Running Head: AATD Patient Experiences With Augmentation Therapy
Funding Support: None.
Date of Acceptance: July 30, 2023 | Publication Online Date: August 4, 2023
Abbreviations: AATD=alpha-1 antitrypsin deficiency; COPD=chronic obstructive pulmonary disease; QOL=quality of life; IVs=intravenous lines; PICCs=peripherally inserted central catheters
Citation: Strange C, Allison S, McCathern J, Sandhaus RA, Holm KE. Augmentation therapy for alpha-1 antitrypsin deficiency: patient experiences with self-infusion, home providers, and clinics. Chronic Obstr Pulm Dis. 2023; 10(4): 392-399. doi: http://doi.org/10.15326/jcopdf.2023.0430
Online Supplemental Material: Read Online Supplemental Material (337KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) is a rare genetic disease that is associated with progressive pulmonary emphysema and liver disease.1 The only licensed U.S. Food and Drug Administration-approved therapies for lung disease in AATD are intravenous therapies that have been in place since 1989. The typical dose of alpha-1 augmentation therapy is 60 mg/kilogram given once weekly.2,3 Intravenous administration creates significant burdens on the AATD population and has prompted shared efforts to improve the quality of life (QoL) associated with drug administration.
The administration of these regular intravenous infusions can take place at a medical facility, a dedicated infusion center, at home with a visiting medical professional, or by self-infusion. The location of treatment is often determined by the patient’s insurer. Travel to infusion centers by AATD-affected individuals is dependent upon distance from an infusion center and access to transportation. As a result, many individuals with AATD choose to get their infusions from a home nursing company that provides nurses and scheduled infusions weekly. Notably, core Medicare benefits do not cover self-infusion or home nursing services, although many non-Medicare insurers cover these services.
In the past several years, there has been increasing interest in and emphasis on the need for at-home administration of augmentation therapy for AATD by medical professionals4 and by patients themselves.5,6 Self-administration has been embraced by many in the alpha-1 community because of the known safety profile of augmentation therapies and the short infusion times. There are many details about self-infusion that are often spread by word of mouth and through communication channels in the highly connected alpha-1 community. Therefore, this study was designed to assess patients’ experiences with augmentation therapy for AATD, including demographic characteristics associated with self-infusion, and perceived benefits of, and barriers to, self-infusion in the United States. Since some of these infusions occur through permanently placed central venous catheters, the survey also captured self-reported catheter complications without discriminating between peripherally inserted central catheters (PICCs) and subcutaneous port central venous catheter devices.
The characteristics of AATD patients and their infusion practices have not been the subject of a comprehensive manuscript. Therefore, this paper seeks to characterize the infusion practices of a large AATD population in the United States, as seen through the perspective of the patients who are living this journey. Data were collected by AlphaNet, a not-for-profit organization that provides a telephone-based disease self-management program designed for individuals with AATD-associated lung disease who are prescribed augmentation therapy.7 AlphaNet follows the majority of individuals in the United States who are prescribed augmentation therapy for lung disease due to AATD. As such, AlphaNet is uniquely well-positioned to examine patients’ experiences with augmentation therapy in a large sample of geographically diverse patients.
Methods
Individuals in the AlphaNet disease management program were invited to participate in a survey regarding their experiences with augmentation therapy. The survey defined self-administration as follows: administration of augmentation therapy by the patient (either alone or with help from a spouse, partner, relative, or friend), without regular involvement by a paid medical professional. This definition is consistent with the definition used by Horvath et al.8 The survey contained one set of questions for individuals who self-infuse and a different set of questions for individuals who do not self-infuse. The key topics among individuals who self-infuse were: (1) learning how to self-administer, (2) satisfaction with self-administration, (3) challenges with self-administration, and (4) assistance needs for self-administration. The key topics among individuals who do not self-infuse were: (1) reasons for receiving augmentation therapy in the location where they received therapy (i.e., in the home with a health care professional versus traveling to a facility), (2) reasons why they would or would not consider self-administering augmentation therapy, and (3) prior experiences with self-administration.
