Running Head: Randomized Controlled Trials on COPD in Africa
Funding Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Date of Acceptance: July 9, 2023 | Published Online Date: July 12, 2023
Abbreviations: 6MWD=6-minute walk distance; AECOPD=acute exacerbation of COPD; ALT=alanine aminotransferase; ARF=acute respiratory failure; AST=aspartate aminotransferase; ATS=American Thoracic Society; AUC=area under curve; AVAPS=average volume-assured pressure support; BiPAP=biphasic positive airway pressure; CENTRAL=Cochrane Central Register of Controlled Trials; CG=control group; CI=confidence interval; CINAHL®=Cumulative Index to Nursing and Allied Health Literature; COPD=chronic obstructive pulmonary disease; CRQ=Chronic Respiratory Disease Questionnaire; ERS=European Respiratory Society; FEV1=forced expiratory flow in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; ICU=intensive care unit; IG=intervention group; IQR=interquartile range; IV=inverse variance; IWI=integrative weaning index; MD=mean difference; MIP=maximal inspiratory pressure; MPT=metronome-paced tachypnea; NPPV=non-invasive positive-pressure ventilation; NR=not reported; PACTR=Pan African Clinical Trials Registry; PEF=peak expiratory flow; PEN=package of essential noncommunicable diseases; PImax=maximal inspiratory pressure; PRISMA=Preferred Reporting Items for Systematic Review and Meta-Analysis; RCT=randomized controlled trial; WHO=World Health Organization
Citation: Kroeber ES, Frese T, Kantelhardt EJ, et al. Randomized controlled trials on chronic obstructive pulmonary disease in Africa: a systematic review. Chronic Obstr Pulm Dis. 2023; 10(4): 422-436. doi: http://doi.org/10.15326/jcopdf.2023.0387
Online Supplemental Material: Read Online Supplemental Material (384KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide.1,2 Of the 3.23 million COPD-related deaths in 2019, 80% occurred in low- and middle-income countries.3 With a mean prevalence of 11.7%,4 COPD affects the lives of over 150million people in Africa.5 The reported prevalence of COPD greatly varies across the continent, ranging from 8.4% in Cape Verde to 24.8% in South Africa.6 These variations are attributed to differing local determinants, risk factors, diagnostic criteria, and procedures.7
The varying and increasing burden of non-communicable diseases such as COPD in African countries is attributed to the aging of the populations and lifestyle changes.8 Major risk factors for COPD are cigarette smoking9 and air pollution.10 Despite several measures to raise awareness and tobacco-control policies,11,12 cigarette consumption is increasing2,13-15 and COPD prevalence is high even among non-smoking young people. 7,14,16,17 Major contributing factors include the use of biomass fuels for cooking and heating in enclosed spaces, environmental pollution, exposure to dust in occupational settings, tuberculosis, childhood respiratory infections,7,16,18 and exposure to ambient air pollution in the megacities.19
COPD is an umbrella term describing chronic lung diseases that cause limitations in pulmonary airflow, with common symptoms such as shortness of breath, wheezing, excessive sputum production, and a chronic cough.20 These symptoms persist with only small day-to-day variations, usually starting in middle age and slowly worsening over time.21 Due to the high individual health and financial burdens of acute exacerbations of COPD (AECOPDs), interventions in stable phases and effective tertiary prevention are crucial.21,22 Currently, there are no national guidelines on the diagnosis and treatment of COPD in African countries except in South Africa.7,8,23 Well-established international guidelines24,25 are rarely applicable due to the low availability of diagnostic tools and affordable treatment, especially in rural areas.14,17
The primary aim of this systematic review is to map the best available evidence of interventions for secondary and tertiary prevention, diagnosis, and treatment to achieve symptom control and to prevent exacerbations in African patients.
Methods
We registered a protocol for a systematic review to summarize studies on chronic obstructive respiratory diseases conducted in Africa on PROSPERO (CRD42020145057), an international prospective register of systematic reviews. This systematic review summarizes a subsample of studies on patients diagnosed with COPD from studies found in the systematic search. All steps were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews (Figure S1 in the online supplement).26
Inclusion and Exclusion Criteria
We included full-text publications of randomized controlled trials (RCTs) with COPD patients from African countries in stable or acute phases of COPD that reported results on our predefined primary or secondary outcomes. Included RCTs focused on any preventive, diagnostic, or treatment interventions for patients with COPD. Studies on primary COPD prevention that included participants without COPD, as well as international multi-center studies with fewer than 50% of centers in African countries, were excluded. Table 1 details our inclusion criteria.
