Running Head: Patient-Centered COPD Self-Management Support
Funding Support: This project was supported by a grant from the National Heart Lung and Blood Institute (NHLBI) (1 R34 HL143747).
Date of Acceptance: October 2, 2023 | Published Online Date: October 9, 2023
Abbreviations: 6MWT=6-minute walk test; ADL=activities of daily living; CAT=COPD Assessment Test; CHW=community health worker; COPD=chronic obstructive pulmonary disease; CSM=Common Sense Model of Self-Regulation; DiD=difference in differences; ED=emergency department; FEV1=forced expiratory volume in 1 second; HADS=Hospital Anxiety and Depression Scale; HBPR=home-based pulmonary rehabilitation; IADL=instrumental activities of daily living; MARS=Medication Adherence Reporting Scale; mMRC=modified Medical Research Council dyspnea scale; PAAQ=Physical Activity Adult Questionnaire; PCP=primary care physician; RA=research assistants; SaMBA-COPD=Supporting self-Management Behavior in Adults with COPD
Citation: Federman AD, O’Conor R, Nnemnbeng J, et al. Feasibility trial of a comprehensive, highly patient-centered COPD self-management support program. Chronic Obstr Pulm Dis. 2024; 11(1): 13-25. doi: http://doi.org/10.15326/jcopdf.2023.0419
Online Supplemental Material: Read Online Supplemental Material (5411KB)
Introduction
Approximately 16 million Americans have chronic obstructive pulmonary disease (COPD),1 a disease associated with substantial morbidity and mortality and $37 billion in U.S. health care spending each year.2 Achieving significant reductions in the morbidity of COPD requires high quality, guideline-concordant care from clinicians and adherence to guideline-directed self-management practices by patients.3,4 This includes regular use of disease-controlling medications with correct inhaler technique, action plans to guide self-care in the event of exacerbations, routine primary care and pulmonary care when indicated, regular exercise or pulmonary rehabilitation, and routine vaccination for influenza and pneumococcal pneumonia.5 COPD patients, however, are often unable to achieve the levels of COPD self-management that guidelines recommend. For example, approximately half of patients do not use their medications regularly, up to 85% use their inhalers ineffectively and few use action plans or get recommended vaccinations.6-11 Use rates of pulmonary rehabilitation (PR) are very low, less than 2% following COPD hospitalizations, owing primarily to limited access.12
The factors that contribute to limited success with self-management in COPD are broad and include conditions both intrinsic and extrinsic to the patient, from socioeconomic issues to beliefs to quality of care and others. A comprehensive approach to helping patients achieve better self-management of COPD and better outcomes would consider the full range of barriers to effective self-management. Yet COPD self-management support interventions typically are narrow in their focus, and provide general COPD education and little in the way of tailoring to meet the individual needs of the patient.13-19 None of the cited studies in a Cochrane review19 of COPD self-management support interventions, for instance, were tailored to the complex needs of individual patients. Many of the interventions subjected patients to lengthy education sessions assuming knowledge deficits to be the cause of self-management problems.19 Such approaches may miss opportunities for more productive engagement with patients.
Recognizing the multifactorial contributors to self-management behaviors in patients with COPD, we developed a highly tailored self-management support intervention called Supporting self-Management Behaviors in Adults with COPD (SaMBA-COPD). The intervention is based on a successful program of self-management support for older adults with asthma, SaMBA-Asthma,20 and includes comprehensive screening for barriers to successful self-management in COPD followed by problem-solving and support to address the identified barriers. In this paper, we report the results of a pilot randomized clinical trial designed to test the feasibility of the intervention.
Methods
Participants and Setting
Details of the intervention and clinical trial were previously reported.21 Patients were recruited from the outpatient general medicine and pulmonary practices of the Mount Sinai Hospital in East Harlem and were ages ≥40 years, resided in the community, had chart-documented severe or very severe COPD (International Classification of Diseases-10th Revision diagnosis code for COPD and forced expiratory volume in 1 second [FEV1] <50% predicted) based on Global Initiative for Chronic Obstructive Lung Disease criteria,5 were prescribed daily medication for COPD, and were English or Spanish speaking. We excluded patients with a history of asthma and those with a diagnosis of dementia in their medical records.
