Running Head: Improving COPD Dyspnea With Weight Loss Coaching
Funding Support: Funding provided by National Heart, Lung, and Blood Institute grant HL138150.
Date of Acceptance: August 16, 2023 | Publication Online Date: August 18, 2023
Abbreviations: BMI=body mass index; COLD=chronic obstructive lung disease; COPD=chronic obstructive pulmonary disease; CRQ=Chronic Respiratory Disease Questionnaire; FAA=framed analytic approach; HC=health coach; INSIGHT=Intervention Study in Overweight Patients With COPD; MCID=minimal clinically important difference
Citation: Benzo MV, Barwise A, Clark MM, Dupuy-McCauley K, Roy M, Benzo RP. Improving dyspnea by targeting weight loss in patients with chronic obstructive lung disease and severe obesity through health coaching and remote monitoring. Chronic Obstr Pulm Dis. 2023; 10(4): 444-449. doi: http://doi.org/10.15326/jcopdf.2023.0404
Introduction
Dyspnea is one of the leading symptoms of chronic obstructive lung disease (COLD), such as chronic obstructive pulmonary disease (COPD) and asthma. Notably, patients with COLD and coexisting obesity report more dyspnea, increased health care utilization, poorer health-related quality of life, reduced functional capacity, and low physical activity.1,2 Despite the clinically negative effect of obesity in many outcomes in COLD, studies in COPD populations have shown improved survival in overweight and obese individuals compared with normal-weight counterparts, and increased mortality in the underweight individuals: the "obesity paradox."3
Obesity is rising in the general population and in patients with COPD, with rates of obesity as high as 42% (body mass index [BMI] ≥30kg/m2) in COPD cohorts,4 and despite optimal medical care, patients with COLD and obesity remain breathless.
Targeting weight loss to improve dyspnea and quality of life may be effective in patients with COLD, severe obesity, and clinically meaningful breathlessness. Obesity may represent a treatable trait that can be targeted through personalized lifestyle interventions for patients with chronic lung conditions, clinically meaningful dyspnea, and severe obesity.4-6 Weight loss may also reduce other risks, such as cardiovascular disease and diabetes.
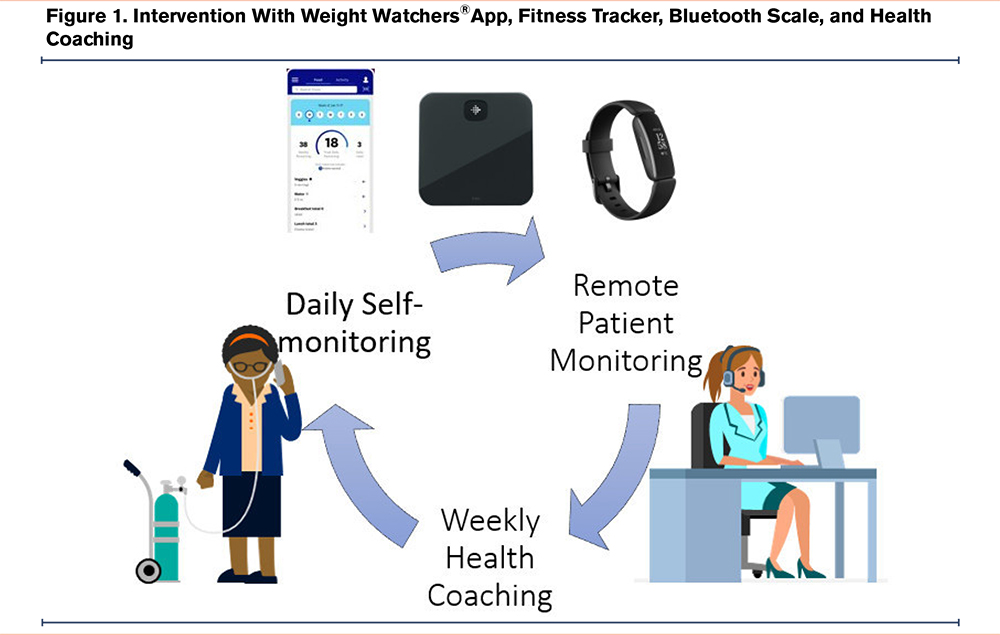
We previously reported the feasibility and possible effectiveness of an 8-week intervention that included the Weight Watchers® online application connected to a fitness tracker, a Bluetooth scale, and weekly health coaching (Figure 1).5 The intervention promoted self-awareness by registering food intake, daily physical activity, and measuring weight. A post-intervention survey indicated that participants preferred a longer program for further health coaching support and accountability. This report aims to extend our previous pilot testing the feasibility and effect size of a 12-week intervention with a 12-week follow-up to evaluate the sustainability of the outcomes and the experience of the patient with COLD and severe obesity through a qualitative inquiry.