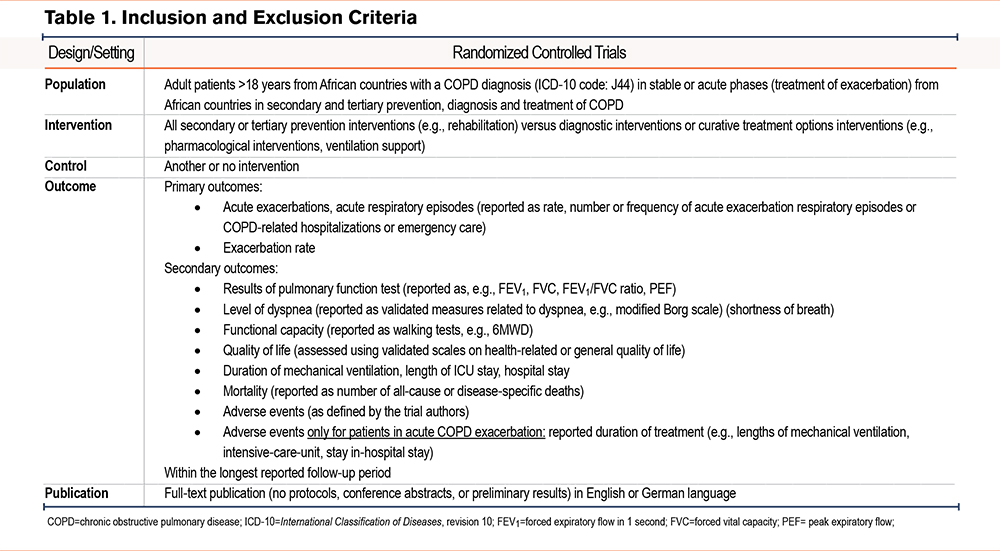
Systematic Search
We performed a systematic search in electronic databases, including MEDLINE®, the Cochrane Central Register of Controlled Trials (CENTRAL), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and specialized African databases such as African Journals Online and African Index Medicus, without time restrictions until January 2022. Furthermore, we checked publications from studies registered in the World Health Organization (WHO) Pan African Clinical Trials Registry (PACTR), screened reference lists, and contacted corresponding authors of included studies as well as members of Guidelines International Network Africa. Search strings based on the National Library of Medicine’s Medical Subject Headings included terms on chronic obstructive lung diseases, including COPD, Africa, all 54 African countries, and terms related to RCTs (see full search strategy in the online supplement).
Study Selection and Data Extraction
Two authors independently screened titles and abstracts of all references as well as potentially eligible full-text articles. Data extraction was done by one author and checked by another author. Disagreements were solved by discussion.
Risk of Bias Assessment
Two authors independently judged the risk of bias for each study using the Cochrane risk of bias tool in 7 specific categories: (1) sequence generation, (2) allocation concealment, (3) blinding of participants/personnel, (4) blinding of outcome assessors, (5) incomplete outcome data, (6) selective outcome reporting, and (7) other sources of bias. They rated the risk of bias as low, high, or unclear.27 Discrepancies were resolved by discussion.
We defined the risk of bias due to incomplete outcome data as high if more than 10% of randomized participants dropped out from analysis. We judged the risk of bias due to selective outcome reporting as low if study protocols with predefined primary and secondary outcomes were available, and high if any results of pre-planned outcomes were missing. Other sources of bias were judged as high risk in cases in which there were missing descriptions of, or relevant deviations from, a pre-planned sample size calculation, no description of a primary endpoint, or relevant differences in main baseline characteristics between the intervention and control groups.
Data Synthesis
We initially subdivided interventions according to the included patients’ conditions (stable versus acute), and then narratively summarized studies according to the type of intervention. We did not perform a pre-planned meta-analysis due to the substantial heterogeneity of interventions and outcomes. We produced forest plots with Cochrane RevMan software28 to visualize treatment effects.
Results
We identified 1819 references, screened 1594 references, read 161 potentially eligible articles, and included 18 studies (reported in 19 articles) on African patients diagnosed with COPD. We excluded 51 articles reporting on asthma patients and 5 articles on patients with other chronic obstructive lung diseases (Figure 1 and “the Included Studies” list in the online supplement). We grouped patients into 2groups: stable patients in outpatient settings, and patients with AECOPDs treated in intensive care units (ICUs) or emergency care settings. The interventions mainly included ventilatory support and pharmacological and rehabilitative interventions.
