Running Head: Coronary Artery Calcium and Exacerbation Risk in COPD
Funding Support: U.S. Department of Defense (W81XWH-15-1-0705)
Date of Acceptance: November 1, 2023 │ Published Online Date:November 13, 2023
Abbreviations: aHR=adjusted hazard ratio; BLOCK-COPD=Beta-Blockers for the Prevention of Acute Exacerbation of COPD study; CAC=coronary artery calcium; CAD=coronary artery disease; CI=confidence interval; IQR=interquartile range; LAD=left anterior descending; LCx=left circumflex; LM=left main; LTOT=long-term oxygen therapy; RCA=right coronary artery; SA=sinoatrial
Citation: Wade RC, Ling SX, Helgeson ES, et al. Associations between coronary artery calcium score and exacerbation risk in BLOCK-COPD. Chronic Obstr Pulm Dis. 2024; 11(1): 101-105. doi: http://doi.org/10.15326/jcopdf.2023.0423
Introduction
Note: A portion of this manuscript was presented at the 2021 American Thoracic Society International Conference as an abstract/poster presentation.
The Beta-Blockers for the Prevention of Acute Exacerbations of COPD (BLOCK-COPD) study investigated whether metoprolol would reduce exacerbation risk in patients with COPD who did not have an evidence-based indication for beta-blocker use, namely coronary artery disease (CAD) with recent reperfusion intervention or congestive heart failure.1 This trial was incepted due to prior reports suggesting beta-blockers may be associated with a reduced risk of acute exacerbations.1 BLOCK-COPD failed to demonstrate exacerbation risk reduction with metoprolol but observed an increase in severe-to-very severe exacerbations leading to early study termination. Since BLOCK-COPD largely sought to enroll individuals without CAD, it is not clear whether participants with subclinical or unrecognized CAD might have a differential response to metoprolol. The coronary artery calcium (CAC) score can be used to assess for CAD using chest computed tomography (CT) scans.2 This imaging biomarker evaluates the deposition of calcified plaque in the coronary vasculature, and a visual score of greater or equal to 7 is associated with incident coronary vascular events in COPD.3 We hypothesized that CAC would be associated with an increased exacerbation risk and differential response to metoprolol treatment within BLOCK-COPD. Some of the results from this study were previously reported in the form of an abstract.4
Methods
The study population includes participants in the BLOCK-COPD study from 5 study sites (University of Maryland, University of Michigan, Northwestern University, Temple University, and University of Alabama at Birmingham) who underwent clinically indicated thoracic CT imaging within a timeframe of 12 months before/after enrollment. Comprehensive clinical, spirometry, exacerbation, and mortality data were collected at regular intervals as part of the BLOCK-COPD protocol.5 An exacerbation of COPD was defined as an increase in or a new onset of 2 or more respiratory symptoms that required treatment with antibiotics or systemic steroids for at least 3 days.5 All participants completed informed consent for enrollment in BLOCK-COPD, and the post-hoc analysis was approved by the institutional review board at each participating institution.
We used the Weston scoring system to quantify CAC using an ordinal scale wherein 0 indicates no vessel calcium and a score of 1–3 indicates increasing calcification severity.6 The Weston score ranges from 0–12 and is derived by summing the individual scores from each of the 4 major coronary arteries: right coronary artery (RCA), left main (LM), left anterior descending (LAD), and left circumflex (LCx). An investigator at each clinical site, blinded to clinical parameters, determined the CAC score for each participant.
Cox proportional hazards models were used to measure associations between CAC in each vessel (and the interaction between CAC and treatment assignment) and time to exacerbation. Adjusted models included age, sex, race, smoking status, baseline FEV1 percentage predicted, number of hospitalizations for COPD during the previous year, number of exacerbations treated with glucocorticoids or antibiotics during the previous year, and treatment assignment and were stratified by center. CAC was evaluated both as a continuous variable and as a binary variable indicated by CAC greater than zero. Analyses were performed using R statistical software (version 3.6.0). P-values were not adjusted for multiple comparisons.
