Running Head: COPD and Clinical Trials
Funding Support: This work was sponsored by the National Natural Science Foundation of China (#81602830).
Date of Acceptance: August 9, 2023 | Publication Online Date: August 11, 2023
Abbreviations: CI=confidence interval; COPD=chronic obstructive pulmonary disease; ICTRP=International Clinical Trials Registry Platform; IQRs=interquartile ranges; NA=not available; OR=odds ratio; SE=standard error; WHO=World Health Organization
Citation: Xu M, Wang J, Shan L, An X. The current landscape of COPD-related clinical trials registered on the World Health Organization's International Clinical Trials Registry Platform: a comprehensive analysis of study characteristics and publication status. Chronic Obstr Pulm Dis. 2023; 10(4): 400-411. doi: http://doi.org/10.15326/jcopdf.2023.0417
Online Supplemental Material: Read Online Supplemental Material (258KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, treatable, but incurable chronic respiratory condition with significant global impact. The age-standardized point prevalence, death, and disability-adjusted life years rates for COPD in 2019 were reported to be 8.7%, 41.7%, and 39.8%, respectively, indicating a decline compared to those rates1 in 1990. Despite this decline, COPD remains the third leading cause of death worldwide, based on age-standardized death rates.2 The economic burden of COPD is substantial and varies across different countries. Research findings suggest that the annual cost per patient ranges from €1,963 in Belgium to €10,701 in Norway.3 The economic burden is positively correlated with factors such as delayed diagnosis, severe COPD, frequent exacerbations, and hospital admissions.3,4 Although the Global Burden of Disease Study 2019 revealed a decrease in the burden of COPD, it remains a critical public health concern, particularly in countries with lower sociodemographic indices.5 As a result, COPD has been included in the World Health Organization’s (WHO) Global Action Plan for the Prevention and Control of Noncommunicable Diseases, and the United Nations’ 2030 Agenda for Sustainable Development.6
Clinical trials play a crucial role in advancing medical knowledge by addressing scientific inquiries and seeking improved methods for disease prevention, detection, diagnosis, and treatment.7 A comprehensive understanding of the current landscape of clinical trials related to COPD is essential to enhance trial designs and identify areas of research that may have been overlooked. However, there is currently a lack of in-depth knowledge regarding clinical studies on COPD. To facilitate access to information on clinical trials conducted worldwide, the WHO established the International Clinical Trials Registry Platform (ICTRP)8 in 2005. The ICTRP serves as a centralized hub connecting various clinical trial registries, including national and regional databases. To enhance the visibility and discoverability of clinical trials, the U.S. Food and Drug Administration mandates the registration of interventional trials and the public disclosure of their results,9,10 fostering a more open and accountable research environment. Publication of trial results in a peer-reviewed journal is considered the most critical form of research dissemination. Timely publication of trial results can provide the best scientific evidence and offer substantial benefits to public health and scientific advancement. Ensuring accessibility to complete trial data is essential for clinicians and stakeholders to make informed and appropriate clinical decisions.
While some studies have examined the publication rates and factors influencing publication outcomes of clinical trials,11-14 they tend to focus on specific diseases, have limited sample sizes, or analyze trials from a single registration platform. Additionally, few studies have investigated the factors impacting the publication time.15,16 Notably, there is a dearth of such information concerning COPD clinical trials. Thus, this study aims to address this research gap by providing a comprehensive overview of interventional clinical trials related to COPD within the ICTRP. Through a thorough analysis of these trials, we seek to identify the factors associated with both publication status and publication time, thereby contributing to a deeper understanding of COPD research, and facilitating the improvement of trial designs in this critical area of respiratory health.
Materials and Methods
Data Source
On April 28, 2022, we conducted a comprehensive search for registered trials related to COPD using the ICTRP search portal (http://apps.who.int/trialsearch) (Table S1 in the online supplement). The search terms included “COPD,” “chronic obstructive pulmonary disease,” “chronic obstructive pulmonary diseases,” “chronic obstructive lung disease,” “chronic obstructive lung diseases,” “chronic obstructive respiratory disease,” “chronic obstructive respiratory diseases,” “chronic obstructive airway disease,” “chronic obstructive airway diseases,” “chronic airflow obstructions,” “chronic airflow obstruction,” “AECOPD,” and “COAD.” A total of 8203 records for 6830 trials were identified and downloaded in the form of XML files.
