Running Head: Circulating Exosomes and Air Pollution in COPD
Funding Support: Funding provided by Tehran University of Medical Sciences grant 97-01-93-38044.
Date of Acceptance: August 22, 2023 | Publication Online Date: September 6, 2023
Abbreviations: CCs=cubic centimeters; CI=confidence interval; COPD=chronic obstructive pulmonary disease; DLS=dynamic light scattering; ELISA=enzyme-linked immunosorbent assay EVs=extracellular vesicles; IFV- 𝛾=interferon gamma; IL=interleukin; kVs=kilovolts; µm=micrometers; MV=microvesicle; nm=nanometer; PBS=phosphate buffered saline; PM=particulate matter; PM2.5 =particulate matter with particulates <2.5 µm in aerodynamic diameter; PM10=particulate matter with particulates <10 µmin aerodynamic diameter; sTNFR1=soluble tumor necrosis factor receptor- 1; TEM=transmission electron microscopy; TGF-β1=transforming growth factor-beta1; TNF-α=tumor necrosis factor-alpha; UTM=Universal Tranverse Mercator
Citation: Soleimanifar N, Assadiasl S, Kalateh E, et al. Circulating exosomes and ambient air pollution exposure in COPD. Chronic Obstr Pulm Dis. 2023; 10(4): 412-421. doi: http://doi.org/10.15326/jcopdf.2023.0400
Online Supplemental Material: Read Online Supplemental Material (163KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive inflammatory airway obstruction mainly caused by sustained exposure to environmental pollutants such as cigarette smoking, occupational smoke, and air pollutants, in genetically susceptible individuals.1 COPD is a leading cause of death among patients with chronic lung diseases. In 2019, the global prevalence of COPD among people aged 30–79 years was 10.3%, which means that nearly 400 million people are affected by this disease.2 The most common cause of COPD, with a prevalence of about 80% in adult patients, is smoking. However, evidence suggests that 17% to 38% of COPD patients are nonsmokers, so other factors such as occupational and air pollution may also play a role.3
Air pollution is one of the most challenging health concerns, particularly in developing countries, and is reported to be associated with inflammatory disease exacerbation and almost 7 million premature deaths annually.4,5 Air pollution is defined as an excessive aggregation of particles, noxious gases, and vapors emitted from industrial and natural sources. The most monitored pollutant is particulate matter (PM), a combination of liquid and solid particles suspended in the air, known as PM10 (particulates <10 micrometers [µm] in aerodynamic diameter) and PM2.5 (particulates <2.5 µm in aerodynamic diameter) according to their size.6 There is also growing evidence of the implication of air pollution in the initiation and progression of respiratory diseases including COPD.7,8 However, due to the lack of sufficient knowledge of the molecular mechanisms that correlate the microparticles with inflammatory responses, no specific modality can be introduced for the prevention or treatment of air pollution-related COPD. Nonetheless, air pollutants have been shown to trigger the production of inflammatory mediators9-11 and extracellular vesicles (EVs).12
EVs are membrane vesicles released from different types of cells and detected in various body fluids such as blood, urine, bronchoalveolar lavage, pleural effusion, and nasal secretions. EVs containing proteins, lipids, nucleic acids, and metabolites are thought to be involved in cell-to-cell interactions.13 EVs are classified into 3categories based on size, biogenesis, and secretory components: exosomes, cellular microvesicles/microparticles, and apoptotic bodies. Exosomes are distinguished from other EV classes by their morphology, their endosomal origin (they are generated by early and late endosomes, and eventually, form multivesicular bodies), and their small size, approximately 30–150 nanometers (nm),14-16 while microvesicles (MVs) and apoptotic bodies are larger in size, > 100 nm and 1–5μm, respectively.17 The CD9, CD63, CD81, and CD82 peripheral membrane proteins (tetraspanins) are fused during release into the extracellular space and used as exosomal markers.18 Exosomes deliver cytokines such as transforming growth factor-beta (TGF-β), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6 (IL-6), IL-8, and IL-10 to the recipient cells, leading to the development of chronic inflammation, oxidative stress, cell apoptosis, and decreased lung function with a pivotal role in the occurrence and progression of COPD.19 Among the cytokines mentioned above, IL-6 is an acute phase protein, a marker of the high systemic inflammation associated with airway epithelium in COPD. Interferon-gamma (IFN-𝛾) enhances COPD by decreasing the number of Th2 cells and shifting the immune response toward COPD exacerbation. TGF-β directs numerous immune cell activities and promotes fibrotic remodeling of airways, which further diminishes lung function in COPD.
