Running Head: COVID-19 and Alpha-1 Antitrypsin Deficiency
Funding Support: None
Date of Acceptance: April 13, 2023 │ Published Online Date: June 26, 2023
Abbreviations: AATD=alpha-1 antitrypsin deficiency; COPD=chronic obstructive pulmonary disease; COVID-19=coronavirus disease 2019; FDA=Food and Drug Administration; ICU=intensive care unit; NS=not significant; SARS-Co-2=severe acute respiratory syndrome coronavirus-2; SD=standard deviation
Citation: Hay M, Holm KE, McCathern J, Sandhaus RA, Strange C. Impact of coronavirus disease 2019 and vaccination attitudes on alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis. 2023; 10(4): 335-342. doi: http://doi.org/10.15326/jcopdf.2023.0406
Online Supplemental Material: Read Online Supplemental Material (163KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) is a genetic disease in which the lack of protease inhibition leads to unregulated protease activity in lung tissue.1 Emphysema is the predominant endotype of chronic obstructive pulmonary disease (COPD) seen in AATD individuals and occurs at an average age 10 years younger than when seen in usual smoking-associated COPD.1 Clinical disease is seen more often when there is an environmental risk of lung disease, particularly in those who smoke.2 The severity of obstructive lung disease and degree of emphysema can vary greatly in the AATD population.3 However, similar to usual COPD, lung function lost is not usually recovered.1 Therefore, at the first signs of emphysema, most individuals in the United States begin weekly intravenous augmentation therapy with one of 4 U.S. Food and Drug Administration (FDA)-approved products to prevent future lung destruction.4,5
The AATD community is supported by 2 prominent disease-specific, not-for-profit organizations: the Alpha-1 Foundation and AlphaNet. When starting augmentation therapy, AATD individuals are given the option to join AlphaNet, a disease management organization.6 AlphaNet assigns each member a peer coordinator who also has AATD, from whom they receive monthly phone calls for education about managing the disease and weekly intravenous augmentation therapy, the only specific FDA-approved treatment for AATD. As part of disease management, the coordinators ask a series of structured questions each month, from which they create a database of information on life with AATD. During the study period, questions regarding coronavirus disease 2019 (COVID-19) were added to the structured questions that the coordinators asked during their monthly phone calls with members.
The COVID-19 pandemic was a novel threat to those with underlying lung disease and comorbidities. Early in the pandemic, COPD was a recognized risk factor for poor outcomes, increasing the risk of hospitalization 5-fold.7 In a systematic review and meta-analysis on COPD and COVID-19 outcomes, COPD was found to be approximately a 4-fold risk factor for hospitalization, intensive care unit (ICU) admission, and increase of mortality.8,9 Therefore, this threat to the AATD community was taken seriously.
We hypothesized that the AlphaNet disease management program6 in which monthly AlphaNet coordinator telephone calls were used to support education, would lower COVID-19 acquisition risks, and that AlphaNet members would have high vaccination intent. Weekly telephone education of AlphaNet coordinators occurred throughout the pandemic. Therefore, coordinators were progressively educated on vaccine issues and COVID-19 severity. Whether this influenced the experience at the level of an alpha-1 patient is speculative. We evaluated the prevalence of COVID-19 infections, severe COVID-19 (defined as hospitalizations and ICU admissions), interruptions in augmentation therapy, intention to vaccinate, and self-reported vaccination rates.
Study Design and Methods
All participants in AlphaNet from the United States and Canada were included in this retrospective observational study of the AlphaNet database. Institutional review board approval for analysis of deidentified data was obtained by the Medical University of South Carolina Institutional Review Board. Dates were fuzzed by +/-7 days to maintain deidentification.
COVID-19 Questions Asked Monthly
Questions regarding COVID-19 acquisition (with follow-up questions regarding the number of days of symptoms and severity if applicable) and missed doses of augmentation therapy due to COVID-19 were asked on a monthly basis by telephone from March 2020 through February 17, 2022. The number of people who answered the questions was variable month-to-month, reflecting enrollments and departures from the AlphaNet program. Available responses were analyzed to evaluate prevalence, symptom duration, severity of COVID-19 infection, and causes for missed doses of augmentation therapy. The questions regarding COVID-19 acquisition were designed to identify individuals who tested positive for COVID-19 at least once. Given the possible persistence of a positive COVID-19 test for months due to a single bout of COVID-19, it was not possible to reliably identify the number of individuals who experienced more than one COVID-19 infection. Multiple responses for causes of missed doses were allowed. Four possible causes were queried: (1) concerns that the augmentation therapy site or personnel involved would increase health risk, (2) infusions switched to longer than 1 week apart, (3) nursing and/or supplies were unavailable, and/or (4) augmentation therapy was unavailable.