Results
Imaging and clinical data were included for 109 participants. The mean age was 65±8 years with 54% female, 33% Black race, and 24% active smokers (Table 1). Sixty-one (56%) participants were assigned to the metoprolol treatment arm. Over a median (interquartile [IQR]) follow-up time of 350 (280 to 352) days, 61 individuals experienced at least one exacerbation.
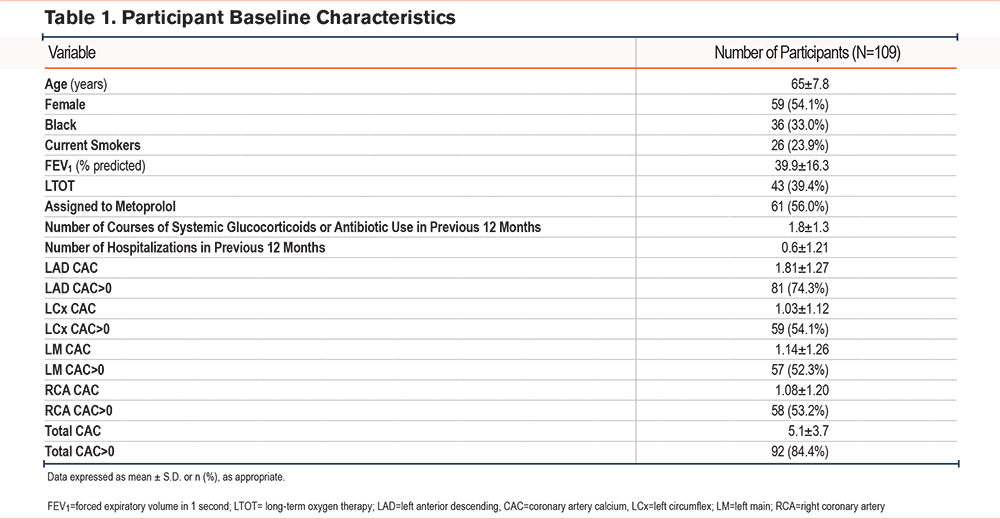
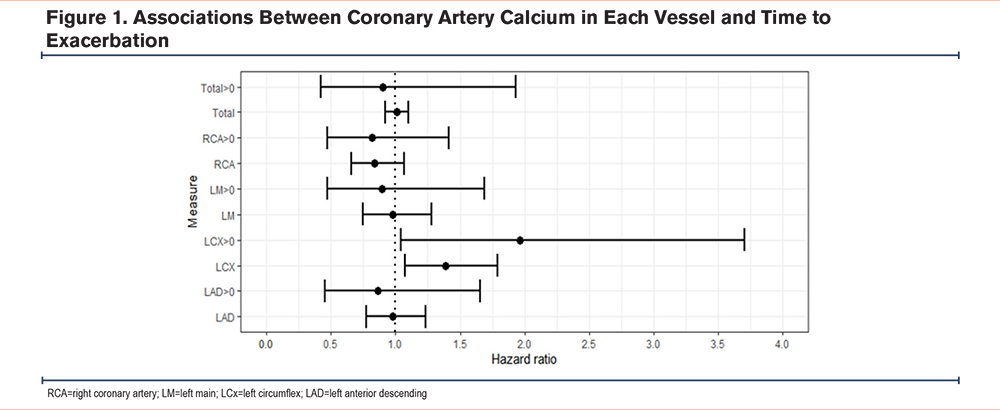
The mean CAC score was 5.1±3.7, with 92 participants (84%) having CAC >0. Associations were observed between CAC in the LCx and shorter time to exacerbation (adjusted hazard ratio [aHR]=1.39 for 1 unit increase in score, 95% confidence interval [CI]: 1.08–1.79, p=0.01). When treating CAC as a dichotomous variable, associations were again observed between LCx CAC and shorter time to exacerbation (aHR= 1.96, 95% CI: 1.04–3.70, p= 0.04). No associations were observed between LM, LAD, or total CAC and time to exacerbation. No interaction was observed between total or any vessel CAC and treatment assignment to either metoprolol or placebo. A summary of the findings is depicted in Figure 1.