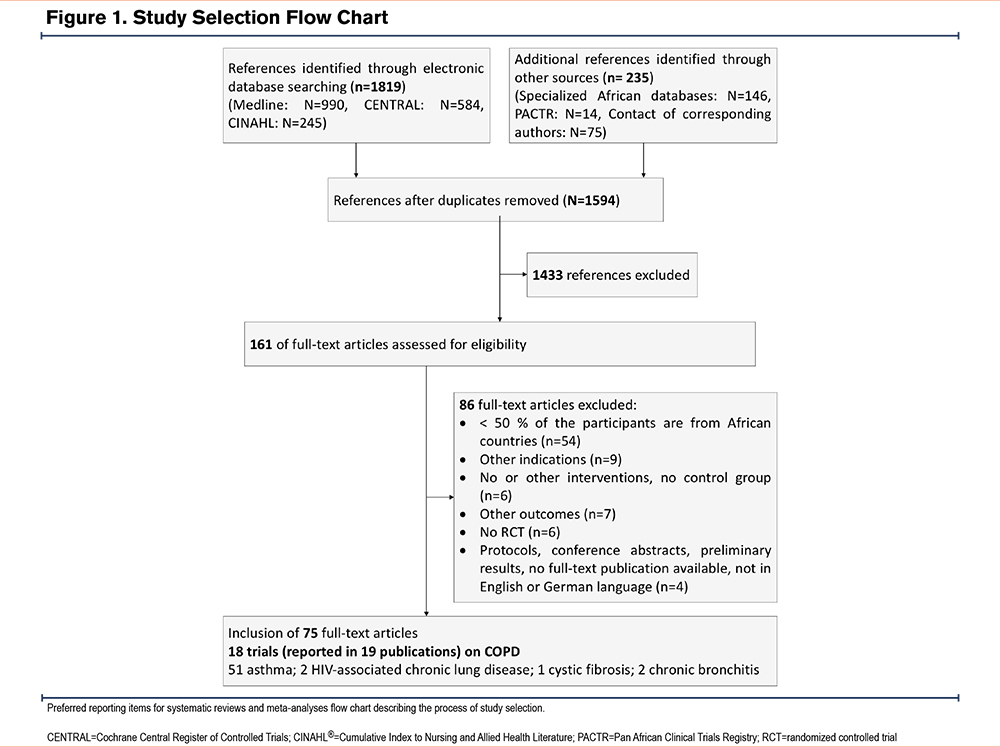
Study Characteristics
Setting
All studies were conducted either in Egypt,29-37 Tunisia,38-44 or South Africa.45,46 Ten studies were conducted in ICUs,29-31,36,38,43,44 emergency units,40 or specialized chest disease departments37 of tertiary hospitals, and included patients in acute conditions. Eight studies were set in outpatient departments and included patients in stable condition.32-35,39,41,42,45,46
Population
A total of 17 RCTs involved 1480 participants, and one crossover RCT that randomized an additional 24 participants to 6 diagnostic procedural variants.46 Eleven studies reported the inclusion of women (between 9% and 45%). The participants’ mean age ranged from 47 to 69 years. Over 90% of the participants had a smoking history, 3 studies excluded recent smokers.29,39,41 Most studies utilized the 2020 Global initiative for chronic Obstructive Lung Disease (GOLD) criteria47 to diagnose COPD,30,38,40,41 and classified airflow limitation as moderate,35,46 moderate to severe,32,34,36,37 or severe.42 Other studies based the diagnosis on the criteria48 of the American Thoracic Society,35,36 pulmonary function testing,39 or a clinical diagnosis and pulmonary function testing.29,31,33,43-45
Interventions and Results
Two studies reported results on our planned primary outcomes of acute respiratory episodes and exacerbation rates. Magdy et al34 compared the effects of 2 nightly ventilation support regimens: spontaneous-timed average volume-assured pressure support (AVAPS), and biphasic positive airway pressure (BiPAP), in stable outpatients with hypercapnic respiratory failure over 6 months. The interventions showed no difference in treatment effects on the change in the number of exacerbations (mean difference [MD] -0.9; 95% confidence interval [CI] -0.9 to 0.7), hospitalizations (MD -0.1; 95% CI -0.6 to 0.4), or hospital days (MD -1.5; 95% CI -5 to 2) over a 6-month period with different support systems. However, patients in the AVAPS group showed slightly better exercise tolerance with a higher change in the 6-minute walk distance (6MWD) of 9.2m (95% CI -1 to 15) and quality of life (QoL) scores. Nouira et al44 compared the effects of the antibacterial agents trimethoprim-sulfamethoxazole and ciprofloxacin in the treatment of severe exacerbations in ICUs, finding no difference in exacerbation-free intervals (14 days; 95% CI -15 to 43).
Interventions for Patients in Stable Phases of COPD
Eight studies included stable patients32,34,35,37,39,41,42,46 with moderate to severe COPD (if reported) 34,35,37,41,46 where interventions were prescribed and conducted in outpatient settings or at home (Table 2).