Vaccination Data
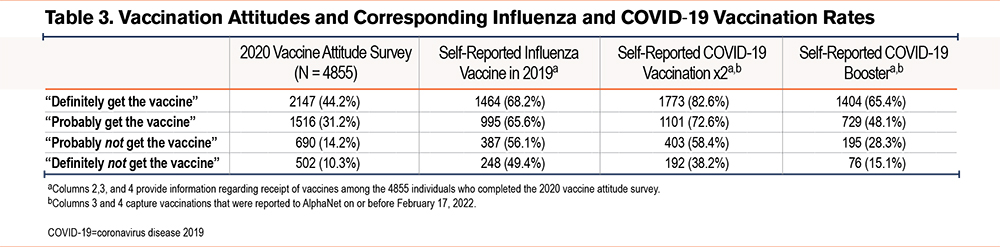
In December 2020, a question was asked regarding intention to get a COVID-19 vaccine, with the following wording: “If a vaccine to prevent COVID-19 were available today, would you…” with 4 response options: (1) definitely get the vaccine, (2) probably get the vaccine, (3) probably not get the vaccine, and (4) definitely not get the vaccine. Starting in January 2021, self-reported COVID-19 vaccination status was captured during monthly contacts with AlphaNet coordinators. In order to examine the association of intention to get a COVID-19 vaccine with past vaccination behavior, self-report of influenza vaccination from the 2019-2020 season was examined.
Data Analyses
Categorical data are presented as numbers and percentages, and continuous data are presented as means with standard deviation and/or medians. Two cuts of the data for the monthly COVID-19 questions were examined: cumulative responses on December 31, 2020, and cumulative responses on February 17, 2022. All available data were analyzed, and missing data were not imputed. The association of vaccination intent, symptom duration, and missed augmentation doses with outcomes was performed with Chi-square analysis or student tests depending on whether variables were continuous or categorical. T-tests were used for comparing means of binary subgroups with a normal data distribution. Analysis of variance was used when more than one comparison of continuous data was performed. Chi-square tests were used for comparing categorical variables. Statistical analysis was performed with JMP 16.0 (Cary, North Carolina) with p-values <0.05 deemed significant.
Results
Responses from 8019 unique individuals with AATD were analyzed, although not all individuals answered all questions. The questionnaire on vaccination intent was answered by 4853 individuals. The demographics of individuals who had a positive test for COVID-19 (N=1473) and those who did not are shown in Table 1. Because entry into AlphaNet is associated with the use of one of the 4 FDA-licensed augmentation therapies (Prolastin-C, Zemaira, Aralast-NP, or Glassia), the majority of respondents had emphysema, an indication for augmentation. The overall cohort was 46.6% male, aged 18–90+ years, with a mean age of 62±11.2 years. Race was predominantly non-Hispanic White, reflecting the known genetics of AATD. Paradoxically, the mean age was younger for individuals who acquired COVID-19 than for the group who never had a positive test.
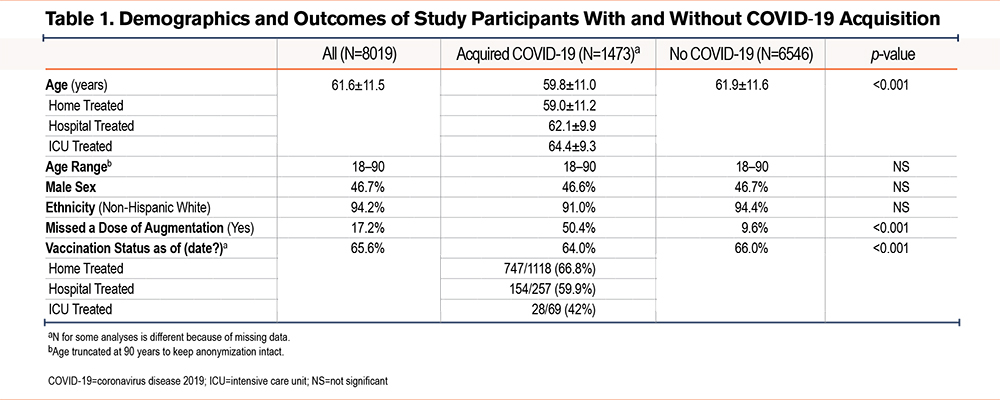
As shown in Figure 1, the prevalence of COVID-19 infection in AlphaNet members mirrored the COVID-19 trends of the general U.S. population, with the largest spike in cases noted during the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Omicron BA.2 variant surge in January 2022. By the end of 2020, 4.% of participants reported a positive COVID-19 test versus 18.6% by February 2022 (Table 2). COVID-19-positive respondents in 2020 noted a long duration of symptoms (13.1±11.5 days), with 37.5% noting a symptom duration > 3 weeks. By February 2022, only 15.6% of those who reported a COVID-19 infection indicated that their symptoms lasted more than 3 weeks, with a mean duration of symptoms of 13.3±11.9 days. Among AlphaNet members with COVID-19 in 2020, 35.3% reported hospitalization, and 8.6% reported ICU admission. By February 2022 these numbers had gone down, with 22.1% of those with a COVID-19 infection reporting hospitalization and 4.7% ICU admission.