Based on the dynamics of the production and release of EVs and their variable contents, they may serve as biomarkers to monitor the inflammatory status of tissue in response to external stimulants. Therefore, EVs, in particular exosomes, may be eligible candidates for investigating the effects of air pollution on inflammatory events in the respiratory system.
Patients and Methods
Patients
Twenty nonsmoker patients with COPD who were admitted to the Imam Khomeini Hospital (Tehran, Iran) were recruited. The diagnosis was approved by a specialist according to the Global Initiative for Obstructive Lung Disease criteria.20 The patients were middle-aged (46.5±13.8 years ). All patients were receiving anticholinergics, long-acting beta2-agonists, and inhaled corticosteroids but none were under systemic immunosuppressive agents at the time of sampling. Twenty healthy age-matched (44.4±12.6 years), nonsmoker individuals with no clinical evidence of COPD were included as controls. None of the studied participants had signs or symptoms of infectious, allergic, or autoimmune disorders at the time of sampling. The study was approved by the Ethics Committee of Tehran University of Medical Sciences (EC: IR.TUMS.CHMC.REC.1397.045). All the techniques carried out in the study were in accordance with the standards of the institutional research committee and with the Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants after describing the process and goals of the study.
Isolation and Illustration of Exosomes
The serum was separated from 5 cubic centimeters (CCs) of fresh peripheral blood by centrifuging the sample at 2500 rpms for 5 minutes. Serum samples undergo the pre-purification stage by 2-step centrifugation at 2000xg for 10 min (4 °C), followed by 10,000xg for 30 min (4 °C) before transferring to a 0.22µm centrifugal filter (Sigma-Aldrich, Missouri) for removing cell debris and particulate impurities from samples.
Exosomes were isolated from serum using the Exocib kit (Cibzist, Tehran, Iran).21,22 Based on the sedimentation method, according to the manufacturer's procedure, the collected supernatant was mixed with pre-heated reagent A (ratio 5:1), and vortexed for 5 min. Then, the mixture was incubated for 24 hours at 4 °C. After vortexing for 1 minute, the mixture was centrifuged at 3200×g for 40 minutes at 4 °C and the supernatant was discarded. The exosome pellet was resuspended in 500µL of reagent B and stored at - 80°C. Then isolated exosomes were first analyzed by dynamic light scattering (DLS) to determine the distribution of the exosomes’ particle size. Then the morphological assessment of the isolated exosomes was performed using transmission electron microscopy (TEM). Briefly, exosomes were deposited onto formvar-carbon coated grids for 20 minutes, blocked with 0.5% bovine serum albumin/phosphate-buffered saline (PBS) for 10 minutes. Grids were fixed with 1% glutaraldehyde/PBS for 5 minutes, washed with distilled water, and contrasted using 0.5% uranyl acetate for 2 minutes. Excess stain was blotted off and grids were dried for ≥2 days. Observations were carried out using a JOEL 2100 electron microscope at 120 kilovolts (kVs).
CD-81+ Exosomes Counting
A preferred method of counting exosomes was performed using the flow cytometry technique.23,24 In brief, 0.01 dilution of extracted samples were stained based on the manufacturer's procedure of perfect-count microspheresTM (Cytognos, Salamanca, Spain) which is a microbead-based single-platform system combined with monoclonal antibodies conjugated with fluorochrome.