We reviewed each trial and excluded observational clinical studies. Trials that did not involve COPD patients were also excluded based on a thorough examination of the “condition,” “public title,” and “scientific title” fields provided in the trial information. Subsequently, all relevant information was imported into an Excel spreadsheet for further processing and analysis.
Data Processing
We extracted key fields from the clinical trial records within the ICTRP. However, certain fields were not available in the ICTRP and were obtained through additional searches on each primary registration website (Table S2 in the online supplement).
The source of support was classified as either industry or nonindustry (including hospitals, universities, and other nonprofit research organizations). If an industrial institution was listed as the lead funder of the trial, the source of support was categorized as industry. Furthermore, dissemination lag was defined as the time interval between the completion of the study and the first public disclosure of the results (through submission to registration websites or publication in scientific journals).
Although ICTRP has attempted to remove duplicate clinical trial records from primary registries, our study still identified duplicate records. To address this issue, we adopted 2 deduplication methods: one involved removing duplicates through secondary identifiers from multiple registries, and the other utilized the same PubMed (the National Institutes of Health’s search engine) ID for published papers in conjunction with clinical trial titles and sponsor information for deduplication.
To identify publications of the included trials, we considered trials completed by April 28, 2020, taking into account that investigators require at least 2 years to analyze the data, prepare a manuscript, and publish their findings in scientific journals. As a result of incomplete links between the ClinicalTrials.gov database and PubMed, semi-automated methods have been developed to identify clinical trial publications.17,18 To further ensure the accuracy and reliability in retrieving the publications of clinical trials, we developed a specific search strategy (Table S3 in the online supplement).
In cases where multiple publications were found for the same clinical trial, the earliest one reporting primary outcome results was retained. The date of electronic publication, if available, was recorded as the date of publication; otherwise, the issue date of the publication was used. When specific day information was not provided, the publication day within the month was assumed to be the 1st. Study protocols, interim analyses, commentaries, meeting abstracts, and other nonrelevant publication types, as well as articles published before trial completion, were excluded.
Statistical Analysis
For categorical variables, frequencies and percentages were calculated, while medians and interquartile ranges (IQRs) were computed for continuous variables. Fisher's exact tests were used to compare categorical variables, and Mann-Whitney U tests were employed for comparing continuous variables. Cumulative publication rates after trial completion were analyzed using the Kaplan-Meier analysis. A logistic regression model was applied to examine the factors associated with the publication status of trials (published and unpublished). A linear regression model was used to explore factors associated