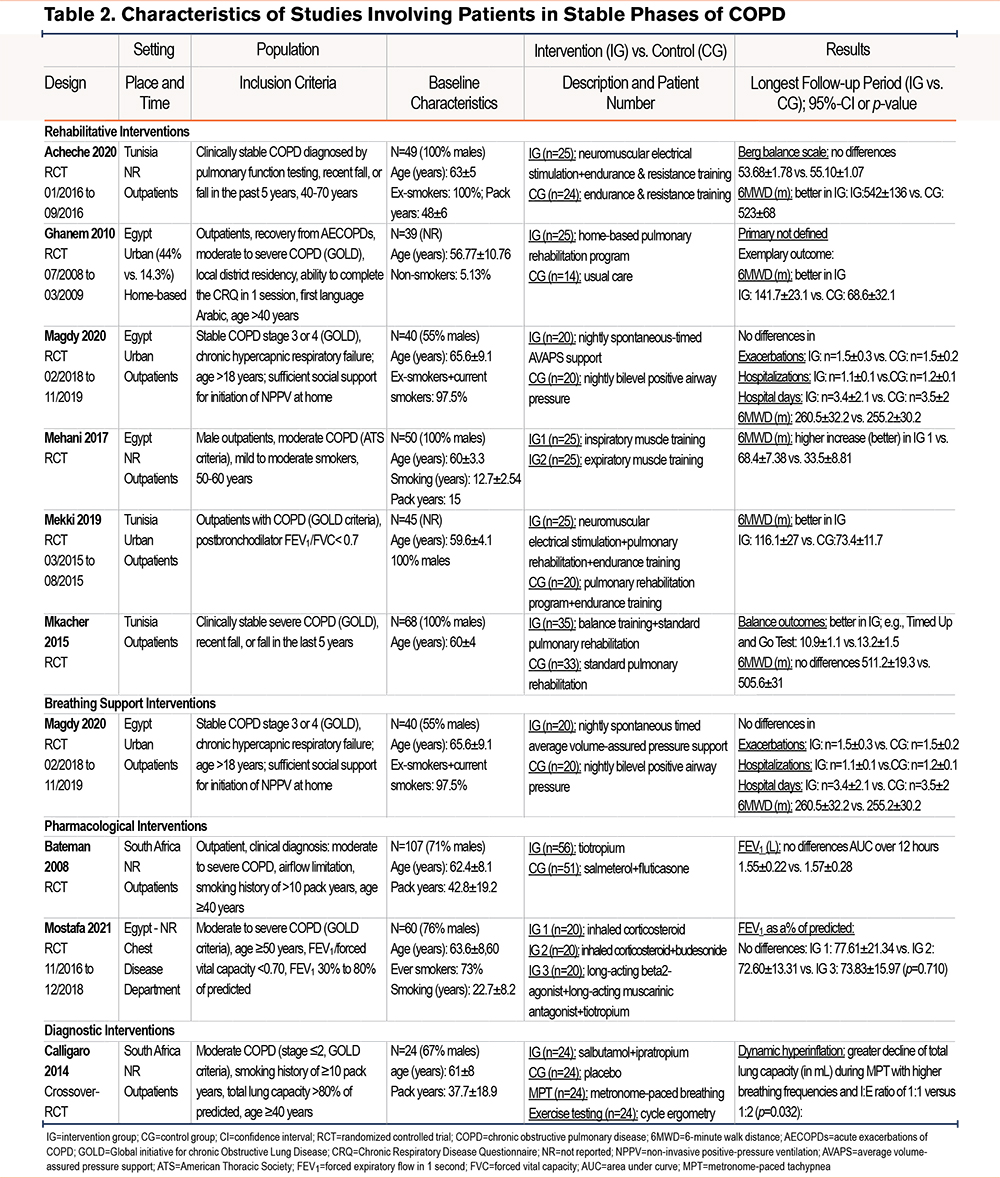
Rehabilitative Interventions
Six studies tested rehabilitative interventions including physical activity, balance training, home-based pulmonary rehabilitation, neuromuscular electrical stimulation, inspiratory muscle training, and nightly spontaneous-timed AVAPS ventilation,32,35,39,41,42 and compared these interventions to usual care or other active component (Figure 2).