Trained coordinators (who also have AATD) administered the survey by telephone and through an invited online portal from February 8, 2022 through July 1, 2022, with responses collected centrally. The dataset was deidentified and analyzed at the Medical University of South Carolina with JMP PRO Version 16 (Cary, North Carolina). Institutional review board approval for the analysis of deidentified data was obtained through the Medical University of South Carolina Institutional Review Board. Statistical analysis was performed with summary statistics reporting demographics of the study population. Correlational statistics were performed using simple linear regression or logistic fit for categorical and continuous data. T-tests or Chi-square analysis were used to compare continuous and categorical values respectively. P-values of less than 0.05 were considered significant.
Results
Among the 5925 individuals who were sent the survey, 628 did not return it, 5 individuals were not on augmentation therapy, and 26 individuals did not supply data on the central question regarding whether they self-infuse (and therefore, also did not answer the remaining survey questions). Therefore, the study population consisted of 5266 adult individuals receiving alpha-1 augmentation therapy (88.9% of those invited) (Figure 1).
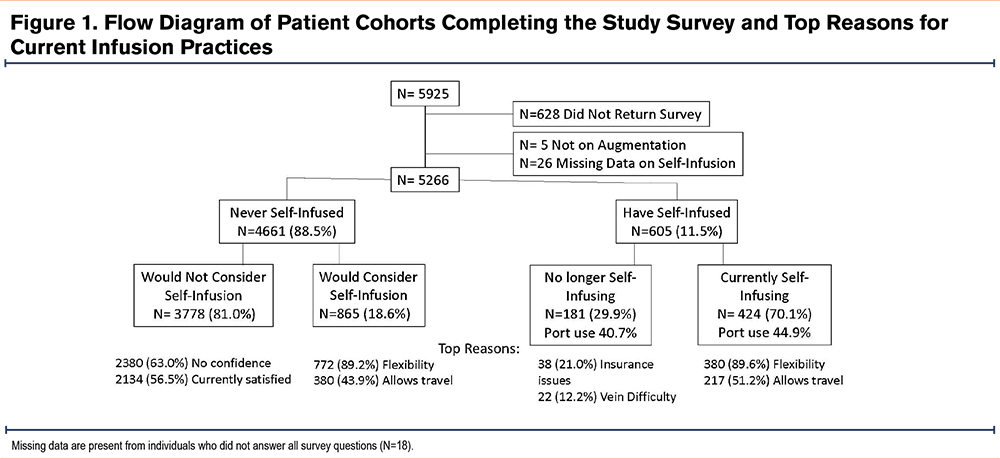
Intravenous augmentation therapy was received weekly by 93.0% of participants. Infusions occurred: (1) with a home health nurse (N= 3169, 60.2%), (2) at an infusion clinic (N=1609, 30.6%), and (3) by self-infusion (N= 424, 8.1%). (Table 1). Multiple demographic differences are present among the 3 cohorts. Clinic infusing participants were older (likely from Medicare coverage at infusion clinics), less likely to be receiving infusions on a weekly infusion schedule, and the least satisfied with their infusion practice. They were more likely than home nursing infused participants to consider self-infusion. Those who self-infused were more often male, more commonly used a port or PICC, had the highest number of infusion years of any group, and had the highest percentage of responses in the “very satisfied” range (93.1%) for infusion choice.