Anti-CD81 conjugated antibodies (349505, BioLegend, Austria) were used to stain the exosomes due to the accumulation of CD81, a tetraspanin molecule used as exosomal markers, in small EVs. Exosome counts were calculated using conventional flow cytometry by the addition of a known amount of counting beads and the formula: MV/μl=(MV count/bead count)×final bead concentration. Flow cytometry was performed by FACSCalibur (BD FacsCalibur, Becton Dickinson, Franklin Lakes, New Jersey ) and data were analyzed with FlowJo v10.7 software (TreeStar Inc., Ashland, Oregon).
PM2.5 and PM10 Monitoring
Tehran, the capital and most populated city of Iran, is located in a relatively limited area of the Alborz mountains at Universal Transverse Mercator (UTM) coordinates 35° 34–35° 50′ N and 51° 08–51° 37′ E. The city includes a total area of 730 km2 with around 9 million dwellers frequently facing metropolitan air pollution and dust storms. The heavy and steady vehicle usage and traffic jams of Tehran, the use of substandard and poor-quality fuels, and poor air recycling because of the geographic location lead to particulate air pollution that reaches usually above the permissible levels. The concentration of airborne particles PM2.5 and PM10 are measured and recorded regularly (every hour) with 32 air quality monitoring stations for measuring PM2.5, and 19 stations for measuring PM10 (Tehran Air Quality Control Company). In the present study, we used the mean of the daily, weekly, and monthly values based on the longitudinal measurements of the monitoring stations. Each missed or unavailable data was modeled with GIS software and satellite information, and the correct values of conflict were obtained for each person. A residence area was defined as a place where the participant spends at least 10 hours during the daytime. The participants resided in different districts of the Tehran province.
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay (ELISA) tests were performed using human IFN-γ (ZB-0105-H9648, ZellBio, Germany), human IL-6 (ZB-0090-H9648, ZellBio, Germany), and human TGF-β1 (ZB-0134-H9648, ZellBio, Germany) kits according to the manufacturer's instructions with the sensitivity of 1.5ng/mL, 3.5pg/mL, and 5.11ng/mL, respectively. All samples were examined in duplicate. Absorbance was read using the Hiperion MPR4 ++ Microplate Reader (Medizintechnik GmbH & Co., KG, Lenzkirch, Germany). The calibration curves were drawn to determine the concentration of the cytokines.
Statistical Analysis
Data were displayed as mean±standard deviation or mean±standard error of the mean. The Mann-Whitney test was used to compare the continuous values within the categorical variables. The correlation was determined as correlate bivariate and Pearson correlation coefficient (r). The graphs were presented as box plots and dot plots. P-values less than 0.05 were considered significant (SPSS 26.0; SPSS Inc., Chicago, Illinois).
Results
Patients
The demographic, paraclinical, and clinical findings of the study are summarized in Table 1.