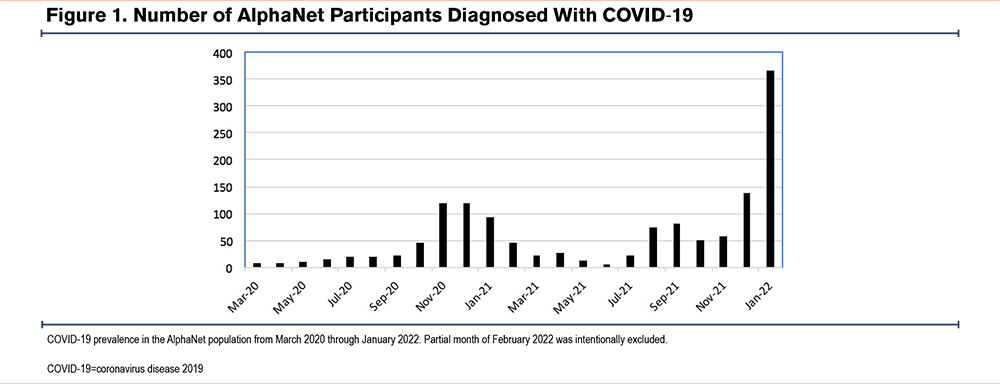
Historically, at least 1 weekly dose of augmentation therapy is missed by 5% to 8% of the AlphaNet population in most years (data not shown). However, 10.2% reported missing at least 1 dose in 2020 and this number had risen to 17.2% by February 2022. Among this group, 70.2% reported only 1 month in which a dose of augmentation therapy was missed. In both data analyses, the most common reason for missed therapy was concern about increased exposure to COVID-19 through home infusion nurse exposure or infusion center travel exposure. This concern was reported by 61.8% of individuals who had missed a dose by the end of 2020 and by 56.2% of individuals who had missed a dose by February 2022. However, missed augmentation therapy due to nursing or supply shortages (22.7% versus 29.0%) or unavailability of augmentation therapy (9.3% versus 13.9%) became more common over time (Figure 2). Missing a dose of augmentation therapy was correlated with COVID-19 acquisition (p<0.001) and with longer duration of symptoms (p<0.001).
