Running Head: Improving Referrals for Lung Volume Reduction
Funding Support: This study was supported by the Clinical Science Research and Development Career Development Award IK2CX001882 from the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service. The contents of this work do not represent the views of the Department of Veterans Affairs or the United States government.
Date of Acceptance: September 3, 2023 | Published Online Date: September 7, 2023
Abbreviations: BLVR=bronchoscopic lung volume reduction; COPD=chronic obstructive pulmonary disease; DLCO=diffusing capacity for carbon monoxide; EMR=electronic medical record; FEV1=forced expiratory volume in 1 second; GOLD=Global initiative for chronic Obstructive Lung Disease; IQR=interquartile range; LVRS=lung volume reduction surgery; PCP=primary care provider; PFT=pulmonary function testing (tests); QI=quality improvement; RV=residual volume; SD=standard deviation; TLC=total lung volume capacity
Citation: Di Felice C, Strumpf ZB, Edmiston EA, et al. Improving referral patterns for bronchoscopic lung volume reduction: a quality improvement initiative. Chronic Obstr Pulm Dis. 2024; 11(1): 95-100. doi: http://doi.org/10.15326/jcopdf.2023.0397
Introduction
The health and economic burden of chronic obstructive pulmonary disease (COPD) is profound, with global prevalence1 estimated at roughly 10%. Disease prevalence is even higher in certain at-risk populations, with both male and female United States military veterans demonstrating higher rates of COPD compared to non-veterans.2 Hyperinflation is one of the many pathophysiologic changes seen in COPD that is known to contribute to patients’ symptom burden and is an independent risk factor for mortality.3 Lung volume reduction surgery (LVRS) can reduce the impact of hyperinflation on pulmonary mechanics in patients with advanced COPD refractory to optimized medical care.4 While LVRS was shown to improve several patient-centered outcomes in a large clinical trial, the procedure requires relative surgical fitness and many patients are ineligible.
Bronchoscopic lung volume reduction (BLVR) is a minimally invasive alternative to LVRS for select patients with advanced emphysema and is supported by the Global initiative for chronic Obstructive Lung Disease (GOLD) treatment guidelines.1 Patient selection for this procedure follows the inclusion and exclusion criteria of the associated clinical trials investigating BLVR.5,6 A key step in the evaluation of potential procedure candidates is pulmonary function testing (PFT) to identify severe airflow obstruction, hyperinflation, and gas trapping. Patients with hyperinflated, severely emphysematous type COPD who undergo BLVR show improved lung function, exercise performance, quality of life, and survival.5-7 In a survey of advanced emphysema patients queried about their treatment preferences, most respondents opted for BLVR over continued medical management.8
Although many patients may be interested candidates, strategies to improve identification and referral for BLVR are lacking. This quality improvement (QI) project explores the implementation of a referral strategy using PFT reports and provider education to identify BLVR-eligible patients in a large Veterans Affairs academic medical center.
Methods
The Veterans Affairs Northeast Ohio Healthcare System has been offering BLVR at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center location since January 2022. This QI project aimed to identify potentially eligible BLVR patients for referral and evaluation. This QI project was exempt from IRB review. Before our QI initiative, BLVR evaluations primarily relied on word-of-mouth referrals within the pulmonary section. Our approach was to: (1) develop a process to communicate eligibility to ordering providers using PFT reports, and (2) educate providers about BLVR and how to refer patients to our program. We tracked BLVR referrals for 8 months pre- and postintervention to assess effectiveness in improving referral patterns.
In September 2022, we launched a restructured PFT template in the electronic medical record (EMR) to indicate potential BLVR qualification and outline steps for referral. When applicable PFT parameters were met (prebronchodilator forced expiratory volume in 1 second [FEV1] <50% and residual volume [RV] >175%), the following comment would be included in the PFT report: “The combination of gas trapping and severe obstruction may qualify patients for lung volume reduction. Consider pulmonary referral for evaluation if clinically indicated. Consults -> Pulmonary -> COPD -> BLVR.” The EMR sent an automated notification to the PFT-ordering provider of the completed results for review. To improve referral tracking, a separate BLVR consult order was added to the EMR. An overview of the PFT review and referral process is provided in Figure 1.