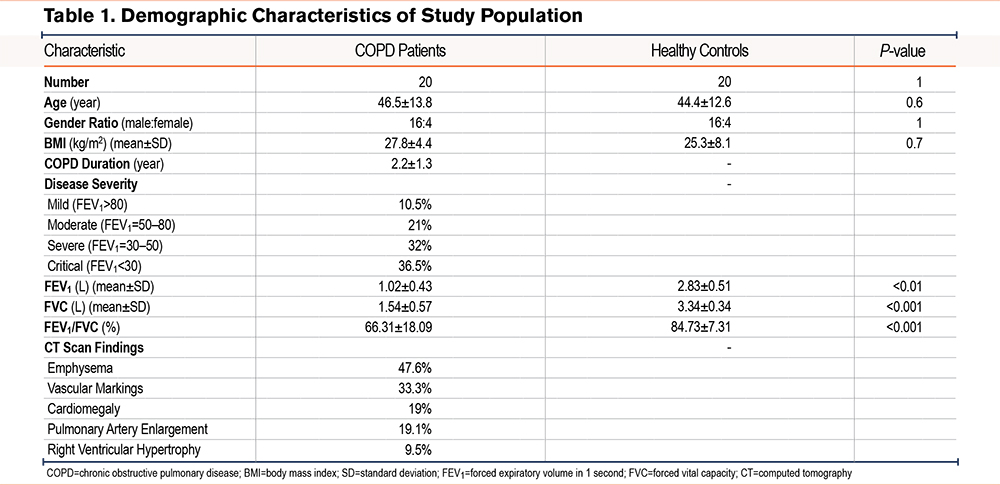
Exosomes Analysis
The size of exosomes in the DLS graph was presumed to be between 100–300 nm. The peak of the graph was 234.2±39.2 nm covering 99.7% of particles (Figure 1a). According to the literature, exosomes are typically 30–150 nm in diameter. However, we observed a slight increase in size in the DLS result, and it is important to note that DLS measures the hydrodynamic diameter of particles, which can be larger than their actual physical diameter due to the presence of a hydration layer. Therefore, it's important to use other techniques, such as electron microscopy, to confirm the size and morphology of exosomes (Figure 1b). The results of the TEM in 2 scales of 50 and 100nm indicated the size of the particles as approximately 30-150nm as well (Figure 1).
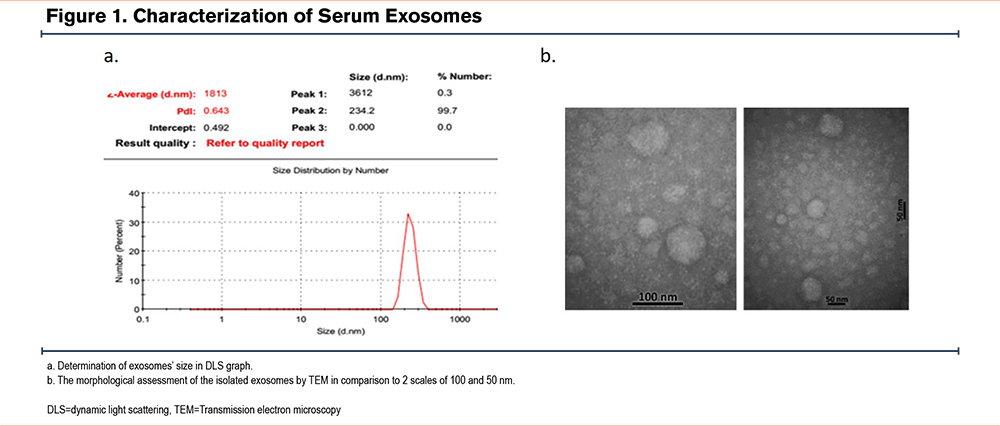
Higher Serum CD81+ Exosome Counts in COPD Patients
The count of serum exosomes in the group of COPD patients was significantly higher than the healthy controls (4789±960.2 versus 2284±367.8 [mean±SEM] [P-value: 0.02]) (Figure 2)
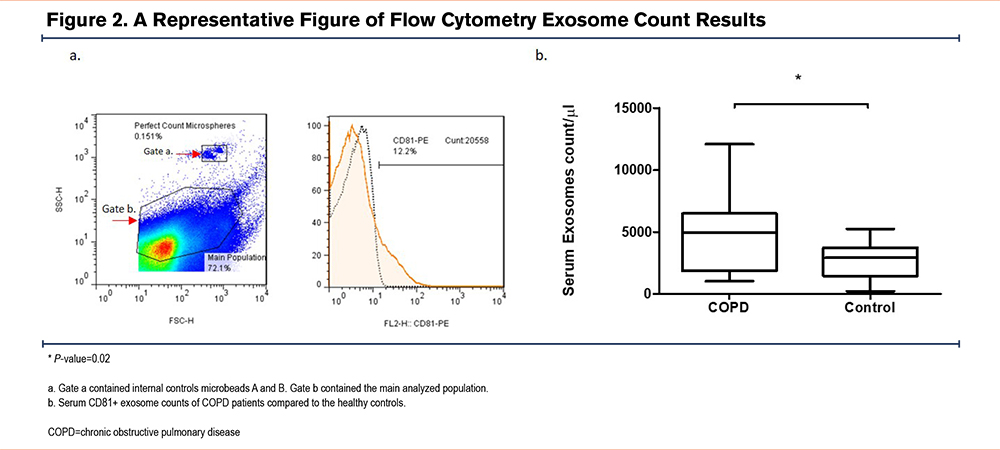
Exposures to PM2.5 and PM10 in Patients and Healthy Controls