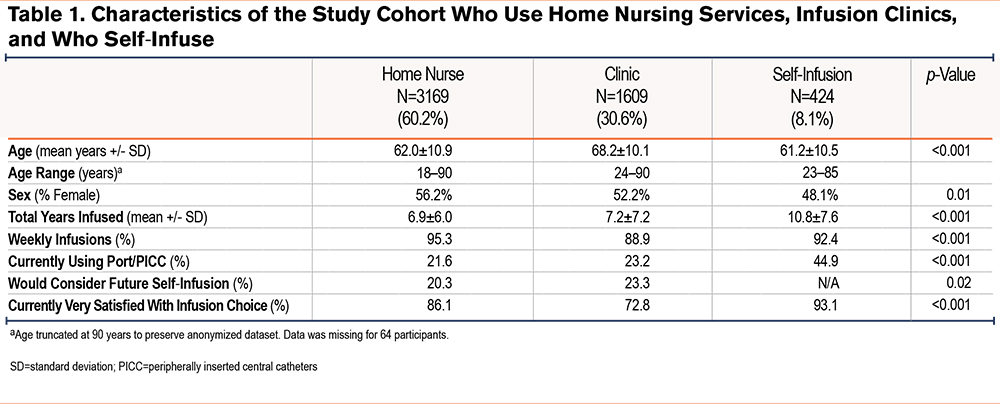
Current self-infusion prevalence increased with time on therapy and yet was more prevalent in younger individuals (61.2 ± 10.5 years) compared to users of other infusion practices (64.1 ± 11.0 years), (p<0.001). The first mention of self-infusion most frequently came from physicians (32.9%), nurses (28.4%), or AlphaNet coordinators (18.7%). Among current self-infusers: (1) 20.6% began self-infusing within the first month of alpha-1 augmentation therapy, (2) 28.4% began between 1 and 12 months after starting augmentation, (3) 40.5% began between 1 and 10 years after starting augmentation, and (4) 8.8% began after they had been infusing for more than 10 years. Training was usually provided by a nurse from a home nursing agency (60.4%), or a hospital/office/infusion center (15.9%). Other sources of training included the internet (9.7%), friend or family member (8.1%), or a physician/advanced care provider (2.7%). Three or fewer training sessions were given to 68.0% of individuals. The benefits perceived by self-infusers were: (1) freedom and flexibility (77.9%), (2) ability to travel (44.5%), (3) avoidance of travel to infusion clinics (41.8%), (4) time-savings (35.9%), (5) less absence from work (26.6%), (6) less exposure to infections (22.1%), and (7) less cost (16.4%). Self-infusion was done through permanent intravenous catheters in 41.2% and peripheral intravenous catheters in 58.3%. Most self-infusing individuals (93.1%) were very satisfied with their decision. Among individuals currently infusing with home nurses or in clinics, 21.4% would consider home infusions in the future, and this increased to 34.4% among those not currently “very satisfied” with home nurses or in-clinic infusions.
There were individuals who previously self-infused but who now use a health care practitioner for infusions (N=152). Past infusion duration was <6 months (33.6%), 6–12 months (8.6%), 1–5 years (24.3%), 5–10 years (18.4%) or >10 years (10.5%). Among the reasons given for stopping home infusions, the most common were: (1) lack of insurance coverage (25.0%), (2) difficulty with venous access (14.5%), (3) difficulty with the port (13.8%), (4) lack of home support (10.5%), and (5) fear of adverse events (4.6%). Port use was not different for this cohort (40.7%) compared to the larger group that had continued home infusions (45.0%), p=0.63.