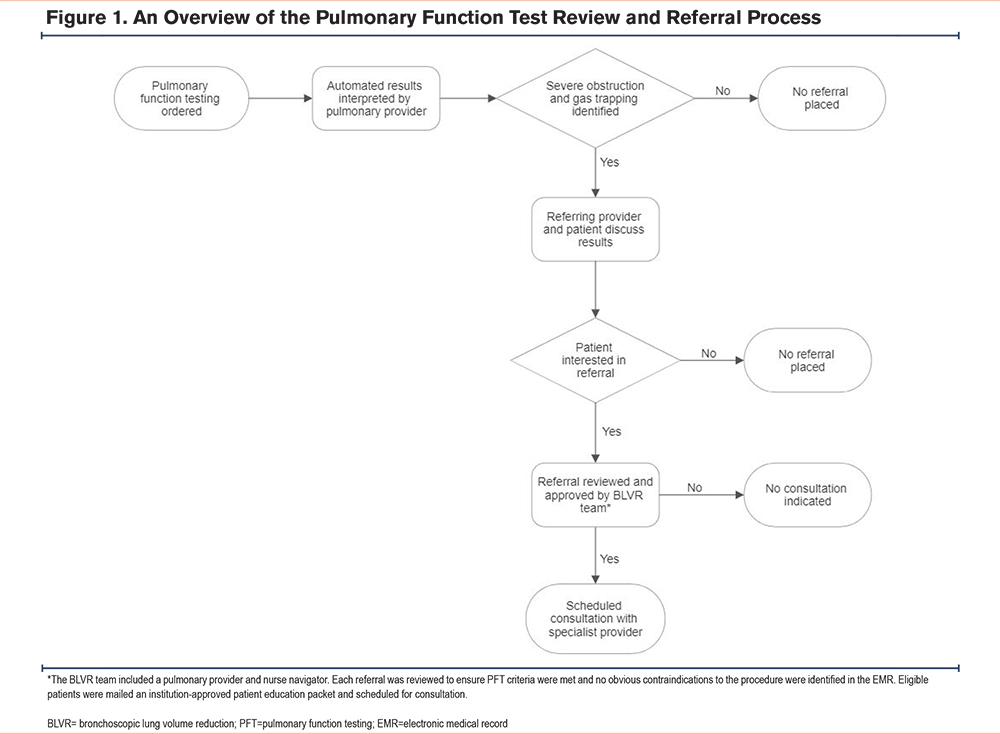
As part of the implementation process, pulmonary and primary care medicine staff were invited to a lecture focused on BLVR eligibility and the referral process specific to our institution. All BLVR evaluation referrals were reviewed by an interventional pulmonary provider and nurse navigator. Upon referral, patient eligibility for BLVR was determined based on previously published inclusion and exclusion criteria.5,6 As an internal quality check, our PFT system was queried biweekly to ensure the BLVR referral comment was added by the interpreting pulmonologist when PFT criteria were met.
Data Collection & Analysis
Baseline demographic information including age, gender, smoking status, PFT results, referring provider type, and comorbidities were reviewed. The comorbidity burden was calculated using the Charlson Comorbidity Index score.9 Charlson Comorbidity Index estimates the 10-year survival based on patient comorbidities. Comorbid conditions include age (1 point for every decade age 50 years and over), myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular accident or transient ischemic attack, dementia, COPD, connective tissue disease, peptic ulcer disease, liver disease (mild or moderate/severe), diabetes (with end-organ damage or uncomplicated), moderate to severe chronic kidney disease, hemiplegia, localized solid tumor, metastatic solid tumor, leukemia, lymphoma, and AIDS. Summary statistics for the postintervention cohort are presented as mean ± standard deviation (SD), median [interquartile range(IQR)], or n (%), as appropriate. An interrupted time series analysis was used to evaluate the change in referrals relative to the intervention and the time after intervention. The 2-proportion z-test was used to compare eligibility before and after intervention. Data analysis was conducted using R software10 version 4.1.2.