The prespecified recruitment target was 60. The electronic medical record was queried to identify a pool of patients meeting eligibility criteria. Research assistants (RA) then reviewed their medical records to verify eligibility, contacted the patients’ primary care physicians for permission to recruit, and then mailed a recruitment letter to a random selection of patients. Two weeks later, the RAs called patients to administer an eligibility screener and invite them to participate in the study. Informed consent was obtained at the time of the baseline (in-person) interview. Patients were recruited between August 2020 and March 2023. All study procedures were approved by the institutional review board of the Icahn School of Medicine at Mount Sinai.
Conceptual Framework
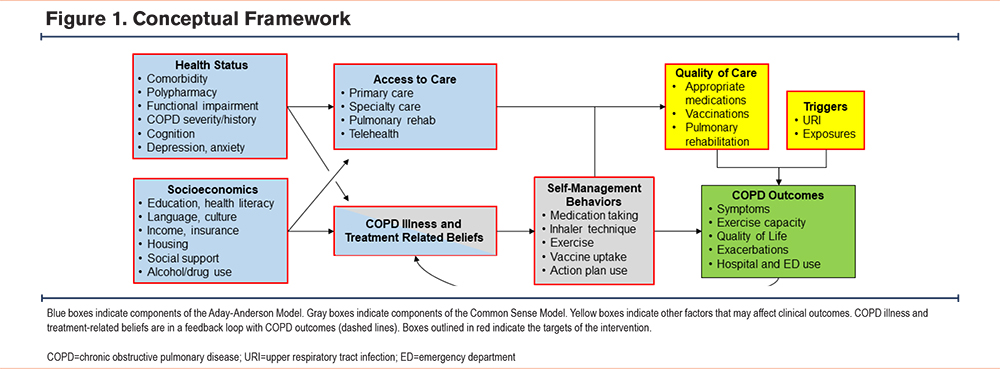
The intervention design was guided by a conceptual framework that integrates the Common Sense Model of Self-Regulation (CSM) and the Aday-Anderson model of health services used to explain health-related behaviors in the context of COPD (Figure 1).22,23 The CSM proposes that 5 domains of beliefs, or illness representations, drive health-related behaviors: identity or attribution of symptoms, timeline, cause, consequences. and control. Illness representations affect behavioral responses (self-management behaviors),24,25 which, in turn, influence COPD outcomes like symptoms and quality of life. Beliefs are in a feedback loop with behaviors and outcomes. The Aday-Anderson model addresses the determinants of behaviors and health outcomes that are not explicitly addressed by the CSM.23 The model encompasses 3 domains of determinants of health care utilization: need (health status factors), enabling (for example, insurance coverage, health literacy), and predisposing factors (beliefs). The enabling and predisposing factors recognize that self-management behaviors function within and are influenced by the surrounding environmental and social contexts. For example, patients with significant social stressors, such as financial insecurity and housing and employment instability, may be unable to prioritize and consistently engage in routine self-management. The SaMBA-COPD intervention is designed to act on the modifiable elements of these interactions.
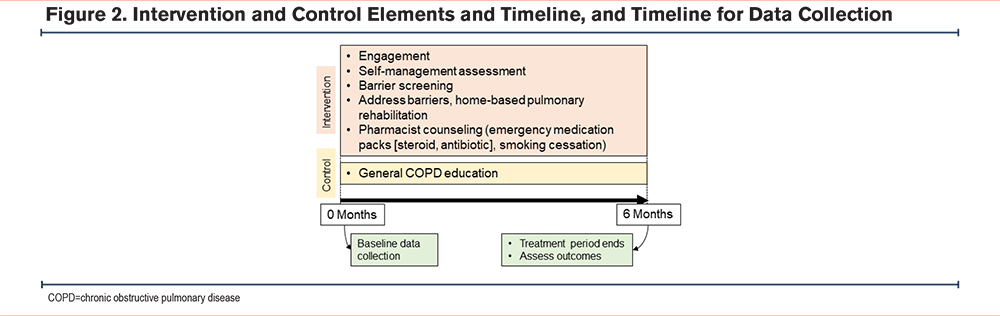
Intervention
Details of the intervention are provided elsewhere. A schematic depiction of the intervention, control, and timeline for data collection is shown in Figure 2, and all intervention materials are provided in the online supplement.21 In brief, the intervention has 3 core elements: (1) patient-centered, comprehensive screening for and specific targeting of barriers to successful self-management of COPD, (2) delivery of the intervention by community health workers (CHW) to optimize cultural concordance and support sustainability, and (3) incorporation of home-based PR (HBPR) to improve access to this highly effective but underused treatment modality. The patient-CHW interaction begins with an assessment of the patient’s COPD-related goals using a strategy based on patient priorities of care.26,27 Progress toward the expressed goals is routinely evaluated during the intervention. The CHW also assesses their COPD symptoms, reviews their medications, and assesses adherence. Errors in inhaler technique are identified and corrected. Next, the CHW administers the SaMBA-COPD screener to identify barriers to successful self-management of COPD.