Methods
This study design is a pre-post intervention with a mixed-method component. It was approved by the Mayo Clinic Institutional Review Board (IRB 18-001832) and registered with clinicaltrials.gov NCT03836547. This study was initially designed as a randomized clinical trial with waitlist control. The waitlist period severely affected recruitment, given that participants were unwilling to wait for the intervention. The study was modified to the current pre-post-intervention design. All patients signed a written informed consent to participate in the intervention and oral consent to the qualitative interview.
Patients were recruited from our pulmonary outpatient practice. Inclusion criteria were > 17 years of age, BMI >34kg/m2, clinically significant dyspnea defined by a modified Medical Research Council dyspnea score >1, and a clinical diagnosis of COPD and/or asthma.
In this 12-week intervention, participants agreed to log their food daily in the Weight Watchers app, wear a Fitbit tracker wristband to capture their daily steps, and weigh themselves daily. Health coaching was weekly and based on motivational interviewing and mindful, active listening. It included reviewing the patient’s weekly food log, daily steps, and weight progress in the Weight Watcher app and collaboratively setting a weekly goal. Outcomes included percentage of weight loss, BMI, and dyspnea measured by the Chronic Respiratory Questionnaire (CRQ) dyspnea domain.7 Outcomes were measured at baseline, 3 months, and 6 months. Descriptive statistics were used to summarize the study cohort's demographic characteristics and primary outcomes. Paired 2-sided t-tests were used to assess the change in participants' weight and CRQ dyspnea domain scores from baseline to 3 months. The minimal clinically important difference (MCID) for the CRQ dyspnea domain is 0.5. The percentages of patients who reach this threshold at 3months and maintain it at 6 months were reported. All tests were 2-sided, and p-values <0.05 were considered statistically significant.
Qualitative Methods
We conducted one-on-one semi-structured interviews with participants who had taken part in the intervention to understand their perceptions about the facilitators, barriers to participating, and suggestions for improving the intervention. At the time of the interview, participants were past 6-months of completion. We also included participants who dropped out of the study. All interviews were conducted over the phone following verbal consent, audio-recorded, and transcribed verbatim. Interviews typically lasted 30–40 minutes. All interviews were coded independently and in duplicate by AB and MB (co-authors), who met weekly to reach a consensus. We used the framework analytic approach (FAA) to organize and analyze the data.8 The FAA is an analytic approach by which the verbatim content of interview transcripts is summarized in a matrix. Within this matrix (commonly a spreadsheet), deductive themes from interview guides create the columns, and individual interviews create the rows. Inductive—emerging—themes may add additional columns.9 Transcript page numbers are noted with paraphrased content within the relevant cells. This approach has several advantages over other qualitative analytic methods: it can be deployed in multidisciplinary research teams where some members have limited qualitative experience but can still engage in sensemaking under the guidance of an experienced qualitative researcher.4 We discontinued data collection once we reached data saturation.
Results
Nineteen participants started, and 13 completed the intervention. Dropout was attributed to other comorbidities that became a higher priority for patients and the lack of measured readiness to commit to a weight loss lifestyle intervention.
Participants’ mean age was 63.6 (10.6) years; 11 participants (85%) carried a diagnosis of COPD; 2 had asthma; 58% were female; 95.7% were White race; forced expiratory volume in 1 second percentage (FEV1%) was 68.7 (18.4) (mean [standard deviation (SD)]), and FEV1/FVC % was 77.6 (1.8) (mean[SD]). The mean baseline weight was 266.4 lbs with a mean BMI of 42kg/m2.