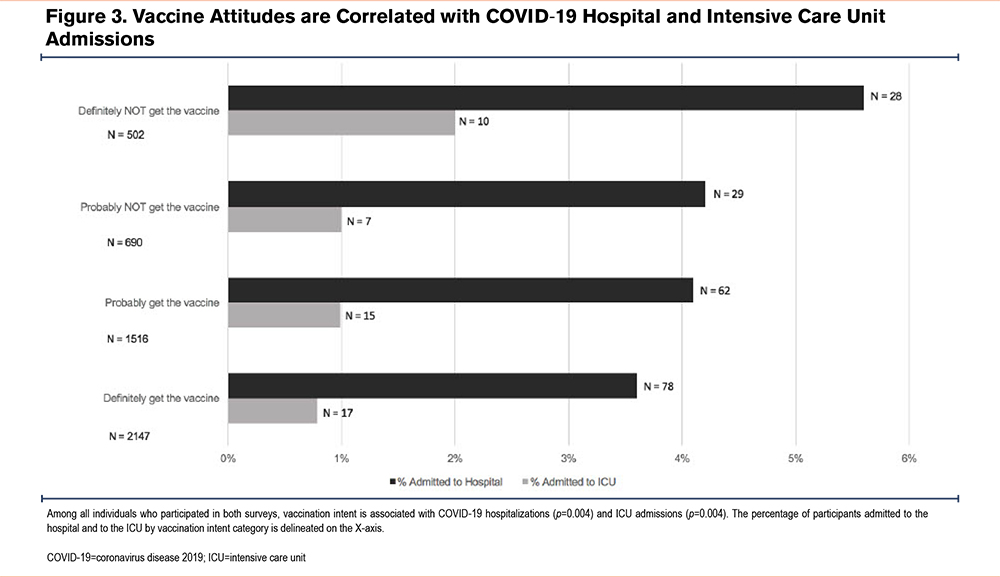
In December 2020, AlphaNet participants were asked whether they would get the COVID-19 vaccine. Of the 4855 respondents, 44.2% responded they would “definitely get the vaccine,” 31.2% “probably get the vaccine,” 14.2% “probably not get the vaccine,” and 10.3% “definitely not get the vaccine”. There was no difference between categories for race or sex (p=NS). Those with definite vaccination intent were older (mean 64.7+/-10.5 years) compared to those with absolutely no vaccination intent (59.0+/-11.2 years) (p<0.0001). Table 3 shows the subsequent COVID-19 vaccination rates and the prior influenza vaccination rates, according to intention to get a COVID-19 vaccine. Figure 3 demonstrates the correlation between vaccination intent and subsequent admission to the hospital or ICU (both p<0.001). There was no difference in gender (P=0.94) or age (P=0.42) between those who answered the vaccine attitude questions (N=4855) and those who did not (N=1896).
Discussion
In the first phase of the COVID-19 pandemic (before the vaccine became available), the AlphaNet population reported lower case rates than the general U.S. population in 2020 (estimated at 31%), with only 4% reporting a positive COVID-19 test.10 Although the cause of low acquisition rates can never be proven, the monthly peer education on COVID-19 transmission given to each member of the disease management program was likely a major cause. The education of coordinators by the medical team occurred weekly and included social distancing measures, optimal mask use, and updates on publications from the COPD literature. Hopefully, without generating unfounded fear, the AlphaNet population was educated on the increased risk for severe COVID-19 disease associated with underlying emphysema-predominant COPD compared to the general population. However, 35.4% of those diagnosed with COVID-19 in 2020 reported hospitalization, significantly higher than the published estimates ranging from 2% to 7% for the U.S. general population11,12 in 2020. Education that both the severity of COPD and age were correlated with severe COVID-19 allowed social distancing measures to be effective.
There are likely many reasons for an increased COVID-19 prevalence by February 2022 to 18.6%. A more contagious virus, requirements to resume work, less perceived risk, less social distancing rigor, and less mask wearing after vaccination likely contributed.13 Also, there were a higher number of cases in 2021 and early 2022 despite the vaccine, similar to the general population. This is likely due to the SARS-CoV-2 Omicron BA.2 variant, which was more contagious and more effective at breakthroughs in vaccinated individuals.
Interestingly, by February 2022 only 15.6% of individuals who had COVID-19 reported symptoms lasting over 3 weeks versus 37.5% in 2020. We suspect that vaccination-associated attenuation of the immune response or a less clinically severe virus contributed to the difference in the duration of persistent symptoms. However, it should be noted that the mean length of symptoms stayed the same at 13 days. The hospitalization and ICU admission rates were slightly higher in 2020 compared to the data collected through February 2022, reflecting similar national demographics14 associated with COVID-19.
Regarding augmentation therapy, the most common reason AlphaNet members missed a dose of therapy was due to a perceived risk of acquiring COVID-19 through contact with health care providers who serve as home nurses or work at an infusion center. However, nursing shortages, supply shortages, and augmentation therapy shortages became more common over time, highlighting both staffing and supply chain disruptions that disrupted regular health care processes.15 Additionally, the group that did have COVID-19 missed a higher percentage of infusions than those who never acquired infection. This observation is worrisome because pneumonias and other clinical respiratory events drive an excess protease burden that augmentation therapy is designed to control. Our opinion is that all efforts should be used to obtain infusions during this time when pneumonia and other exacerbations put AATD-affected individuals in the hospital. Missing an infusion was associated with a longer duration of symptoms.