At the completion of screening, the CHW and patient review the identified barriers and decide which ones are most important to address to achieve their individual goals. The SaMBA-COPD workbook provides a menu of possible actions to take to address each barrier. Examples of actions include the following:
- For patients who forget to routinely use their inhaled controller medications, the CHW discusses varied reminder strategies like keeping the medication next to their toothbrush.
- For smokers, the CHW counsels on quitting, discusses medical treatment options and offers to arrange a visit with their primary care physician (PCP) or a pharmacist for medication prescribing.
- If the patient screens positive for depression and is not already on depression medications, the CHW talks about how depression may affect self-care and offers to schedule a visit with their PCP.
- Alternatively, the patient and CHW may devise courses of action that are not listed in the menus.
The CHW also discusses the options of HBPR and an action plan to treat COPD exacerbations. Patients who express a willingness to participate in HBPR meet in person with a respiratory therapist to undergo an evaluation of their exercise capacity, for both aerobic and resistance exercises. Following the evaluation, the respiratory therapist creates an exercise plan for the patient. The CHW observes the patient performing the exercises at home to ensure safe and proper technique and provide encouragement. Patients interested in action plans for COPD exacerbations are seen by a clinic-based pharmacist, in person or remotely. The pharmacist discusses the option of treating COPD exacerbations in their early stages with oral steroids and antibiotics (a rescue pack) and reviews a written action plan with instructions on when and how to use the medications. The pharmacist also discusses medications for smoking cessation with the patient if they are an active smoker. If the patient agrees to any of these treatments, the pharmacist prepares and pends the prescription order and notifies the patient’s physician. The physician is responsible for signing the order but has the option of modifying or canceling it.
The intervention was originally designed for in-person initial and follow-up encounters supplemented with telephone calls. However, the COVID-19 pandemic necessitated a switch to all-remote procedures (telephone and/or video) for the intervention and control treatments before any patients were recruited for the trial. To accommodate patients for the remote intervention, we provided those in both arms with internet-connected tablets if they lacked this technology (n=26 [44%]). Content of the intervention and control treatments did not change. The CHW recommended meetings 1, 2, and 4 weeks after intake then monthly until month 6 when the intervention ended. However, patients and the CHW had the option of tailoring the frequency and timing of their encounters. Patients who chose to participate in HBPR were required to have an encounter with the CHW, by video conference, if possible, before starting the home exercise program. CHWs used an educational booklet suitable for persons with low health literacy, “COPD 1-2-3,” to facilitate their counseling and provide to the patients as an at-home resource. CHWs in both the intervention and control arms provided counseling in English or Spanish.
Time and Attention Control Treatment
The attention control consisted of 6 sessions with the CHW (approximately 1 per month) via video conference or telephone, per patient preference, for general COPD education using the “COPD 1-2-3” booklet. To avoid contamination, CHWs in the control arm were not trained in the SaMBA-COPD methods. During visits 1–3, they walked the patient through “COPD 1-2-3”; in visits 4–6, they made “check-in” visits to discuss symptoms, answer questions, and review specific sections of “COPD 1-2-3” as requested by the patient.
Randomization and Blinding
Patients were block-randomized in strata of COPD severity (severe and very severe) to the intervention and control arms using an algorithm implemented in Research Electronic Data Capture (REDCap) survey software.28 The study project manager notified patients of their treatment assignment by telephone. The research coordinators conducting patient interviews and the investigators were blinded to the assignment.