with time lag from study completion to publication for trials. The multivariate regression model included variables associated with p≤0.10 in univariate analysis. Statistical significance was defined as 2-sided p<0.05. All statistical analyses were performed using R Statistical Software, version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Distribution of COPD-Related Interventional Clinical Trials
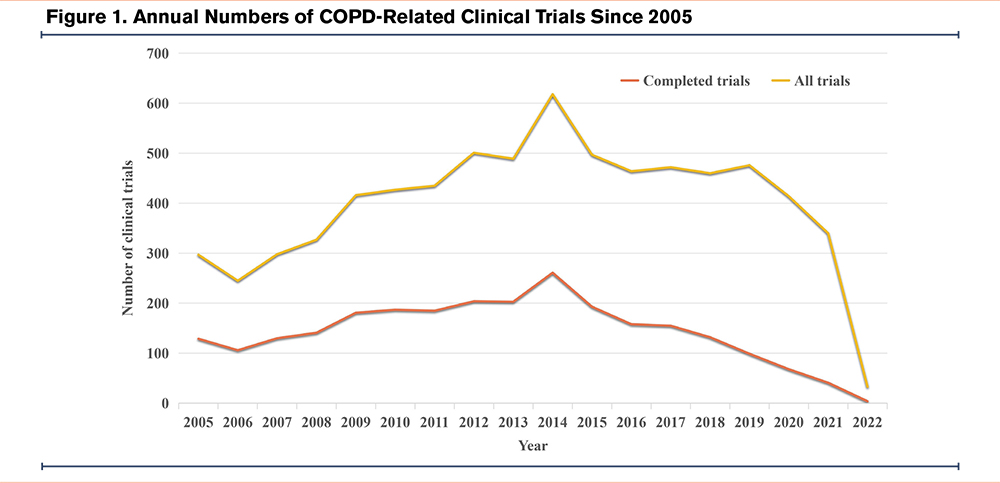
Overall, 5100 registered, interventional clinical trials were retrieved from the ICTRP. Sixty-one trials that started before 2005 were excluded. Trials that were not completed (n=2055), involving non-COPD patients (n=133), or identified as duplicates (n=274) were also excluded. A total of 2577 clinical trials remained eligible for analysis (Table S4 in the online supplement). The distribution of trials (as of April 28, 2022) is summarized in Figure 1 according to the registration year. It was observed that there is a consistent trend in the annual number of both registered and completed COPD-related interventional clinical trials, with the highest number occurring in 2014.
General Characteristics of COPD-Related Interventional Clinical Trials
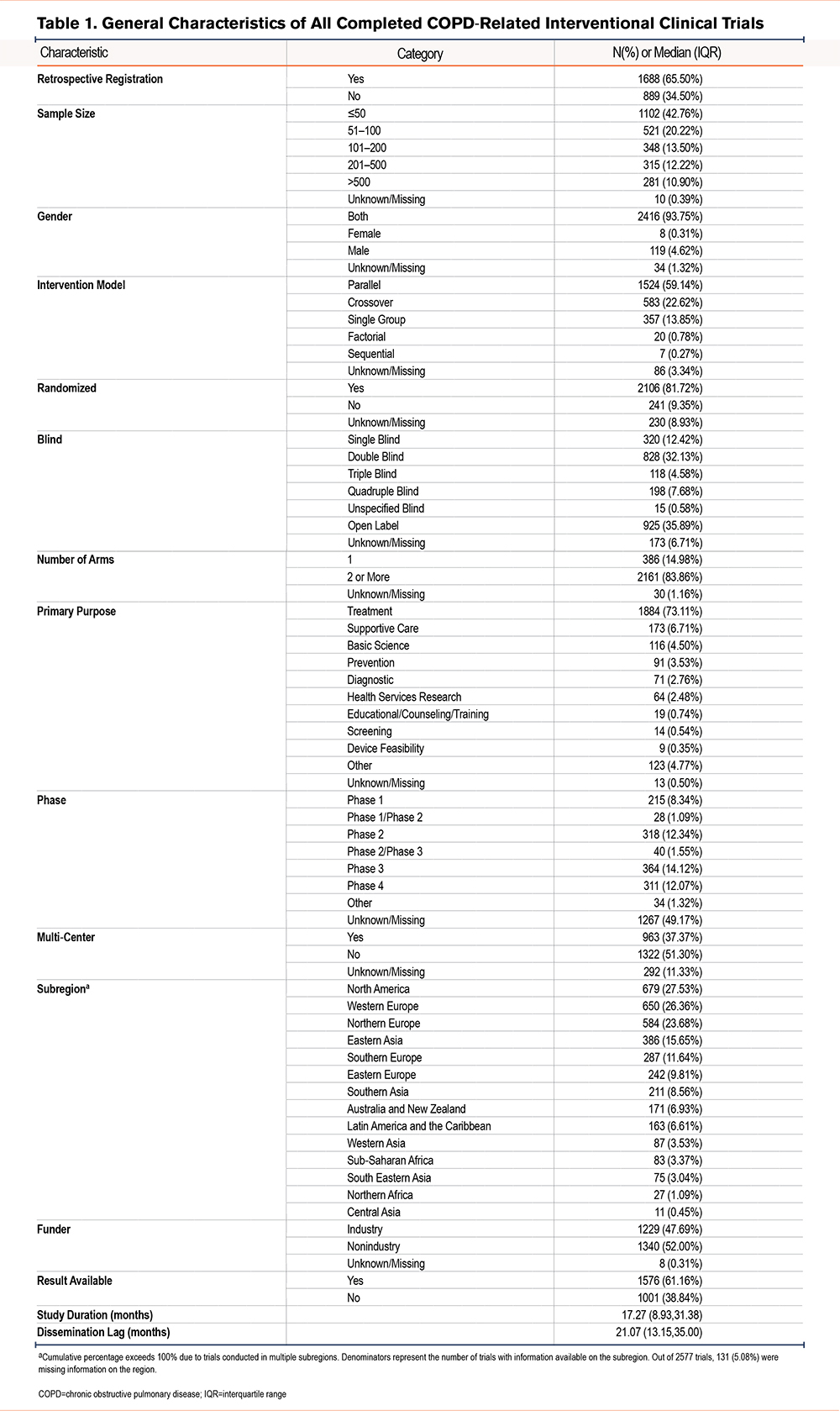
Table 1 presents the characteristics of all completed COPD-related interventional clinical trials registered after 2005. Over 60% of the trials were retrospectively registered. In total, 42.76% of the trials enrolled ≤50 participants. Regarding the study design, the majority of the trials were randomized (81.72%), blind (57.39%), conducted at single-center sites (51.30%), and involved multiple arms (83.86%). The most commonly employed intervention model was parallel assignment (59.14%), followed by crossover assignment (22.62%). Treating the disease was the primary purpose of most trials (73.11%). Nearly half of the trials (49.17%) did not specify a particular phase, while 14.12% were classified as phase 3 trials. Geographically, the trials were conducted in 14 subregions, with the majority being carried out in North America (27.53%), western Europe (26.36%), and northern Europe (23.68%). Nonindustry sources funded slightly more clinical trials (52.00%) compared to industry-funded trials (47.69%). A total of 61.16% of the trials had their results available in registration websites or publications. The median time of study duration was 17.27 months, and the dissemination lag was 21.07 months.
Publication Status of Completed COPD-Related Interventional Clinical Trials
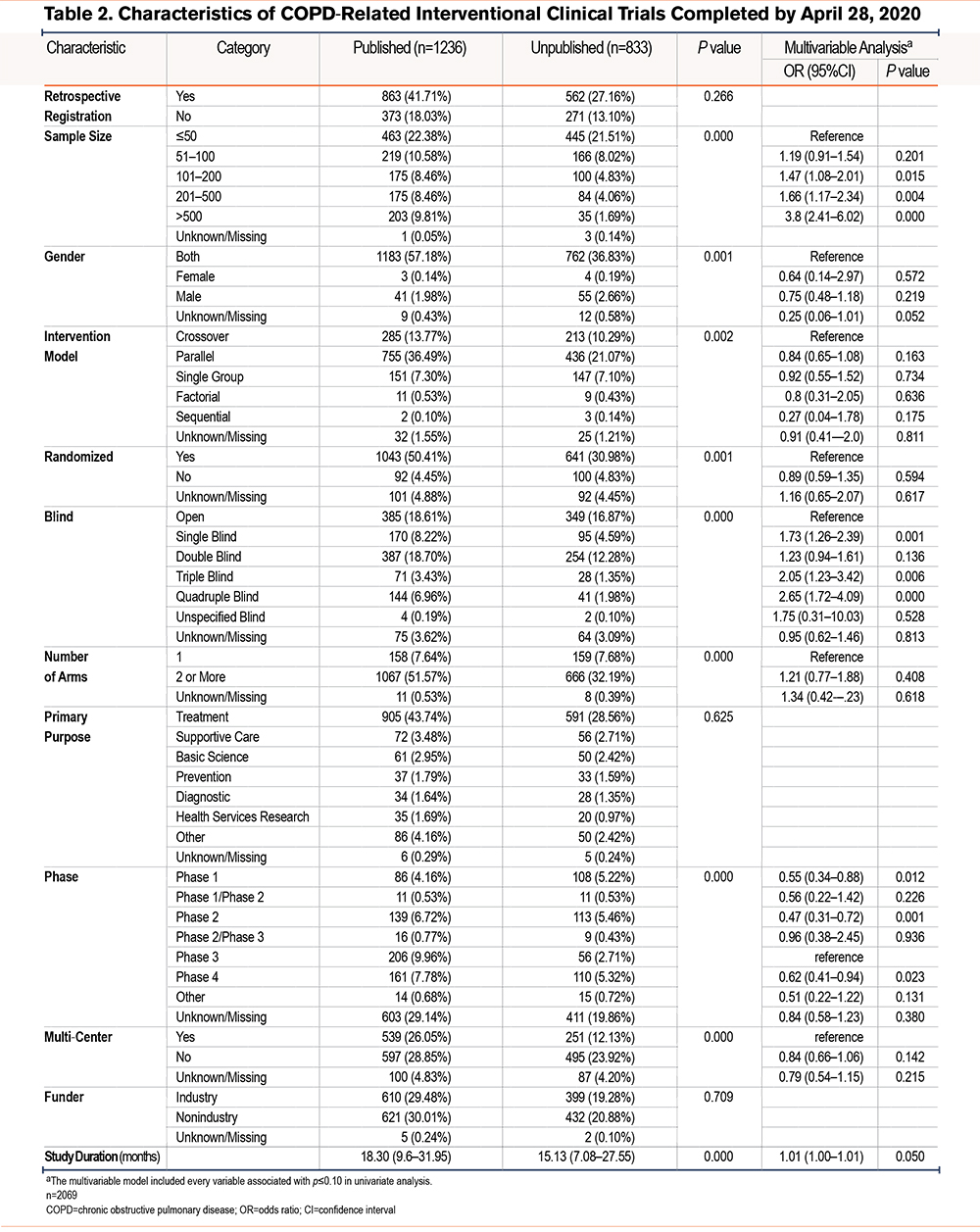
Among 2069 interventional clinical trials completed before April 28, 2020, a total of 1236 (59.73%) were published by October 1, 2022. For the remaining unpublished trials, abstracts were found for 43 of them (5.16%). The characteristics of both published and unpublished interventional clinical trials are presented in Table 2. The results of the univariate analysis of factors associated with publication status can be found in Table S5 in the online supplement. In the multivariate analysis, it was observed that significant factors associated with published trials were limited to sample size, blind design, and study phase (p<0.05).
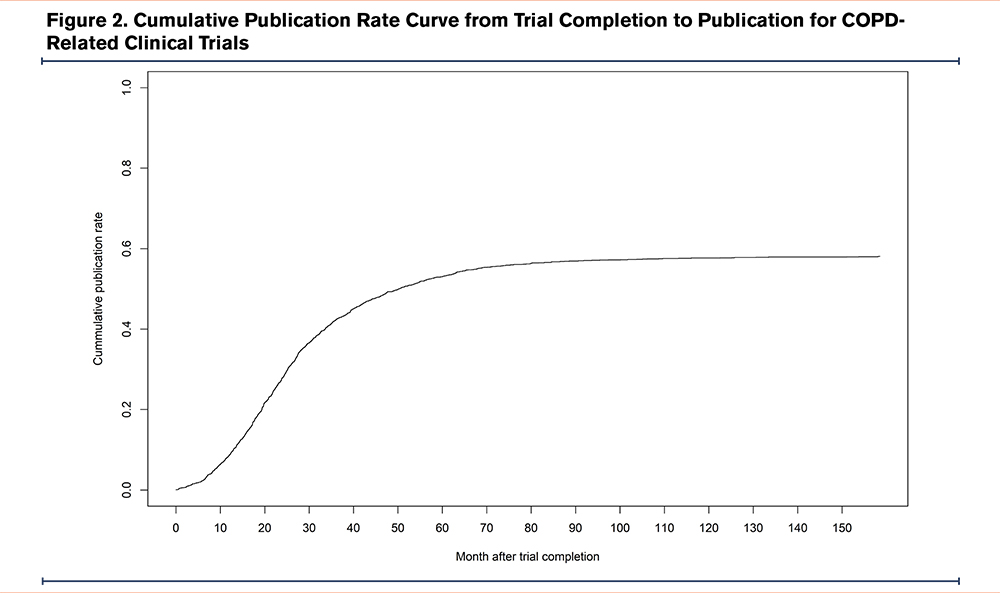
The median time to publication was 24.70 months (IQR: 13.27–38.30 months), and the cumulative publication rates at 1, 2, 3, and 5 years were 8.7%, 27.9%, 42.3%, and 53.0%, respectively (Figure 2).
