but RMSSD (IRR 1.49; 95% CI 0.99 to 2.25) did not meet the threshold for statistical significance.
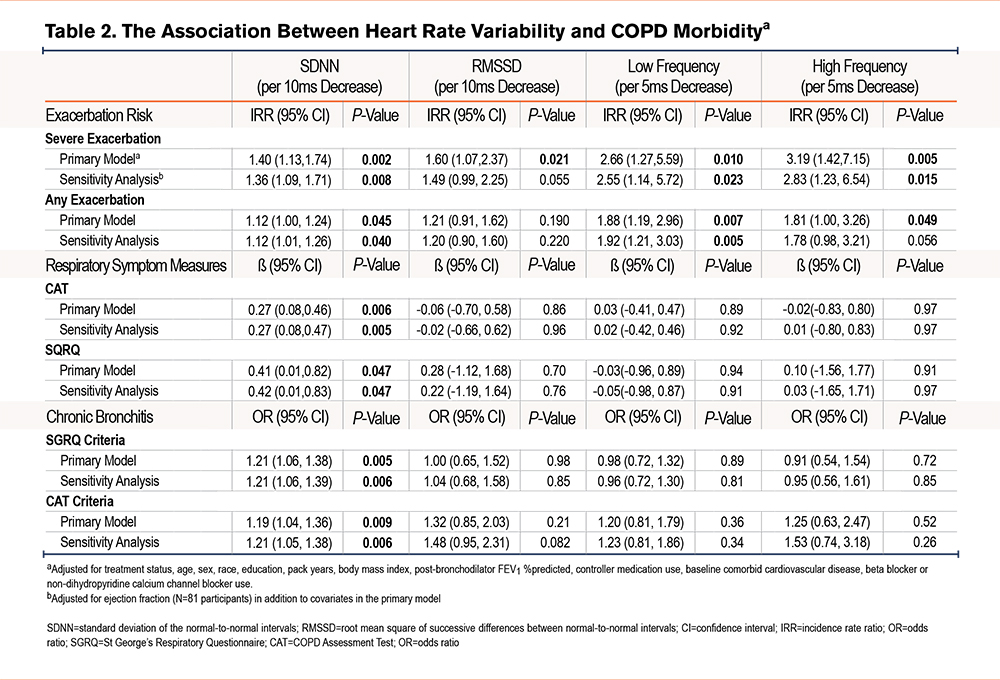
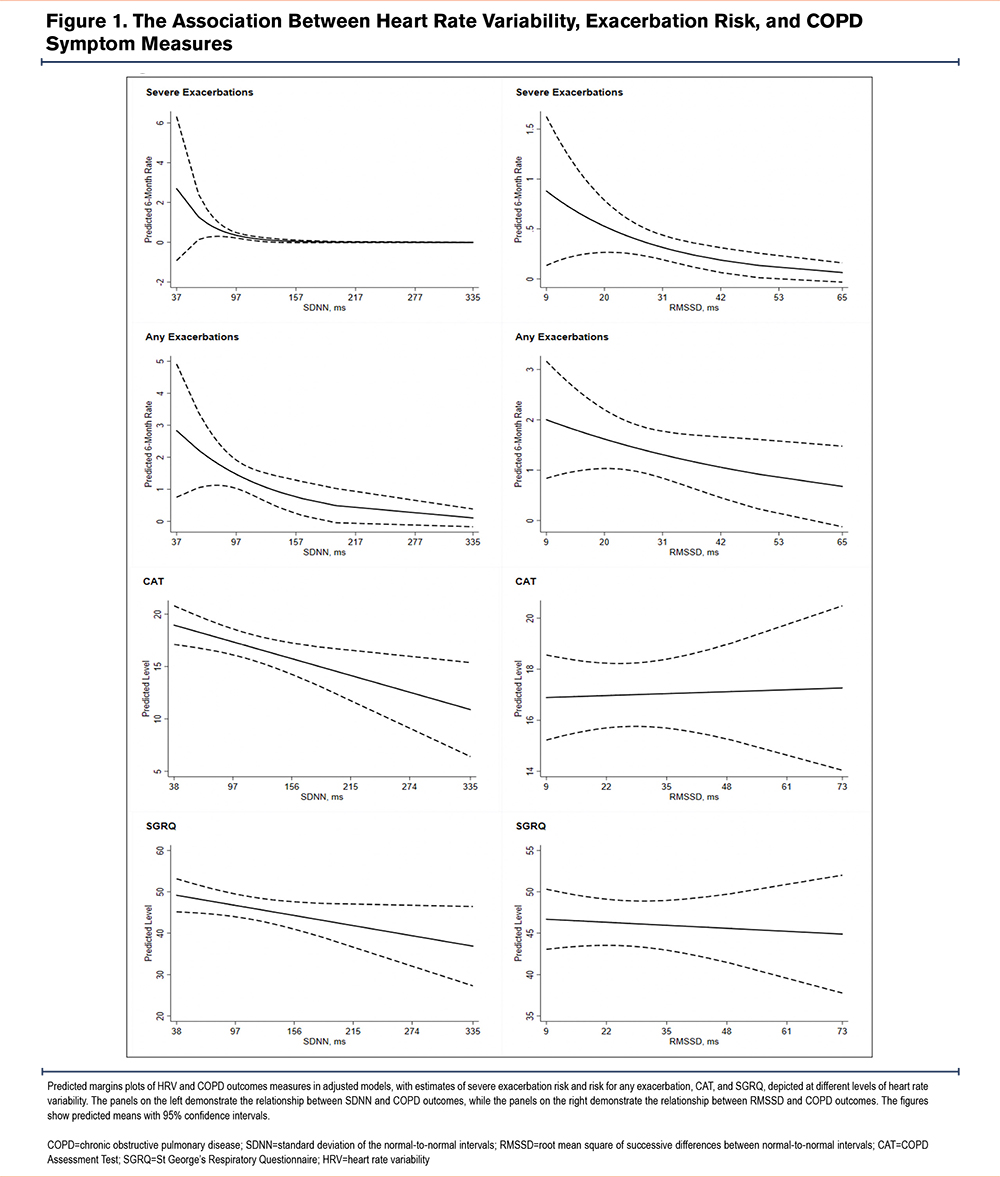
Furthermore, lower HRV, as measured by SDNN, was associated with worsened COPD symptom measures, including SGRQ and CAT (Table 2, Figure 1). Lower SDNN was also associated with higher odds of chronic bronchitis by both SGRQ (odds ratio [OR] 1.21; 95% CI 1.06 to 1.38) and CAT (OR 1.19; 95% CI 1.04 to 1.36) criteria. Other measures of HRV were not associated with symptom measures.
Interaction models did not detect significant effect modification by randomization group (intervention, placebo) on the relationship between SDNN (p-interaction 0.50) or RMSSD (p-interaction 0.31) and exacerbation risk. There was effect modification by randomization group on the relationship between SDDN (p-interaction 0.018) and RMSSD (p-interaction 0.020) on SGRQ score. Lower SDNN was associated with higher SGRQ scores in the placebo group (ß 1.01; 95% CI 0.37 to1.65) but not the active intervention group (ß -0.01; 95% CI -0.51 to 0.53). Similarly, lower RMSSD was associated with higher SGRQ scores in the placebo group (ß 2.12; 95% CI 0.04 to 4.21) but not the active intervention group (ß -1.17; 95% CI -3.01 to 0.67).
Discussion
Findings from the CLEAN AIR Heart study demonstrate that impaired cardiac autonomic function, as measured by HRV, is associated with higher exacerbation risk and symptom burden in COPD. These results depict a pathway implicated in both the respiratory and cardiovascular aspects of the multi-morbidity in COPD.
HRV holds promise as a non-invasive biomarker to predict disease morbidity and exacerbation risk, with novel digital health platforms increasing feasibility of routine assessment.9 Guidelines, including the Global initiative for chronic Obstructive Lung Disease (GOLD), emphasize the importance of multimorbidity care and cardiovascular disease assessment in COPD.3 HRV has previously been tied to cardiovascular risk.16 Results from the current study, provide further evidence that HRV is also a predictor of respiratory morbidity in COPD. The results demonstrate an association between multiple measures of HRV and COPD morbidity, including severe exacerbation risk, COPD-related quality of life and chronic bronchitis symptoms after accounting for traditional markers of COPD severity and cardiovascular disease.