Results
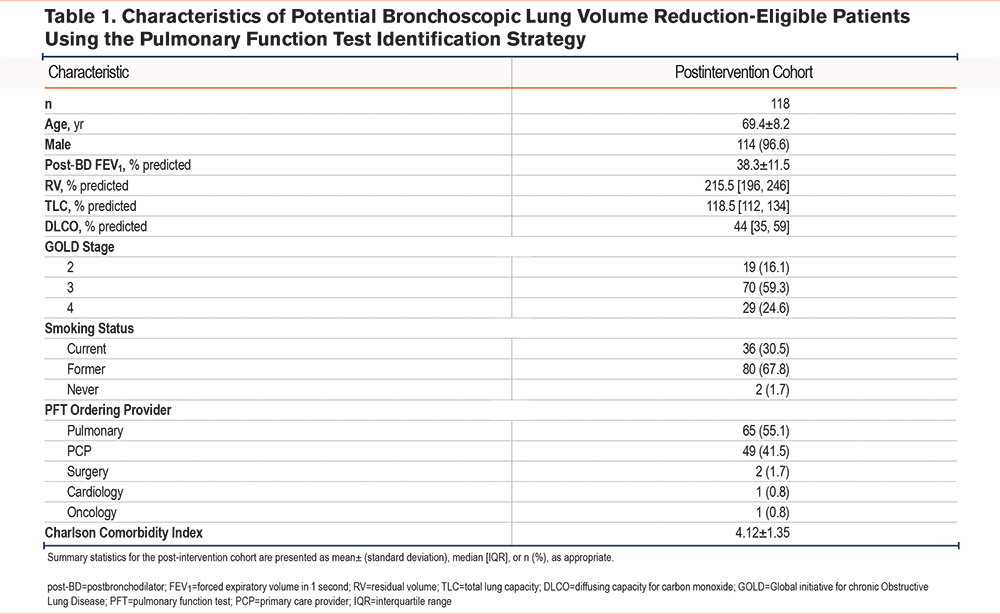
The postintervention cohort included 118 patients who were identified as potentially BLVR-eligible based on PFT criteria between September 2022 and April 2023 (Table 1). The mean age of the population was 69 years old, of which 97% were male. More than 80% were GOLD stage 3 or 4 with a mean postbronchodilator FEV1 of 38.3% ± 11.5% and an RV of 216% (196, 246). Most patients were former smokers with a mean Charlson Comorbidity Index of 4.12 ± 1.35. The PFT-ordering providers were predominantly pulmonary (55.1%) and primary care (41.5%) specialists.
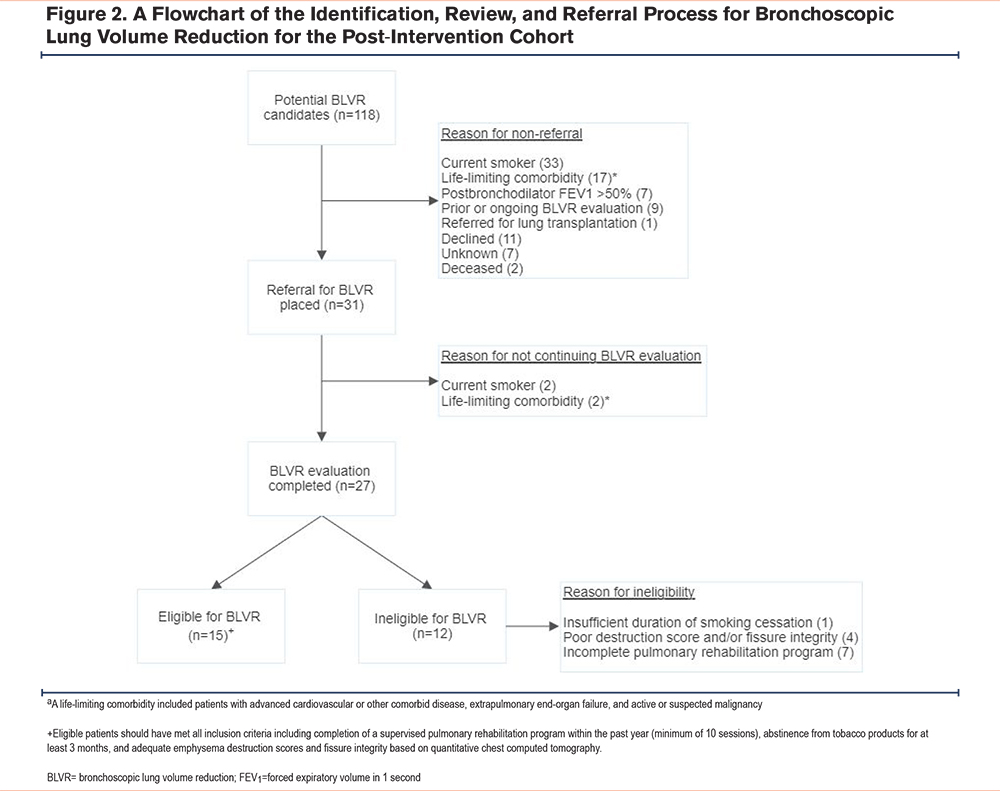
Before the intervention, 14 patients were referred to our program between January and August 2022 for BLVR evaluation. Of the 14 BLVR evaluations, 7 patients were candidates for the procedure, a rate of 0.9 eligible referrals per month. After implementation of the restructured PFT template, 31 patients had a referral placed for BLVR, 4 of whom were not sent for evaluation due to a procedure contraindication. A total of 27 patients were evaluated for BLVR, of which 15 were candidates for the procedure, a rate of 1.9 eligible referrals per month (Figure 2). All evaluations for BLVR were referred by either a pulmonary (70%) or primary care (30%) provider. Two of the 12 BLVR-ineligible patients were referred for evaluation of surgical lung volume reduction and lung transplantation.