Self-reported COVID-19 vaccination rates directly correlated with stated intent to vaccinate in December 2020. Also, intention to get a COVID-19 vaccine was associated with a higher self-reported rate of influenza vaccination in 2019-2020. The most surprising finding related to intention to get a COVID vaccine is that among the 10.3% of respondents who stated in December 2020 that they would “definitely not get the vaccine,” 38.2% self-reported that they had received 2 COVID-19 vaccinations in 2021. The factors causing 38.2% to change their minds remain unknown. We speculate that any combination of personal COVID-19 experiences, family members and friends becoming seriously ill from COVID-19, increased perceived risk from COVID-19, education and recommendations regarding vaccination from physicians, and work vaccination requirements played a role.16 A more focused evaluation that includes education level, income level, political affiliation, religiosity, and urban versus rural home site could be helpful in understanding vaccine hesitancy.17,18 From a European study on AATD and COVID-19 vaccination attitudes, overall the fear of severe illness was the major motivation to vaccinate; however, when evaluating those under 50 years old, a desire to socialize was a major motivator.19 Identifying the most effective ways to motivate people to get vaccinated has broad implications even outside of the COVID-19 pandemic. These data could be applied to other vaccinations, such as the annual influenza vaccine, and help with education and motivation should future pandemics and/or the need for other novel vaccinations arise.
Unsurprisingly, we found a direct correlation between favorable attitudes to vaccinations in 2020 and lower rates of reported hospitalization and ICU admissions for COVID-19. Even prior to the COVID-19 pandemic, the World Health Organization named vaccine hesitancy as one of the top 10 threats to global health20 in 2019. Prior studies have shown that despite distrust of the medical system, clinicians are still considered a trusted source of information, and the strength and quality of their recommendations can reduce vaccine hesitancy.21 A major adversary in increasing vaccination rates is social media. On Twitter, anti-vaccination tweets have been shown to be retweeted 4 times more than neutral tweets.20 Studies during the pandemic show that vaccine hesitancy fluctuates over time, corresponding to people whose decision on vaccination is often driven by risk aversion.22 Risk aversion is often linked with anxiety, with individuals more or less sensitive to specific types of risk.22 Our data suggest that peer-to-peer support may play an important role in vaccine decisions.
There are several limitations to this study. AATD is predominantly found in people who identify as White. The median age of this population (63 years) is older than the U.S. population median age (38 years). The number of individuals who provided data in any given month fluctuated over time due to the degree of engagement with AlphaNet services, new participants joining, and other participants leaving AlphaNet services over the course of time. Although these impacts are likely small, they contribute to missing data. All vaccination rates and COVID-19 acquisition rates were self-reported in this population. Thus, COVID-19 prevalence, hospitalization, and ICU admission rates are likely underestimated in this study. Another limitation is that we did not perform modeling of our experience on the basis of genotype, socioeconomic status, geography, severity of underlying disease, or mortality. We were unable to define if COVID-19 occurred more than once in a given participant or if missed augmentation therapy preceded or followed infections. One of the weaknesses of the AlphaNet database is that everyone has AATD, and we do not have a control group.
Interpretation
Being a member of the AlphaNet disease management program was correlated with a COVID-19 prevalence below the acquisition rates in the general U.S. population. Individuals with AATD on augmentation therapy had higher rates of severe COVID-19 (hospitalization and ICU admissions) than the general U.S. population, similar to other populations with COPD. This rare disease population supported by peer interaction had high vaccination intent that was likely enhanced through telephone education. Weekly telephone education of AlphaNet coordinators occurred throughout the pandemic. Therefore, coordinators were progressively educated on vaccine issues and COVID-19 severity. However, a surprising number of individuals who had vaccine hesitancy before the vaccine was widely available ultimately received 2 doses of the vaccine. Peer education in rare disease communities appears to improve health.
Acknowledgements
Author contributions: All authors contributed to the conception and design of the study, acquisition of data, data analysis and/or interpretation, writing the article, and revisions prior to submission. The authors are grateful to AlphaNet, Inc., for providing support for manuscript generation and the coordinators responsible for collecting the survey data.
Declaration of Interest
MAH has no associations or conflicts of interest to disclose. CS has consulted for Bronchus, CSL Behring, Dicerna, Inhibrx, Pulmanage, and Vertex for Alpha-1 and/or COPD. RAS has grants to National Jewish Health from the Alpha-1 Foundation, Grifols, Vertex, and the National Institutes of Health /National Center for the Advancing Translational Sciences. He is a consultant/scientific advisor to Dicerna, Grifols, CSL Behring, Takeda, and Vertex and a medical director with AlphaNet. KEH and JMn receive financial support as research staff employed by AlphaNet.