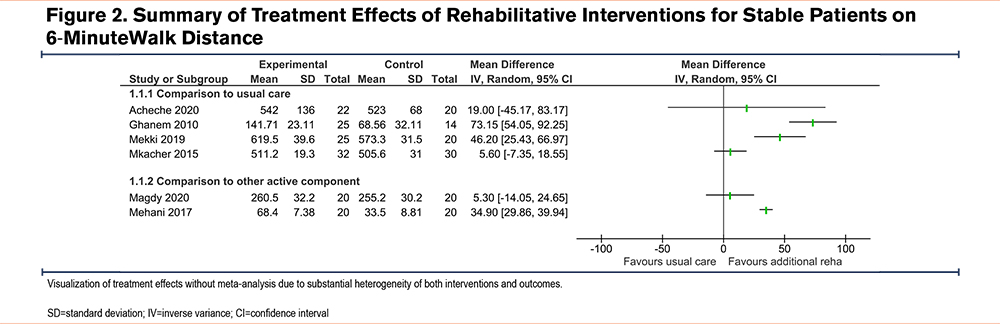
All these studies reported on exercise tolerance (all studies reported on 6MWD) showing beneficial, yet very heterogeneous treatment effects, with an MD between 5.3m (95% CI -14.0 to 24.6) and 73.2m (95% CI 54.0 to 92.2) (Figure 2). A clinically relevant difference in the 6MWD of over 25m, which was judged as clinically relevant for patients with COPD,49 was gained in 3 studies.32,35,41
The highest exercise tolerance benefit was reported from a home-based pulmonary rehabilitation program that included educational lectures and muscle training, MD 73.2 m (95 %CI 54.1 to 92.2). The study also described improved health-related QoL scores.32 Two studies trialing neuromuscular electrical stimulation39,41 in addition to standard pulmonary rehabilitation programs, showed improved balance and exercise parameters. Finally, a comparison of training inspiratory versus training expiratory muscles35 showed improved pulmonary function parameters and 6MWD in both groups but showed little to no between group differences. Magdy et al tested 2 nightly breathing support regimens in stable COPD outpatients with chronic hypercapnic failure.34 Both interventions had beneficial outcomes with AVAPS showing additional improvements in exercise tolerance and in several QoL domains.
Other Pharmacological and Diagnostic Interventions
Two studies compared pharmacological interventions with comparable results in pulmonary function and dyspnea parameters.37,45 Calligaro et al tested ways to provoke dynamic hyperinflation showing the feasibility of metronome-paced tachypnea as an alternative to exercise testing.46
Treatment of Patients in Acute Exacerbation Phases of COPD
Ten studies studied patients with AECOPDs in the ICU29-31,36,38,43,44 or in emergency departments40 requiring mechanical ventilation or included outpatients.33,45 Seven studies29,33,38,40,43-45 investigated the efficacy of medication, and 3 studies compared different ventilation treatments.30,31,36 The studies reported results on ventilation support outcomes,30,31,38,43,44 length of ICU31,36,38,43,44 or hospital stay,30,31,40,43,44 mortality,29,31,36,38,40,43,44 and adverse events including hyperglycemic episodes, ventilator-associated pneumonia, nausea, tremors, and headaches29,36,38,40,43,44 (Table 2 and Table 3 and Figures S1 to S4 in the online supplement).