The mean daily exposure to PM2.5 and PM10 in COPD patients was slightly lower than healthy controls (93±7.9 and 84±4.2 mean±SEM for PM2.5 and PM10, respectively). However, weekly exposure to PM10 was mildly higher in the patient group (77±5 versus 69±7.1 mean±SEM). Comparing the monthly exposure, the COPD patients had a significantly higher exposure to PM2.5 in the preceding month (53±4.1 vs. 41±4.0 mean±SEM) (P-value=0.02) (Figure 3).
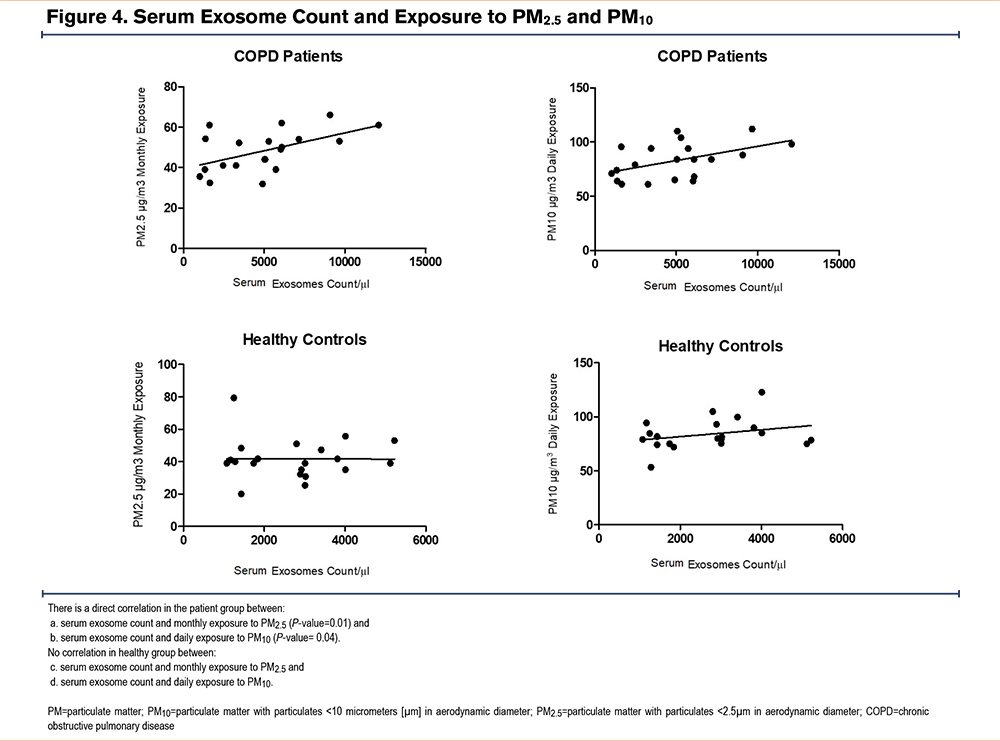
Direct Correlation Between Exosome Count and Monthly Exposure to PM2.5 and PM10 in COPD Patients
Peripheral blood exosome frequency in COPD patients showed a direct significant correlation with monthly exposure to PM2.5 with a Pearson correlation coefficient of 0.58 (confidence interval [CI] 95%: 0.086 to 0.85 and P-value=0.02) (Figure 4a). The result of correlation analysis in these variables was not significant in the healthy group with a Pearson correlation coefficient of -0.005 (CI 95%: -0.44 to 0.43 and P-value=0.9) (Figure 4c). In addition, serum exosome counts of COPD patients showed a direct significant association with PM10 exposure on the day of sampling with a Pearson correlation coefficient of 0.61 (CI 95%: 0.12 to 0.86 and P-value=0.02) (Figure 4b). The result of correlation analysis in these variables was also not significant in the healthy group with a Pearson correlation coefficient of 0.28 (CI 95%: -0.18 to 0.64 and P-value=0.2) (Figure 4d).