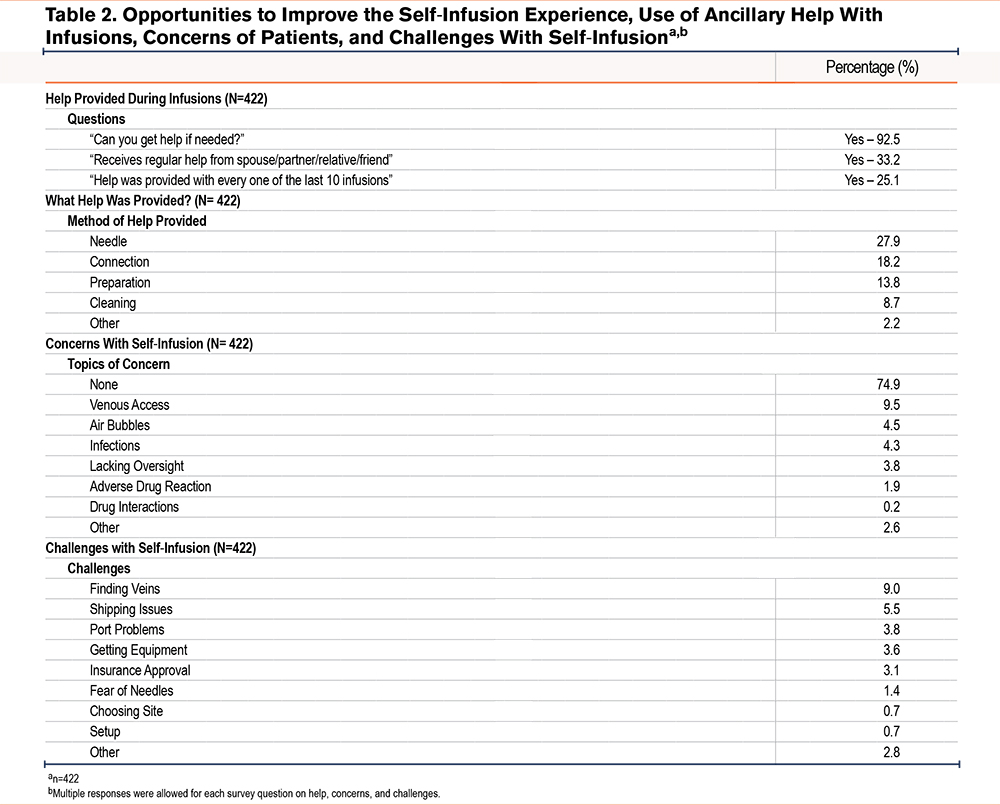
Opportunities to improve the self-infusion experience, use of ancillary help with infusions, concerns of patients, and challenges with self-infusion are enumerated in Table 2. In short, the majority of patients felt empowered to self-infuse and appeared to do so safely.
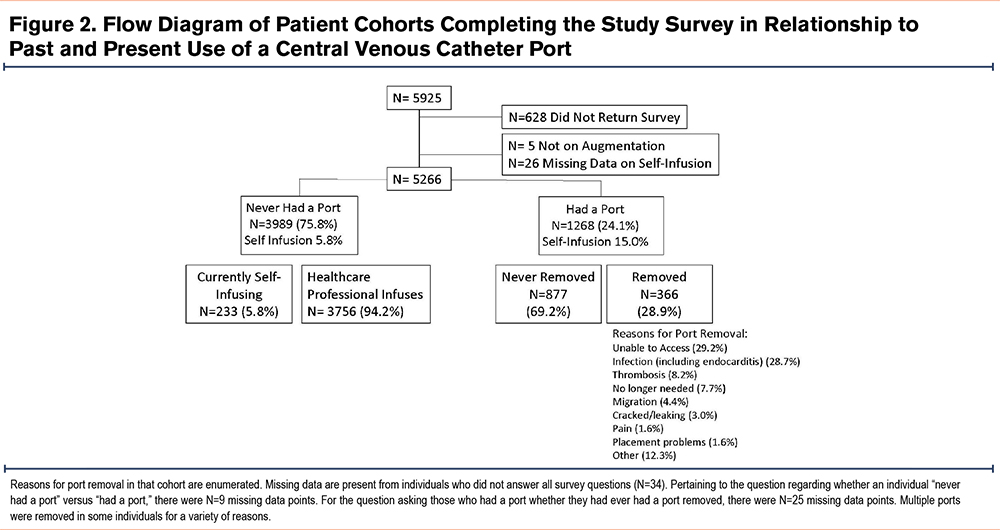
Additional information was obtained from the 1268 individuals who had ever had central venous access lines placed (usually a subcutaneous port) (Figure 2). Self-infusion prevalence was higher among individuals who ever had a port (15.0%) compared to those who never had a port (5.8%) (p<0.001). Among all individuals with current or past port placements, 366 (28.9%) had 1 or more removed. The most common reasons for removal were: (1) lack of blood return (29.2%), (2) infection including endocarditis (28.7%), (3) thrombosis (8.2%), (4) migration (4.4%), and (5) mechanical catheter problems (3.0%). Infection was more common in self-infusers (39.2%) compared to those with nursing administered care (25.7%), p=0.02. In the cohort with port removal, self-infusion rates remained high (17.9%).