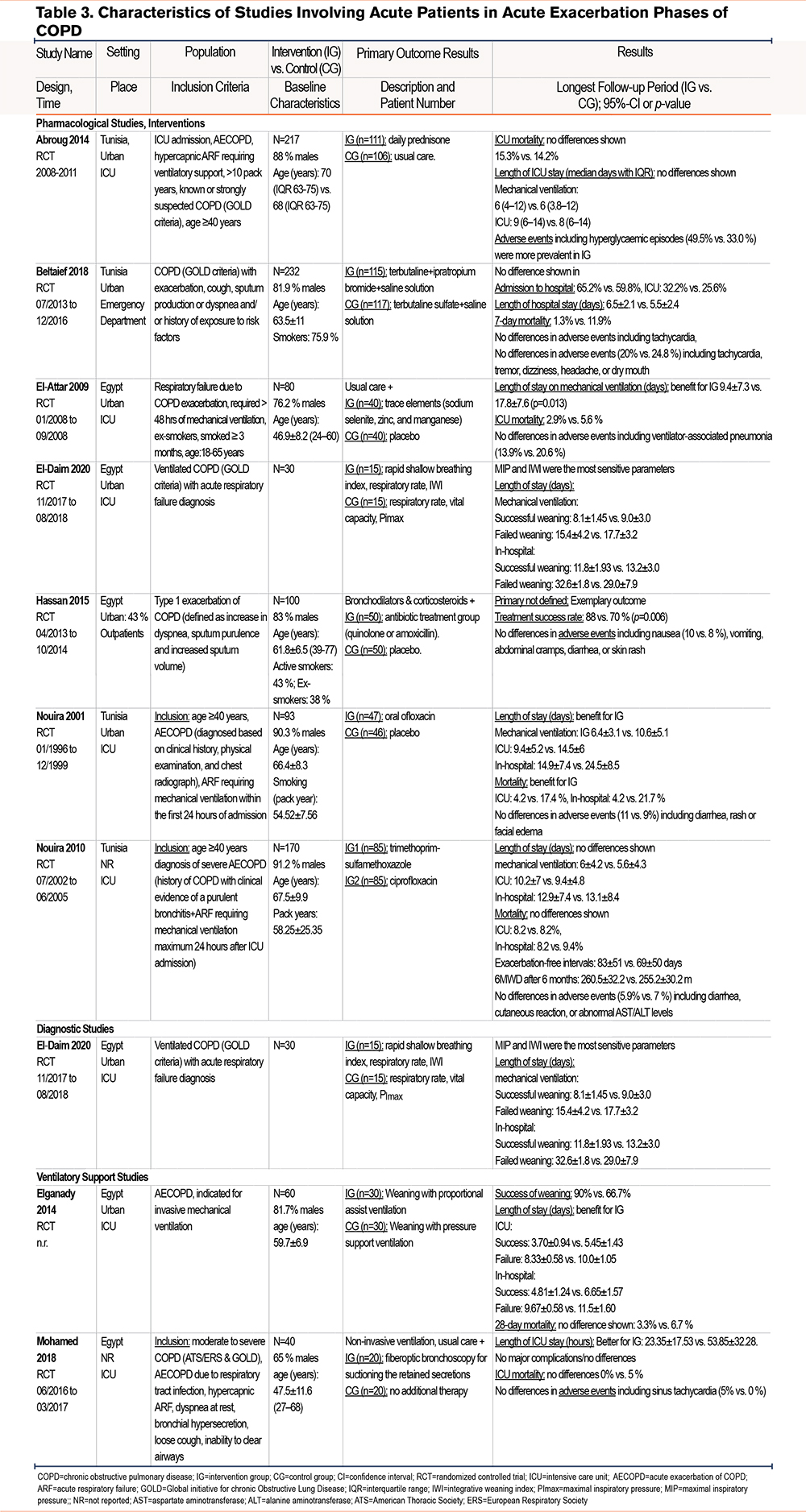
Pharmacological Interventions
Three studies33,43,44 investigated the effects of antibiotics. Two of them stated the benefit of antibiotic therapy for patients with exacerbations Nouira et al43 compared ofloxacin to placebo and stated reduced mortality, time on mechanical ventilation, and hospital stay. Additional treatment with quinolone or amoxicillin resulted in a shorter treatment time and a higher treatment success rate.33 No differences were shown between distinct antibacterial agents (trimethoprim-sulfamethoxazole and ciprofloxacin).44
Only one of the other pharmacological studies29,38,40 showed some benefit for patients with exacerbations. A small study including 80 patients with COPD exacerbations showed that intravenous supplementation of the trace elements selenium, manganese, and zinc during mechanical ventilation can reduce the length of ventilation by 8.4 days (95% CI 5.1 to 11.7) and slightly reduce mortality and adverse events in the ICU29 (Table 2).
Breathing Ventilatory Support
Weaning was more often successful with proportionally assisted ventilation than with pressure support ventilation, resulting in shortened duration of mechanical ventilation and hospital stay (relative risk: 1.35; 95% CI 1.02 to 1.79).31 Due to the importance of successfully weaning patients from mechanical ventilation, El-Daim et al 30 investigated different predictors of successful weaning and stated sensitive parameters. Another study36 investigated the therapeutic utility of fiber-optic bronchoscopy to suction retained secretions in patients with noninvasive ventilation as an alternative to intubation and was able to reduce stay in the ICU by 30.5 hours (95% CI 14.4 to 46.6) without major complications.