Discussion
We identified that CAC, as a reflection of underlying CAD, was associated with COPD exacerbation risk in a well-characterized COPD population. However, we did not observe a beneficial effect of metoprolol on exacerbation risk reduction in BLOCK-COPD participants with any visible CAC. Interestingly, we found that CAD (by CAC >0) was highly prevalent in this subgroup of patients despite the BLOCK-COPD design excluding patients with CAD requiring recent revascularization. Our study is novel in that it links an imaging biomarker of CAD with COPD control and further investigated associations between CAC and beta-blocker treatment. CAC is an important imaging biomarker that is implicated in COPD morbidity.3 The observation between LCx CAC and exacerbation risk is thought-provoking with several potential mechanisms at play. Elevated CAC scores in the LCx have been implicated in large myocardial ischemia deficits on nuclear perfusion scans.7 Additionally, the LCx can perfuse a large portion of the left ventricle including the mitral valve. Several publications report associations between total CAC and left ventricular diastolic dysfunction.8,9 Given that diastolic dysfunction is associated with an increased risk of severe exacerbations, it is possible that impaired left ventricular perfusion increases diastolic dysfunction and increases exacerbation risk.10 Interestingly, the LCx perfuses the sinoatrial (SA) node in up to 25% of individuals.11 Existing reports indicate associations between atrial arrhythmias and increased COPD exacerbation risk, so it is reasonable to posit that poor perfusion of the SA node may increase the incidence of arrhythmias and subsequently contribute to exacerbation risk.12 Thus, poor myocardial perfusion and resultant impairment in cardiac function may lead to increased respiratory symptoms. Our study is limited by its post hoc design and small sample size, which precludes matching between groups or inferences regarding causality. In addition, given that the study was terminated early, some participants had limited follow-up time. Further, we are limited to participants who underwent thoracic CT for clinical reasons outside of the BLOCK-COPD protocol, which may introduce confounding by indication.
Conclusions
We found associations between CAC in the LCx and exacerbation risk, expanding the relevance of CAC measurement in COPD. Future investigations should incorporate the use of CAC measurement to better understand exacerbation risk prediction as well as to disentangle complex heart-lung interactions.
Acknowledgements
Author contributions: All authors provided substantial contributions to the conception and design of the study as well as the acquisition, analysis, and interpretation of data for the work. RCW and JMW drafted the work and all authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published. RCW and JMW agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Sharing Statement: Requests for de-identified participant data and study-related documents can be requested through the corresponding author and data-coordinating center.
Declaration of Interests
RCW, SXL, HV, DM, OC, JYS, RR, and JMW report no conflicts of interest to declare. WWL reports personal fees from Konica Minolta and Continuing Education Alliance. ESH has received grant support from the National Institutes of Health (NIH) and the Department of Defense and clinical trial support from Fisher & Paykel Healthcare. GC reports grants from Boehringer-Ingelheim, Novartis, Astra Zeneca, Respironics, MedImmune, Actelion, Forest, Pearl, Ikaria, Aeris, PneumRx, and Pulmonx, and personal fees from HE Health Care Solutions, Inc, Amirall, Boehringer-Ingelheim, and Holaira. RK receives grants and personal fees from AstraZeneca and GlaxoSmithKline, and personal fees from CVS Caremark, Aptus Health, Boston Scientific, and Boston Consulting Group. MKH reports receiving grants from the NIH and the COPD Foundation and personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva Pharmaceutical Industries, Verona Pharma, Merck, Mylan, Sanofi, DevPro Biopharma, Aerogen, Polarian, Regeneron, Amgen, UpToDate, Altesa Biopharma, Medscape, the National Association of Colleges and Employers, MDBriefCase, and Integrity. She also reports research support paid to her institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, AstraZeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association; data and safety monitoring board funds paid to the institution from Novartis and Medtronic; and stock options from Meissa Vaccines and Altesa BioSciences. MTD reports grants from the American Lung Association, the Department of Defense, and the NIH, along with consulting fees from AstraZeneca, GlaxoSmithKline, Novartis, Pulmonx, and Teva.