Overall, the intervention led to a significant increase in the number of BLVR referrals (β=4.6, p=0.007). There was no significant trend (e.g., regression toward the number of referrals before intervention) in the 8 months after intervention (β=-0.61, p=0.07). In addition, there was no significant difference in the percentage of eligible patients before and after the intervention (p=0.99).
Discussion
The severe emphysema population continues to grow, making advanced therapeutic options like BLVR an important adjunct treatment strategy.11 Developing a system to identify eligible patients and educate providers about these therapies may significantly impact population morbidity and mortality. After implementing an institution-specific QI initiative, we observed a marked increase in the number of BLVR evaluations, and twice as many eligible referrals, a finding that was sustained in the 8 months of monitoring after the intervention. The positive change in referral patterns was partly attributed to the efforts of primary care providers who prior to the intervention did not account for any BLVR evaluations. In fact, we were pleasantly surprised that the percentage of eligible referrals was similar before and after implementation, suggesting many primary care specialists, like pulmonary, are providing optimal COPD care. Even for ineligible patients, this was a value-added consultation as they were assessed for optimal COPD therapy and other advanced interventions including surgical lung volume reduction and lung transplantation. Moreover, those yet to complete pulmonary rehabilitation or abstain from tobacco products for at least 3 months are still being longitudinally followed for BLVR eligibility.
There are some limitations to our work. While the number of BLVR referrals increased after project implementation, we cannot definitively attribute this change to restructuring the PFT report alone. It could be argued that increasing awareness and education drove the improvement in referrals; however, educational interventions tend to fade over time, meaning if the improvement in referrals was only due to education, we would expect decreasing effectiveness the longer from the intervention12,13 —a pattern not observed in our post-implementation cohort. Therefore, the PFT identification strategy, combined with provider education, is likely what accounts for the sustained improvement in our referral rates.
A potential drawback of our implementation approach was the inclusion of prebronchodilator, instead of postbronchodilator, FEV1 values <50%. This led to inaccurately identifying a small proportion with significant postbronchodilator improvement as potential BLVR candidates. It is also worth noting that our cohort was predominantly older males, limiting the generalizability of our results. Finally, although an increase in referral volumes was anticipated, this required close coordination with our referring providers and nurse navigator to ensure timely evaluation. It is foreseeable that with a growing advanced lung failure program, leveraging the support of nurse navigators, coordinators, and advanced practice providers will be instrumental to the program’s success.14
Conclusion
Implementing a restructured PFT template in conjunction with provider education was effective in improving case identification and referral rates for BLVR. Future quality improvement efforts should explore larger-scale VA and non-VA medical center implementation, with careful attention to institutional preferences and cultural norms.
Acknowledgements
Author contributions: CD and AMM contributed to the conception and design of the study, project implementation, data acquisition, data analysis and interpretation, and writing the manuscript. ZBS, EAE, CFCP, LCE, JLM, MAS, and SDW contributed to the conception and design of the study, project implementation, and writing the manuscript. All authors read and approved the manuscript for publication.
The contents of this work do not represent the views of the Department of Veterans Affairs or the United States government.
Declaration of Interest
CD has a consulting agreement with Pulmonx. AMM has received payment from LivaNova, Inc., for participation on a data safety monitoring board. The remaining authors have no relevant conflicts of interest to disclose.