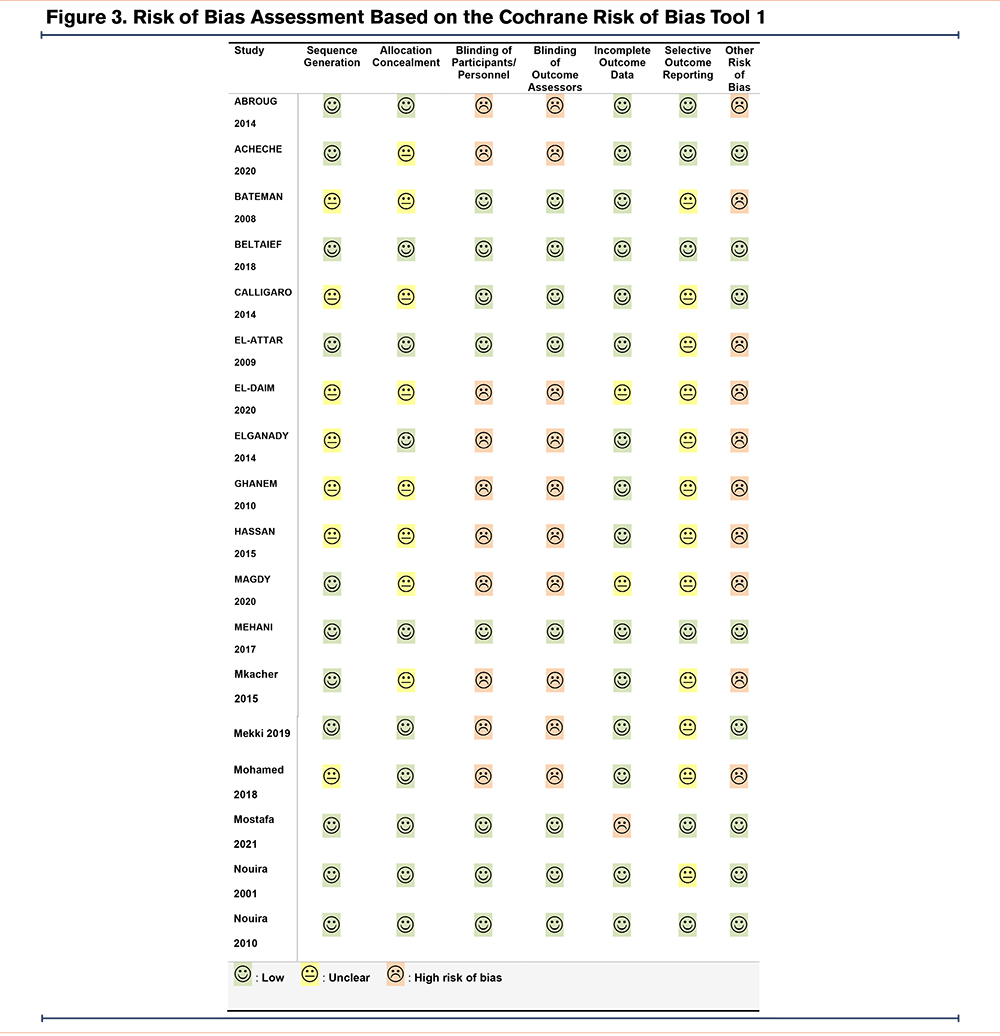
Risk of Bias
Adequate information on sequence generation and allocation concealment was reported for 8 studies. Most studies analyzed more than 90% of randomized participants in their primary analyses. The risk of selective outcome reporting was checked in 7 studies with published protocols and judged as low. Other sources of bias were identified in 10 studies, either due to missing descriptions of primary endpoints or pre-planned sample sizes, relevant deviations from the pre-planned sample sizes, or missing descriptions of main baseline characteristics (Figure 3).
Discussion
This systematic review aims to map the available high-quality evidence of management options for patients with COPD conducted in Africa. The included studies investigated patients in stable phases of COPD treated in outpatient settings and those experiencing AECOPDs treated in emergency or intensive care settings. The interventions involved ventilatory support and pharmacological and rehabilitative interventions. At present, RCTs are sparse and heterogeneous, but the number of studies and the frequency of publications have grown over the last 2 decades. As of today, all RCTs were conducted in 3 countries with comparably high infrastructural development. No studies have been conducted in Central, East, or West Africa.
Early Detection, Diagnosis, and Initial Assessment and Diagnosis of COPD
Reliable diagnostic interventions are crucial to initiate adequate treatment, inform patients about their condition, monitor the disease, and prevent exacerbations.50 Diagnostic studies with patient-related outcomes are generally rare.51 We identified only 1 small crossover trial that tested a simple standardized alternative to usual exercise testing for early diagnosis and assessment.46 The WHO recommends spirometrically measuring the peak expiratory flow rate for patients presenting typical COPD symptoms. Underutilization due to high costs and the need for trained staff is a major reason for the under- and over-diagnosis of COPD.52 There is a need for implementation research on how to effectively implement high-quality diagnostic infrastructure, especially spirometry, for chronic lung diseases in Africa.53,54
Treatment of Patients in Stable Phases of COPD
The 6 included studies that tested rehabilitative interventions in the management of COPD32,34,35,39,41,42 all showed some beneficial results as an additional component to usual care. Even though the studies were very heterogeneous in both intervention type as well as outcomes, these rehabilitative efforts are diverse and are showing promising results, offering a first glimpse into a future where COPD interventions that have been trialed in African countries can be used in continent-specific guidelines. Stating this positive trend, it has to be said that these 6 studies are currently only being conducted in Tunisia and Egypt, and 4 of them were conducted solely with male participants in urban areas,35,39,41,42 leaving out vulnerable groups such as women and people living in rural areas, who have a higher exposure to indoor air pollution and the associated increased COPD risk.55-57 This leaves a big leap to be taken to implement research structures that represent different African populations more distinctly.