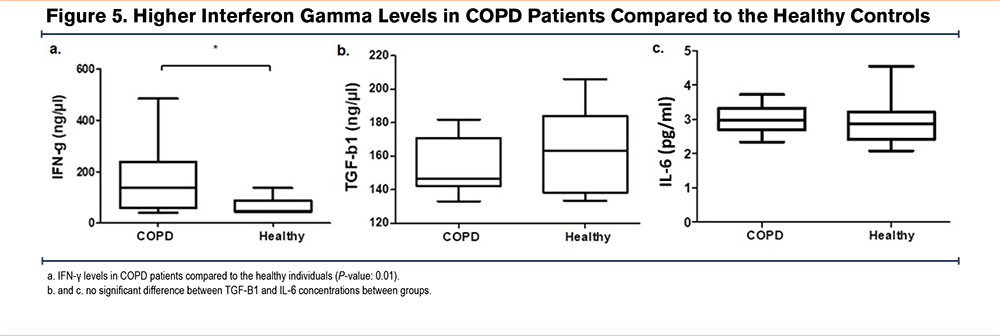
Higher Interferon Gamma Levels in COPD Patients Compared to the Healthy Controls
The serum level of IFN-γ was significantly higher in COPD patients than in the healthy controls (171.9±126.7 versus 63.7±30.8 [P-value=0.01]). However, neither TGF-β1 (153.3±17 versus 163.6±24) nor IL-6 (2.99±0.4 versus 2.94±0.66) amounts showed a significant difference between the 2 groups (Figure 5).
No Correlation Between Exosome Counts and Cytokine Levels
The correlation analysis showed no significant correlation between exosome counts and IFN-γ, TGF-B1, and IL-6 levels. There was also no statistically significant association between the concentration of air pollutants PM2.5 or PM10 and studied cytokines.
Discussion
Chronic obstructive pulmonary diseases are chronic inflammatory disorders leading to irreversible airway obstruction. COPD includes clinical subgroups such as chronic bronchitis, bronchial hyperresponsiveness, and emphysema. They are presented with hyperinflation, cachexia, chronic bronchitis, frequent exacerbations, and systemic inflammation. COPD is considered the most common cause of death from respiratory disorders.25
Air pollution is one of the most important health concerns in developing countries that causes millions of deaths per year and is estimated to become the third cause of death in the near future.26 Air pollutants include sulfur dioxide, nitrogen dioxide, carbon monoxide, ozone, methane, hydrogen chloride, aromatic hydrocarbons, dioxins, organic volatiles, and particulate matter. Particulate matter is classified into PM0.1, PM2.5, and PM10 based on the diameter size of the particulates.27 Air pollutants have been shown to be involved in the initiation, development, and exacerbation of bronchopulmonary,6 cardiovascular,28 allergic,29 and autoimmune diseases.30 They can affect healthy populations but the hazard is greater for vulnerable individuals, such as the elderly, children, and those with chronic conditions, e.g., asthma and emphysema.31 It has been shown that prolonged exposure to air pollutants increases the risk of developing COPD. There is also some evidence that exposure to particulate matter exacerbates COPD symptoms, thereby, increasing the risk of death.32 Similarly, the present study showed a significant correlation between PM2.5 exposure in the preceding month of sampling and increased exosome counts in COPD patients. This finding might be suggestive of an association between chronic exposure to air pollutants and exacerbation of inflammatory conditions in these patients.