Discussion
Individuals who self-infused reported being highly satisfied with self-infusing, and the most frequently reported reasons for self-infusing were flexibility and the belief that self-infusing makes it easier to travel. The optimal candidate for self-infusion has a good support system, good venous access, baseline knowledge that is usually acquired over the first year of infusions, and insurance that allows drugs to be shipped to the home. Although this survey did not collect actual or estimated costs, the lack of ancillary costs associated with a home nursing agency or infusion clinic necessarily saved money for the health care system. Therefore, we were disappointed to see that the most frequent reason for stopping self-infusion was due to insurance issues, presumably since traditional Medicare only authorizes intravenous infusions in health care facilities, despite the cost savings of other methods.
The success of home infusion in the United States and the satisfaction of patients who receive home augmentation therapy is important for other parts of the world where the ability to receive augmentation therapy in the home may be more limited. In Europe, one study that included 15 physicians from 13 European countries found that all of the respondents would consider self-administration for at least some of their patients, if it were available.8 In addition, 78.6% of respondents believed that 3 or fewer training sessions would be needed for patients to be able to self-administer independently. The belief of these physicians aligns with the findings of the current study, in which 68.0% of the participants who self-infused had 3 or fewer training sessions.
While home infusion by a nurse has been widespread in the United States, this has not been the case in other countries. In Italy, where augmentation administration has largely been done in a medical setting, one study examined nurse-administered augmentation therapy in the patient’s home. This study found an improvement in QoL, specifically with regard to the decrease in perception that augmentation therapy interferes with life activities.4
A study conducted in the United States examined patient perspectives regarding transitioning to self-infusing in a small sample of 22 individuals with AATD.6 In this study, 40% of participants indicated that they would consider self-infusing if training and education were provided. Among those who were not willing to consider self-infusing, the most frequently cited reasons were lack of skills and fear of problems.6 These findings suggest that more patients might consider self-infusion if a formalized training program were available. Such education could be developed, formalized, refined, and tested for optimal use in AATD community programs.
In addition to training, access to ongoing support is likely to be a key to success with self-infusing. In our study, 25.1% of self-infusers indicated that they had some type of help with all 10 of their most recent infusions. The vast majority of self-infusers (92.5%) indicated that they could get help if needed. Support in the home appears to come from many sources, but many individuals cited family, friends, and telephone support as being equally as important as help from health care providers. In contradistinction, those who chose to use a nursing infusion service cited the lack of confidence in their own skills as the primary reason not to consider self-infusing.
Use of a central venous catheter was present in less than 50% of the self-infusing patients. Community discussions have suggested that port use is heavily physician directed. The AlphaNet experience is that placement of peripheral intravenous lines (IVs) is usually successful and can be sustained for many years. There was no difference in the mean or longest duration of self-infusion by peripheral IV (range 1–32 years) compared to by port (range 1–40 years). The training required for peripheral IV placement is manageable with 3 or fewer sessions and IV placement is a skill that can be acquired in a variety of home or health care settings.
There are opportunities to improve the education, training, supply chain, and details of infusion practice. Some of these are within the mission of AlphaNet. In particular, the finding that self-infusing port users have higher rates of central venous infections than those using a home nursing service is an educational opportunity for the community of self-infusers. Furthermore, there is likely a self-selection of individuals who choose self-infusion because of ease in finding a vein, lack of anxiety, and better support networks, compared to individuals who choose to have a nurse administer infusions. Whether all of the same issues will remain if subcutaneous preparations or longer half-life preparations of alpha-1 augmentation become available is not clear.