Data Collection and Measures
Interviews and assessments were conducted in person, in English or Spanish, at baseline and 6 months. The feasibility evaluation followed an established framework that includes acceptability among patients and clinicians, implementation and practicality (or fidelity of execution and completion of intervention tasks), and limited efficacy (intervention effects should be consistent with hypotheses, though effects need not be statistically significant).29 For acceptability, we evaluated study enrollment, clinicians’ assent to recruit their patients and allow rescue pack prescribing, and patients’ satisfaction and experiences with the intervention, which were assessed during an exit interview. Intervention implementation and practicality measures included the proportions of patients who underwent barrier screening, had 4 or more encounters with the CHW, underwent evaluation for HBPR, were prescribed rescue packs, and completed the study.
For efficacy, we assessed clinical (symptoms, functioning, and health services use) and self-management (medication adherence) outcomes. COPD symptoms were measured with the 8-item COPD Assessment Test (CAT) (score range 0–40).30,31 Exercise capacity was measured with the 6-minute walk test (6MWT).32 Exercise in the past 7 days was measured with the 13-item Physical Activity Adult Questionnaire (PAAQ), which correlates well with accelerometer-measured activity. Higher scores indicate higher levels of physical activity.33 Hospitalizations and emergency department (ED) visits in the prior 6 months for COPD exacerbations or any respiratory issues were assessed by self-report. Self-reported adherence with inhaled controller COPD medications was measured with the Medication Adherence Reporting Scale (MARS), a 10-item scale validated for use with asthma and COPD medications.34, 35 Adherence was defined as a score ≥4.5. Medication adherence was also measured using electronic monitoring devices, the Doser CT (Meditrack, Hudson, Massachusetts) for metered-dose inhalers and the Smartdisk (Nexus6, Franklin, Ohio) for dry powder inhalers. Patients used the devices over a 4-week period of observation. Adherence was defined as use of ≥80% of doses prescribed.
To characterize the study population, we collected data on basic demographics, social support, COPD history, physical and mental health, and functional and cognitive status measures that might influence self-management of COPD and COPD outcomes as suggested by our conceptual model. Health literacy was measured using a 3-item screener.36 Self-perceived social support was measured with the Social Provisions Scale, with higher scores indicating