In our analysis, the association between decreased HRV and severe exacerbations appeared stronger than associations observed between HRV and risk for any exacerbation. We hypothesized that individuals with less adaptive autonomic function demonstrate limited resilience and have exacerbations that are more likely to require acute care interventions to facilitate recovery. Individuals with decreased HRV may additionally be more susceptible to the combined impacts of COPD and concomitant cardiovascular disease.
The autonomic nervous system also plays an important role in regulating airway function and mucus secretion.17 We note that the association between respiratory symptoms, including chronic bronchitis, was only seen for SDNN, a measure of overall HRV in the primary analysis. It is possible that chronic bronchitis symptoms are more tied to the overall balance between sympathetic and parasympathetic function. We also note that the association between RMSSD and severe exacerbations, after accounting for ejection fraction, approached but did not reach significance. We postulate that this was a sample size effect, as high frequency power, another marker of parasympathetic activity, remained associated with severe exacerbations.
Interaction models do show a greater association between both RMSSD and SDNN and SGRQ scores in the placebo group. While the sample size limits our ability to draw larger conclusions, one potential is that the benefit of improvements to respiratory quality of life gained from the air cleaner intervention blunted the relationship between HRV and SGRQ scores. Previous studies from CLEAN AIR demonstrated that an air cleaner intervention has the potential to improve both HRV (particularly RMSSD) and respiratory quality of life, as measured by the SGRQ.10,11 As such, the air cleaner intervention may have limited our ability to determine the full magnitude of the association of HRV with respiratory quality of life among those with impaired cardiac autonomic function.
There are limitations to our study, including a narrow sample population that reduced our ability to extrapolate the results to other groups, notably those with ongoing tobacco use.18 The small sample size limited our ability to conduct subgroup analyses. Future analyses of interest include those focused on the effects of HRV in high-risk phenotypes, including the frequent exacerbator phenotype and individuals living with COPD and pulmonary vascular disease. Our study was also not able to account for measures of hypoxemia, including nocturnal hypoxemia, that can impact HRV and contribute to cardiovascular morbidity among individuals with COPD. While outside the scope of the current analysis, a subset of participants completed sleep study and actigraphy measurements, that will allow us to study the associations between nocturnal hypoxemia, physical activity, and HRV among individuals with COPD in the future. We do note significant strengths, including a well characterized cohort with detailed information on both pulmonary and cardiovascular disease, in addition to repeated measures of HRV and COPD outcomes that allow us to investigate health effects over time.
HRV may represent an important non-invasive biomarker with the potential to identify high-risk populations with COPD. Future studies are warranted to confirm the relationship between HRV and multimorbidity in COPD across diverse populations.
Acknowledgements
Authors Contributions: NNH and RDB helped to design the framework for the study, assisted with analysis, and acquisition of data. AF, NP, and AB contributed to authorship of the manuscript and interpretation of results. HWL contributed to data analysis and figure design. SCM contributed to manuscript authorship and acquisition of data. MCM contributed to study design, data analysis, interpretation of results, and authorship of manuscript. SR contributed to data analysis, interpretation of results, and drafted the original manuscript with input from all coauthors. All authors reviewed and approved the manuscript for publication.
Declarations of Interest
SR reports grants from the National Institutes of Health (NIH) during the conduct of the study. MCM reports grants from the NIH and the Environmental Protection Agency during the conduct of the study, and personal fees from Aridis, GSK, and Celgene outside the scope of the submitted work. NNH reports grants from the NIH, grants from the COPD Foundation, grants and personal fees from AstraZeneca, grants and personal fees from GSK, grants from Boehringer Ingelheim, and personal fees from Mylan during the conduct of the study. HW, NP, AF, AB, SCM, and RDB have no financial relationships or conflicts of interest related to the submitted work.