Treatment of Patients in Acute Exacerbation Phases of COPD
Most of the included studies stated at least one beneficial change in clinically relevant outcomes in the intervention group.29,31-36,39,41,43 The existing evidence is mainly concentrated on different pharmacological interventions (Table 3). Since ICU treatment is cost- and infrastructure-intensive, there are considerable hurdles to its implementation and utilization in many low-resource settings. Self-management interventions with prescribed drugs for AECOPDs are proven to reduce the delay in seeking treatment, lower the risk of hospitalization, and improve QoL.22,58
Association Between Prevalence and Research
The geographic distribution of the randomized studies we included does not reflect the distribution of COPD prevalence with the highest rates in the southern and eastern African regions.4 Only 2 of the included studies were conducted in South Africa, where it is known that prevalence rates are high, while most studies were conducted in Tunisia and Egypt, where prevalence rates are comparably low.4,59 Despite the increase in COPD prevalence research in recent years, and COPD being considered a relevant health problem, there is a lack of awareness of the ongoing burden of COPD that exists in many African countries.4,6,60,61 Research activities are affected by infrastructural conditions including access to essential medications, ICU capacities, research-supportive environments, funding, or trained personnel than by the burden of disease.62-64
Infrastructural Aspects of COPD Research
Hospital care for COPD is often costly due to acute medical treatment in ICUs.65 Egypt, South Africa, and Tunisia rank second, fourth, and sixth, respectively on the 2018 African Infrastructure Development Index.66 About half the studies were set in ICUs, studying AECOPD interventions. On average, there are 3.1 ICU beds per 100,000 capita throughout the African continent,64 whereas the European mean is 11.5. Egypt, South Africa, and Tunisia have an estimated 11.2, 5.7, and 4.3 ICU beds, respectively, per 100,000 capita. Other African countries have much lower ICU capacities (e.g., Nigeria: 0.2; Ethiopia: 0.5). Moreover, the availability of standard treatment options such as salbutamol in public health facilities varies greatly on the African continent, ranging from 81% to 100% availability in Tunisia, to below 5% in countries like Mali and Nigeria.63 The improvement of medical infrastructure, including ICU capacities, access to medication, funding, and training of research personnel is required to support high-quality research.62,67,68
Low-Resource Contexts
Six out of 7 studies trialing pharmacological treatments33,38,40,43-45 tested management options from the WHO essential medicines list69 and from the WHO Package of Essential Noncommunicable (WHO PEN) Disease Interventions for Primary Health Care50 that can be used in low-resource settings. There is a strong need to provide effective and affordable long-term primary care treatment to prolong the duration of stable clinical periods, manage and provide rehabilitation from AECOPDs, and prevent adverse events. 50
Strengths and Limitations
The main aim of this review was to map, describe, and discuss the characteristics and results of all RCTs related to the prevention, diagnosis, and treatment of chronic obstructive respiratory diseases in African countries. We initiated, registered, and used all methods of a systematic review and visualized treatment effects. Due to this broad question, a scoping review with no synthesis of findings from individual studies might have been an alternative.70 We, therefore, decided to visualize, but not synthesize, treatment effects.
This review is the first to summarize RCTs on the management of patients with clinically diagnosed COPD in African countries. We did not include studies on individual and community-based primary prevention of COPD, since these are generally not COPD-specific.71-73 Nevertheless, these interventions clearly have a large impact on tackling the burden of COPD and should be considered when implementing care. However, the more specific focus on RCTs maps the current research landscape on high-quality quantitative research for COPD patients.
This review aims to emphasize research primarily initiated, planned, and conducted in African countries. We excluded several multinational studies with few African centers that provide training for researchers to improve skills in scientific methodology, study design, and study conduction. The small number of included studies, as well as the heterogeneity of interventions and outcomes, limits the current possibility of building specifically adapted COPD guidelines in African countries.
Conclusion
This systematic scoping review summarizes heterogeneous COPD interventions with a wide range of outcomes and results. The available evidence was compiled in 3 countries with comparably high infrastructural development, highlighting the urgent need for comprehensive technical infrastructure implementation and capacity building in African countries. Due to the increasing COPD burden, studies on early identification approaches and preventive primary care for high-risk populations are highly needed.
Acknowledgements
Author Contributions: SU, BN, EJK, TF, MT, and AS contributed to the conception and design of this systematic review. SU guarantees methodological quality. SU organized and performed the systematic search and updates with support from ESK and BN. SU, ESK, AS, and BN contributed to the title-, abstract-, and full-text screening and data extractions. SU and ESK built the tables. SU and ESK wrote the first draft, and EN wrote sections of the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
Kathleen Denny corrected and proofread the manuscript.
Data availability statement: No additional data available.
Declaration of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.