The role of exosomes in the pathogenesis of COPD has barely been studied.33 In addition, study results have generally been obtained from experimental models or in vitro studies. One research by Moon et al on long lung epithelial cells showed that prolonged exposure to secondhand cigarette smoke could produce CCN1-rich exosomes, an extracellular matrix protein that plays an important role in tissue repair and malformation.34 As a result, the exosomes concentrated with this protein may lead to the induction of paracrine CXCL8 production and the subsequent recruitment of neutrophils and other inflammatory cells to the site, which can eventually enhance the process of fibrosis in the lungs. Indeed, microvesicles with a pro-inflammatory function may be released by a broad range of respiratory cell types subsequent to exposure to various environmental factors such as microbes, cigarette smoke, and oxidative stress.35 These and other findings suggest the contribution of exosomes in sustained inflammation and airway remodeling in COPD. The present study also showed increased counts of serum exosomes in COPD patients. Moreover, the exosome count was significantly correlated with daily PM10 and monthly PM2.5 exposures as environmental triggers.
In addition, there have been some investigations about the association of exosome counts with inflammatory cytokine levels.36,37 For instance, Tan et al reported higher exosome counts in COPD patients compared to the healthy controls and also demonstrated elevated levels of CRP, IL-6, and soluble tumor necrosis factor receptor 1 (sTNFR1) in COPD patients, which was correlated with the serum exosome counts. Furthermore, IL-6 and sTNFR1 levels in patients experiencing an acute exacerbation of COPD were higher than in stable patients.38 Similarly, our study demonstrated a higher number of exosomes in the blood of COPD patients compared to healthy individuals. Moreover, there was a significant association between environmental pollutant PM10 and PM2.5 exposures in patients with the frequency of serum exosomes. There was also a significant difference between the IFN- γ serum levels of patients and healthy individuals. However, we found no association between exosome counts and cytokine levels and this may be due to the administration of inhaled corticosteroids or high baseline amounts of IL-6 in healthy volunteers.
These findings suggest a correlation between air pollution and exosome release in COPD patients. Therefore, it might be helpful to avoid polluted environments in order to reduce the severity of inflammation and exacerbation of the disease. Serum levels of circulating exosomes and their contents might serve as diagnostic and prognostic biomarkers in patient surveillance.
The present study had some limitations, for instance, the sample size was fairly small. Since smoking is a considerable risk factor for developing COPD, finding patients without any history of active or passive smoking was challenging. In addition, due to the coronavirus disease 2019 pandemic, the patients’ presence in the clinics was restricted. Therefore, the number of eligible patients was lower than expected. The other limitation was the lack of information about the biological contents of the detected exosomes. Obviously, to discover the correlation of exosomes with chronic inflammation and disease pathogenesis, it is critical to investigate the probable presence of cytokines, chemokines, and nucleic acids. In brief, the elevated number of exosomes in the serum of COPD patients and the correlation of exosome count with air pollutants suggests the need for further studies to reveal the mechanisms associating environmental factors with the inflammatory process in COPD.
Acknowledgments
Author contributions: NS designed the study, supervised the experimental work, analyzed data, and wrote the manuscript. SA analyzed data and reviewed and corrected the manuscript. EK evaluated patients and the acquisition of patients’ clinical data. MSH and KN contributed to the air quality control data collection and analysis. MS and HM performed the experimental tests. MN conceived the idea and co-supervised the project and obtained funds. ME revised the manuscript and supervised the project. All authors were involved in the final approval of the manuscript.
We thank the Air Quality and Traffic Control Center as well as the Air Pollution and Environmental Research Institute for providing us with the air quality data.
Declaration of Interests
All authors declare no conflicts of interest.