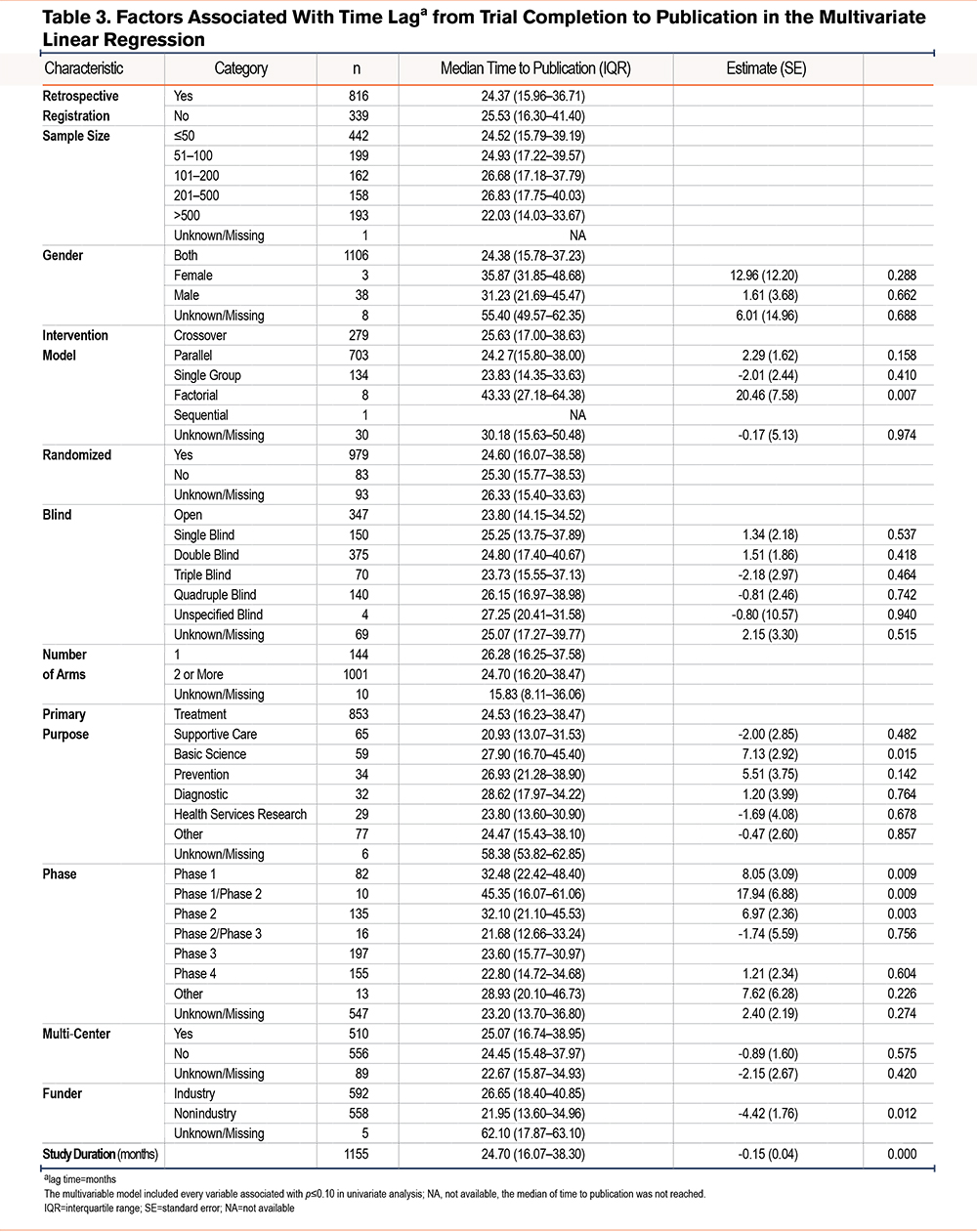
Factors Associated with Time Lag from Study Completion to Publication
The univariate analysis results, showcasing the factors associated with a longer lag from study completion to publication, can be found in Table S6 in the online supplement. In Table 3, the findings of the multivariate linear regression analysis are presented, indicating that specific variables, including intervention model, primary purpose, study phases, funder, and study duration, were found to be statistically significant (p<0.05) in influencing publication time. For instance, compared with industry-funded trials, nonindustry-funded trials demonstrated a shorter time to publication by 4.42 months (standard error [SE]=1.76, p=0.012). Basic science trials exhibited a longer time to publish in comparison with treatment trials, with a difference of 7.13 months (SE=2.92, p=0.015).
Discussion
To our knowledge, this is the first study to provide a comprehensive overview of the current landscape of clinical trials related to COPD, including study characteristics and the factors influencing publication status and time. The findings reveal that a majority of completed interventional clinical trials related to COPD had relatively small sample sizes. The dominant features of these trials included parallel assignment, randomized allocation, treating purpose, single-center design, multi-arm structure, and blind studies. Moreover, more than half of the trials had their results available in the database or were published in scientific journals. The median time to publication was observed to be 24.70 months, with a 2-year cumulative publication rate of 27.9%. Through multivariate analysis, we identified that the significant factors associated with published status were sample size, blind design, and study phase. Additionally, the factors influencing publication time were investigated, with intervention model, primary purpose, study phase, funder, and study duration found to be statistically significant. This study adds significant contributions to the existing knowledge of COPD clinical trials.
The WHO defines timely publication of clinical trial results as having the main outcome of a trial posted to the results section of the clinical trial registry within 12 months of completion and published in a journal within 24 months.19 However, our study revealed that only 27.9% of the COPD-related interventional clinical trials met this criteria for timely publication. The rate is considerably lower than the 56% reported for randomized controlled trials funded by the Health Research Council of New Zealand.20
In comparison with previous studies, our analysis of COPD-related clinical trials revealed a shorter median time to publication (24.70 months). For example, Liu et al reported a median time of 46.5 months from primary completion to publication for interventional studies related to thyroid cancer.15 Shepshelovich et al observed a median lag of 26 months from data lock to publication in phase 1 clinical cancer trials.16 Jones et al conducted a study of large randomized clinical trials and found a median time of 27 months (IQR: 20–37 months) from study completion to publication.11 These discrepancies in findings can be attributed to differences in the definition of publication time, the specific disease fields being studied, the phase of the clinical trials, and the number of enrolled participants.