At 3 months, participants demonstrated an average (SD) weight loss of 6.9% (4.4%) or -18.4 lbs. (11.1), p=0.00007. At 6 months, the average (SD) weight loss was 9.0% (5.26%) or 22.39 lbs. (12.6). The CRQ dyspnea domain had a mean difference (3 months – baseline) of 0.643 (p=0.00919) beyond the MCID of 0.5. From the individual results, results demonstrated that 46% (6/13) reached the MCID threshold (improvement of >0.5 on CRQ dyspnea) at 3 months, and 30% maintained the MCID threshold at 6 months.
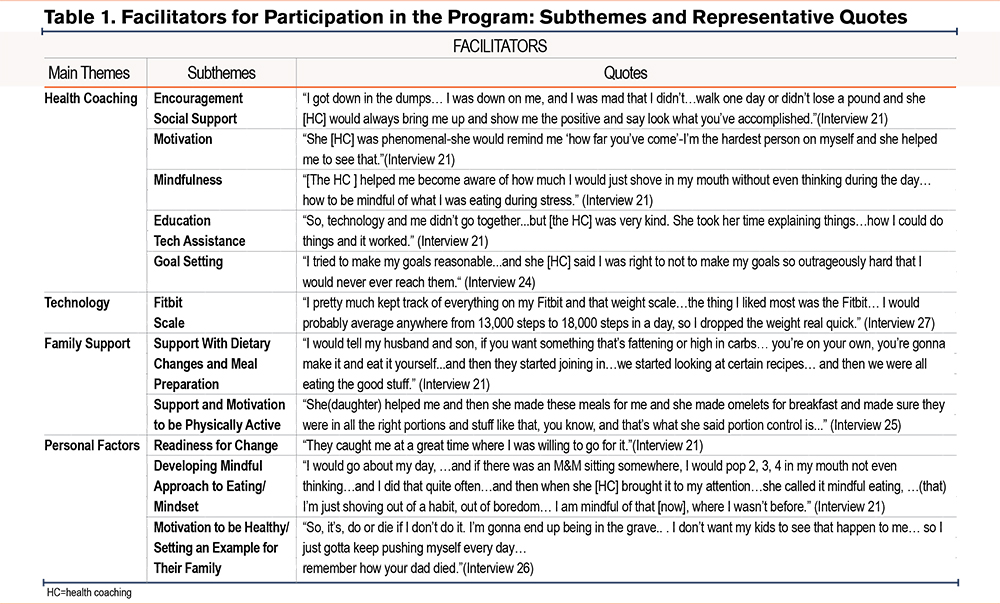
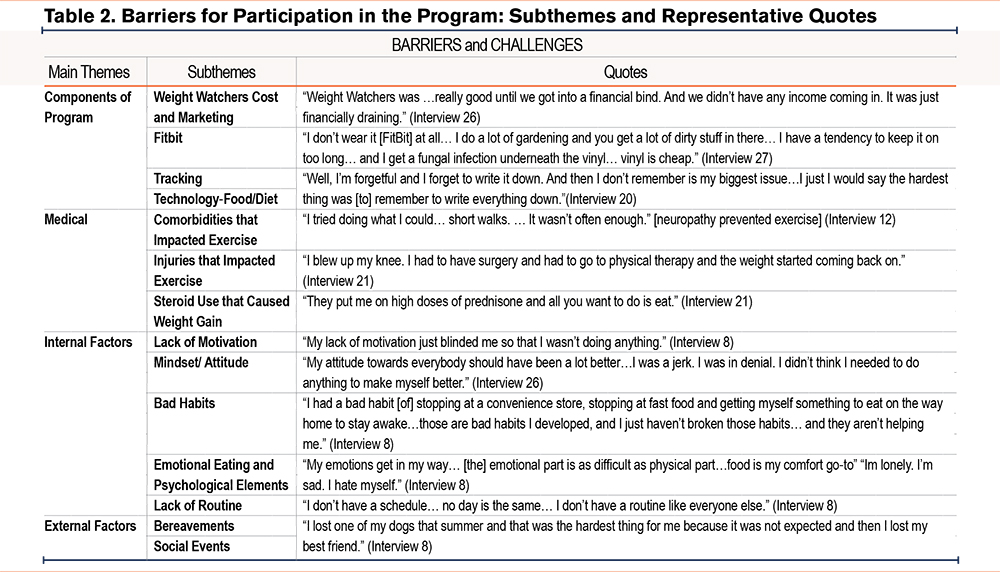
Qualitative interviews were conducted with 9 study participants and 5 were female. The most prominent themes in our qualitative data analysis included facilitators and barriers to the intervention, as well as intervention recommendations. Despite variability in the intervention's success in achieving weight loss and improved symptoms, most participants voiced enthusiasm for the intervention overall and the components of the intervention, especially the health coaching (Table 1). Barriers to success in the intervention included concurrent injuries and comorbidities, internal factors such as lack of motivation, and external issues such as bereavement (Table 2). Participants also expressed their recommendations for future interventions when asked, primarily for more personalization of the intervention components. They recommended a longer program to support: (1) a sustained healthy diet and exercise habits, (2) customized diet, education, and exercise regimens based on benchmarking of physical ability, injuries, and comorbidities, and (3) customized follow-up support from the health coach and clinician. Several participants also stated that the emotional and psychological aspects of eating habits should be addressed within a future modified program. Lack of readiness was identified as a factor in poor uptake and adherence.