greater support.37 The Charlson Comorbidity Index was used to summarize the burden of chronic illness.38 Activities of daily living (ADL) included bathing or showering, dressing, eating, getting in or out of bed or chair, walking, and toileting. Instrumental activities of daily living (IADL) included the ability to use a telephone, shop, prepare food, perform housekeeping chores, do laundry, use public transportation, self-manage medications, and handle finances. Impairments for each were indicated by difficulty performing or requiring assistance to perform the activity. Scores for ADL and IADL impairments were the sum of impairments for the 6 and 8 items of the measures, respectively. Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS).39
Analyses
We compared the baseline characteristics of study participants in the intervention and control groups using a t-test, Wilcoxon Rank Sum test, or Chi-square test, as appropriate. We used a difference-in-differences (DiD) approach to examine the effects of intervention and control over time. Analyses were performed using generalized linear models with a REPEATED statement (SAS Proc GENMOD, SAS Institute, Inc., Cary, North Carolina). As a feasibility trial, the study was not powered to detect statistically significant differences for outcomes between intervention and control arms, though P <0.05 was considered statistically significant for all outcomes analyses. All analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Recruitment
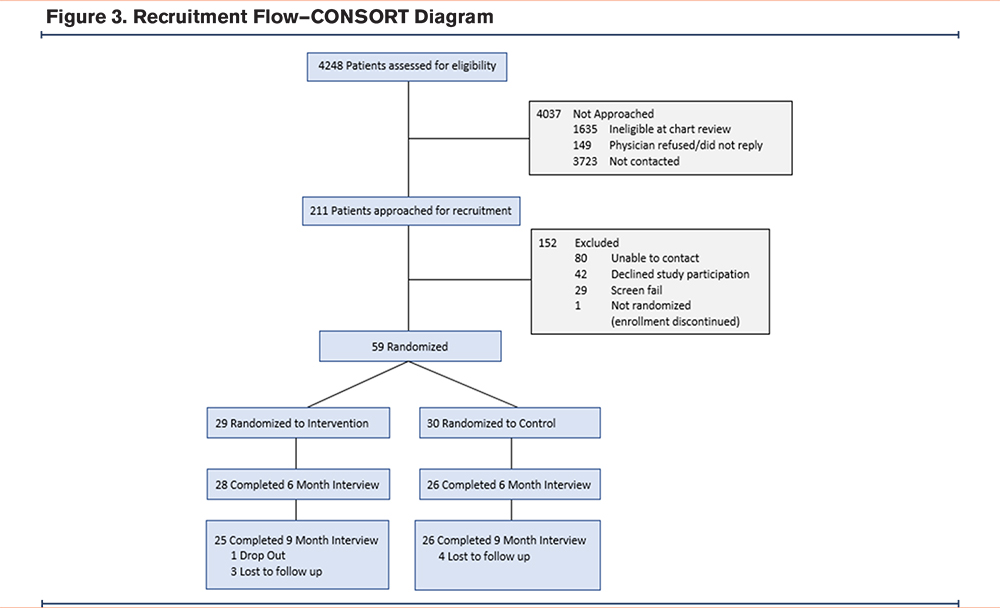
The recruitment results are shown in the CONSORT diagram (Figure 3). Of 4248 patients identified in the electronic health record query, 1635 were ineligible, 149 had a physician who declined patient participation (or did not respond to the request), and 3723 were not contacted; 211 patients were deemed eligible and approached for recruitment. A total of 60 participants were enrolled in the study, 59 of whom were randomized to receive the intervention (n=30) or control condition (n=29). Retention was 88% at 6 months. There was 1 dropout, and 7 patients were lost to follow-up.
Patient Characteristics
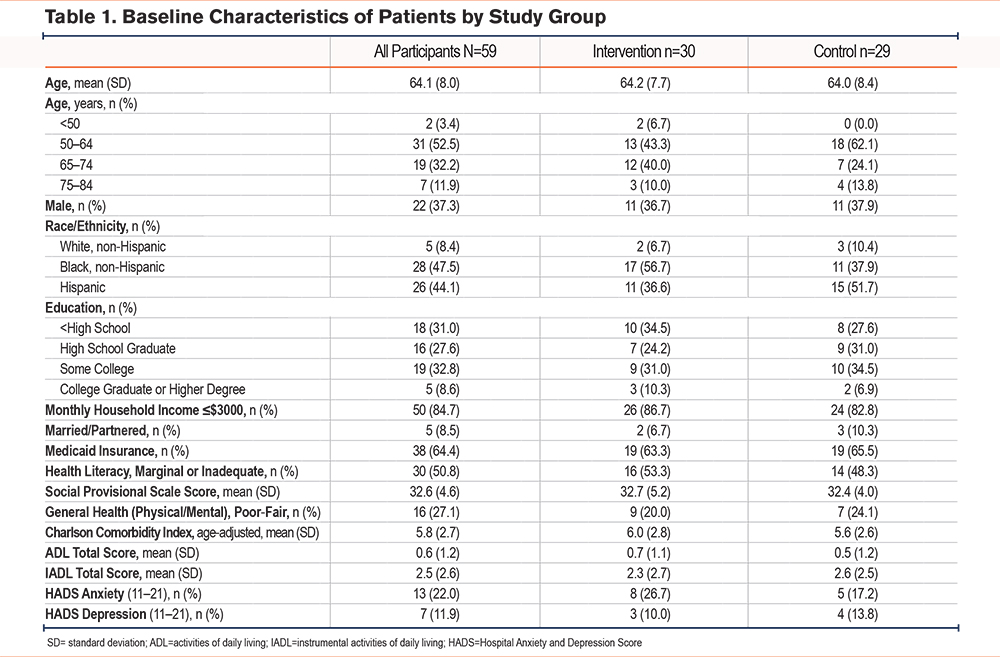
Baseline patient demographics, health status, and COPD-specific measures were balanced between intervention and control arms (Table 1). The mean age of the 59 randomized participants was 64.1 (8.0) years, 22 (37.3%) were male, 28 (47.5%) were Black non-Hispanic, and 26 (44.1%) were Hispanic; 38 (64.4%) received Medicaid benefits and 50 (84.7%) had a monthly income less than $3000. Twenty-four participants (41.4%) had some college education and 30 (50.8%) had marginal/inadequate health literacy.