In our study, we identified several factors independently associated with the publication status of clinical trials, including sample size, blind design, and study phase. Adequate sample size is crucial in clinical trials to ensure statistical power and robustness of the study findings. Trials with inadequate sample sizes are often considered underpowered and may not yield meaningful or generalizable results. As a result, such trials may face challenges in getting published due to their limited scientific impact.21 Regarding blind design, our findings revealed that trials with blind designs were more likely to be published, while the previous studies15,22 did not observe blind design-associated publication status. The clinical trials for different disease fields that have distinct blind design proportions may explain the heterogeneity in results.22 Additionally, we observed that clinical trials in phase 3 were more likely to be published compared to trials in other phases, which is consistent with the findings of Wang’s study.21 Shepshelovich et al also found that compared to the phase 1 cancer trials found on the ClinicalTrials.gov website, phase 2 trials had higher publication rates.16 The observed differences in publication rates between early-phase and late-phase clinical trials can be partly attributed to the distinct nature and objectives of the trials. Early-phase trials, primarily focused on assessing the safety and tolerability of interventions in a small group of participants, may be more prone to demonstrating failed efficacy or inconclusive results. Late-phase trials, particularly phase 3 trials, are designed to assess the efficacy and safety of interventions in a larger and more diverse patient population; these trials are conducted after extensive preclinical and early clinical studies have provided promising results, and they have well-defined research methods and rigorous protocols. The robustness and significance of the findings in late-phase trials make them more attractive for publication.23,24
In the present study, several factors associated with publication time were identified. Trials with a primary purpose of basic science took longer to be published compared to trials with a treatment purpose. This difference in publication time may be attributed to the nature of basic science trials, which are initiated by investigators and involve more intricate research methodologies and data analysis, leading to a more prolonged preparation of manuscripts for publication.25 Regarding the study phase, we found that phase 3 trials for COPD were published sooner than trials in other phases, while Liu et al15 did not observe consistent results in the clinical trials on thyroid cancer. This may be due to the distribution of clinical trials across different phases for different diseases. For instance, oncological diseases often have a higher proportion of early-phase trials26,27 while COPD trials are more frequently conducted in later phases. Furthermore, we observed that nonindustry-funded trials tended to be published more rapidly than industry-funded trials. This finding aligns with previous research13,28 and can be elucidated by potential factors, including slower manuscript preparation due to rigorous internal review processes and commercial considerations within industry organizations, and the need to meet specific publication requirements as part of the funding conditions for nonindustry-funded trials.29 Additionally, trials with longer durations were associated with earlier publication. This phenomenon may be attributed to the complexity of research on new interventions, such as novel drugs, which may require a more extended period for data collection, analysis, and interpretation. The significance and novelty of the findings in such trials may also contribute to their prioritization for publication.30
Our study has several strengths that contribute to the robustness of the analysis regarding factors influencing publication outcome and publication time. Previous studies often focused on data from a single registry,12,17,22 which could lead to potential biases and limitations in the analysis. One notable strength of this study is the inclusion of a large number of trials registered across multiple platforms, providing a more representative and diverse dataset for analysis. The second notable strength is addressing the challenging issue of data deduplication, which is encountered when analyzing clinical trial data from different registries. In this study, multiple methods were employed to carefully deduplicate the collected clinical trials, minimizing the potential biases in the analysis.
Despite the strengths of this study, there are some limitations that should be acknowledged. First, this study relied on the original study fields from the registries, and there was no additional manual screening to identify and verify possibly misclassified information, such as study design. This limitation may introduce some degree of uncertainty in the accuracy of the data. Second, the publication date in some clinical trials preceded the completion date. Given that the date may be entered incorrectly, trials with conflicting dates were excluded from the analysis of median publication time and influencing factors. Third, despite the thorough search for publications, it is possible that some publications related to the included trials were not identified. The lack of complete linkage between trial registration IDs and publications, as well as the large number of trials, made it challenging to trace all publications. Moreover, contacting investigators of these trials to inquire about trial-related publications was not feasible due to the scale of the study.
Conclusion
This study presented the first comprehensive landscape of registered COPD-related interventional clinical trials. The findings revealed that there is a deficiency in large multi-center interventional clinical trials for COPD, and the 2-year cumulative publication rate is relatively low. To address the identified limitations, further efforts are warranted to strengthen collaboration among investigators and promote the adoption of robust scientific designs, particularly for initiating larger phase 3 clinical trials. Improving the timely publication of trial results is imperative to enhance transparency, disseminate critical findings, and facilitate evidence-based decision-making in the field of COPD research.
Acknowledgements
Author contributions: MX designed and performed the study, including data analysis and manuscript writing. JW and LS helped with data collecting and manuscript revision. XA oversaw the implementation of the study, and reviewed and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Interest
The authors have no conflicts of interest to declare.