Discussion
We found that individuals with severe obesity and COLD, mostly COPD, had clinically and statistically significant improvements in dyspnea and weight loss. Although this is a small sample, these results suggest a possible effective intervention to lose weight and improve breathlessness that may be sustainable after the intervention and may fill a knowledge and practice gap. It is plausible that the dyspnea improvement is independent of the severity of the COLD. Future confirmatory research is warranted. We further recognize the possible synergistic effect this type of lifestyle intervention could have with weight management pharmacological treatments.10 Our results confirm and extend our preliminary work5 and hypothesis that weight loss in patients with severe obesity may represent an added treatment to improve dyspnea with chronic lung disease in individuals who otherwise had optimal pharmacological treatment.
This pilot obtained greater weight loss than our previous study possibly because it was a longer intervention. The barriers that emerged from the qualitative analysis suggest opportunities to increase the feasibility of future interventions. Assessing readiness for change and other comorbidities may impact the patient’s success in their weight loss journey. During the recruitment process, patients who were ready for behavioral change proposed by the program wanted to start this intervention immediately. Engaging with the participant immediately after consenting was important when the motivation for the change was high.
We must acknowledge the study limitations: small, single center, and with possible selection/attrition bias, since 13/19 participants completed the study. Our results align with the reported beneficial effect of weight loss in obese patients with COPD and asthma 5,11,12 and provide a method for lifestyle intervention targeting weight loss. The qualitative study indicated that the intervention was well-received but pointed out aspects that can be improved, including a more personalized weight loss approach, physical exercise, and health coaching follow-up support. Our results further confirm the results presented as an abstract of the National Institutes of Health-funded Intervention Study in Overweight Patients With COPD (INSIGHT) study that indicates that lifestyle modification interventions are feasible and possibly effective in COPD.12 However, we tested a more intense intervention for behavior change (health coaching) with a favorable patient experience. Health coaching is supported by our previous research, which has shown the consistent benefit of health coaching and remote monitoring on all aspects of quality of life in COPD.13,14
Our results can inform needed, randomized controlled trials focusing on lifestyle programs, given the scarcity of treatment options for severe obesity in patients with COLD. Addressing weight loss through health coaching and lifestyle modification in patients with severe obesity and COPD may represent a strategy to improve dyspnea, the most prevalent symptom of their condition. Finally, we believe that the proposed behavior change model/intervention is translatable to multiple chronic conditions.
Acknowledgments
Author contributions: MB was responsible for writing and editing the manuscript. AB was responsible for the tables and the study methods. MR was responsible for data analysis and MC and KD were responsible for editing the manuscript. RB was responsible for the study’s design, the study’s funding, editing the manuscript, and overall supervision of the study. All authors reviewed and approved the final version of the manuscript for publication.
We would like to acknowledge the Mindful Breathing Laboratory's team effort that made this research on remote patient monitoring and lifestyle intervention possible.
Declaration of Interest
All authors declare no conflicts of interest.