AATD is not the only health condition in which self-infusion is relevant. There is a long-standing history of self-infusion of intravenous therapies in other health conditions, including hemophilia9-13 and hereditary angioedema.14-21 In hemophilia, self-infusion is associated with a sense of independence and personal freedom,9 improved QoL, and less missed school or work.10 In hereditary angioedema, self-infusion is associated with improved QoL.19,20 Some of the advantages and disadvantages of self-infusion reported by patients with hereditary angioedema are similar to those reported in our study. In hereditary angioedema, advantages of self-infusion included patients’ ability to manage their own disease and the ability to travel.20 Disadvantages included the need for training, decreased contact with medical providers, fear of self-infusion, and difficulty in finding a vein.
There are limitations to our study. This survey included individuals in AlphaNet, a free service offered to all AATD individuals in the United States regardless of the medication they are receiving. However, there are individuals who infuse and are not members of AlphaNet. There was non-participation from 628 individuals in the survey and some missing data with each question. Similarly, bias can result from the patient-reported medical retrospective data that informed some of our questions, particularly if obtained at differing times in the past. Independent validation of reported central venous complications was not performed. Furthermore, this study occurred during the coronavirus disease 2019 pandemic and infusion practices before or after the pandemic may be different. We did not evaluate the severity of chronic obstructive pulmonary disease (COPD), liver disease, AATD genotype, or other clinical characteristics to define associations with the site of infusion.
There are several strengths to our study. This is the largest survey about AATD infusion practices that has ever been assembled. All data were collected via self-report, which is ideal for understanding patients’ experiences with various infusion practices. In addition, this study included more than 400 individuals who self-infuse, which is a rich sample of patients who were able to provide information regarding their experiences with self-infusion. The experience is associated with high patient satisfaction derived from the freedom and flexibility of self-infusion.
Self-infusion is a desirable option for a subset of patients who are on augmentation therapy for AATD. Among self-infusers, satisfaction was extremely high and numerous benefits of self-infusion were endorsed. Three-quarters of the self-infusers in our study indicated that they had no concerns regarding self-infusion. However, not all patients want to, or are able to, self-infuse. The majority of participants in this study were not self-infusing and were highly satisfied with their method of receiving infusions. This highlights the importance of having multiple options for augmentation therapy, including where and how it is administered; i.e., in a clinic versus at home, and with or without the involvement of a medical professional.
Acknowledgements
Author contributions: Study design was envisioned by CS, RAS, and KEH. Data acquisition occurred through help from the AlphaNet coordinators. All authors contributed to writing, revisions, and approval of the final version of the manuscript.
This work could not have been accomplished without data collection from the AlphaNet coordinators. The authors would like to thank CSL Behring for funding a pilot study that led to the development of this project. The authors thank Chloe Jacobs for assistance with final manuscript formatting.
Declaration of Interests
All authors are employees of AlphaNet, a not-for profit disease management company that provides care for participants with alpha-1 antitrypsin deficiency. CS is a medical director at AlphaNet. He has grants in alpha-1 antitrypsin deficiency and/or COPD paid to the Medical University of South Carolina from Adverum, Arrowhead, AstraZeneca, CSA Medical, Dicerna, Grifols, Nuvaira, Takeda, and Vertex. CS has consulted for Bronchus, CSL Behring, Dicerna, Inhibrx, Pulmanage, and Vertex on alpha-1 antitrypsin deficiency and/or COPD. RAS has grants to National Jewish Health from the Alpha-1 Foundation, Grifols, Vertex, and the National Institutes of Health/National Center for the Advancing Translational Sciences. He is a consultant and scientific advisor to Dicerna, Grifols, CSL Behring, Takeda, and Vertex, and a medical director at AlphaNet. SA, JM, and KH have no disclosures.