The mean CAT score at baseline was 22.0 (8.4) and 61% of participants had high impact CAT scores (≥20). The mean modified Medical Research Council dyspnea scale (mMRC) score was 3.1 (1.1). The mean 6MWT distance was 265.9 (98.4) meters. Twenty-nine (49.2%) had a history of hospitalizations and 34 (57.6%) had past ED visits for COPD. Adherence to controller medications was low, 46.4% based on self-report.
Feasibility
Physicians agreed to recruitment for 91% of patients considered for the study, and all physicians who provided their assent for recruitment agreed to HBPR participation and rescue pack prescribing. Among intervention patients, 89% met with a community health worker 4 or more times. Screening for self-management barriers was completed for all intervention patients. Among those intervention patients who agreed to participate in HBPR (n=27, 90%), 75% underwent the HBPR evaluation and were observed on average 7.4 times (standard deviation) by the CHW. CHWs offered to arrange a meeting with the pharmacist to discuss rescue pack medications for 12 (41%) patients but only 5 (17%) had the actual encounter. There were no adverse events.
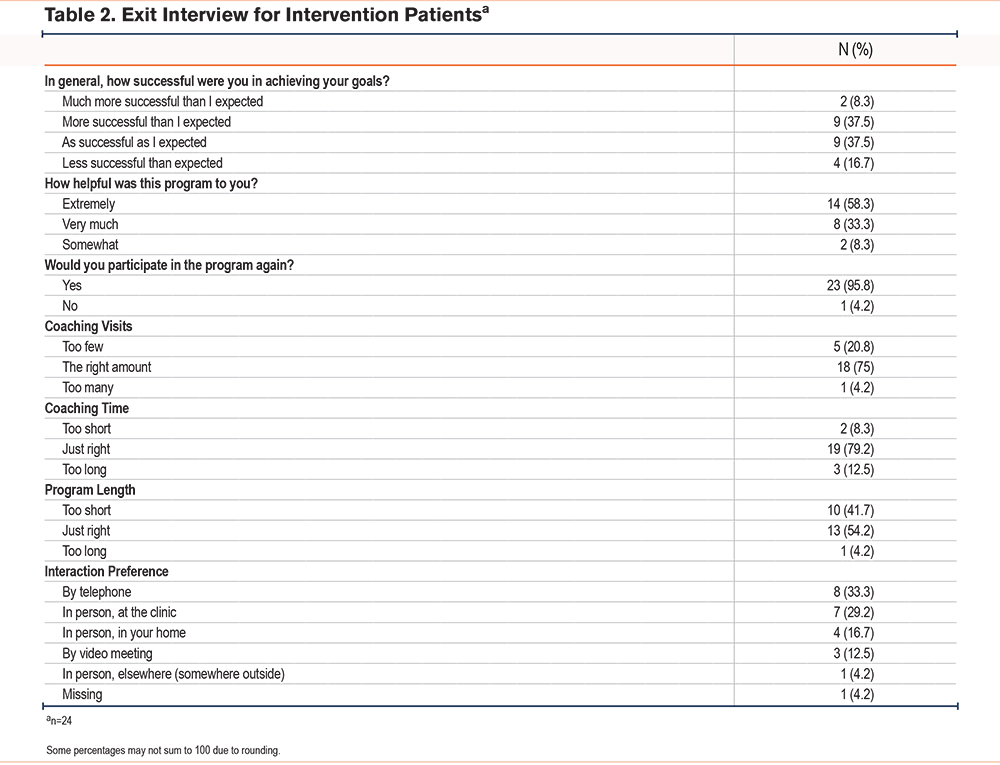
Exit interviews were completed by 24 intervention patients. Patients reported that the program was very or extremely helpful (92%) and 46% reported that they were more successful in achieving their goals than they had expected; 96% said they would participate in the program again (Table 2). Most participants believed the number and duration of coaching encounters were appropriate, although 42% believed that the 6-month program was too short. Approximately half (52%) preferred interactions to take place in person.
Evaluation of Clinical Outcomes
For clinical outcomes, the results of analyses generally favored the intervention (Table 3). On average, CAT scores in the intervention group declined from baseline to 6 months, whereas they increased in the control group, with a DiD of -1.1 (95% confidence interval [CI] -5.9 to 3.6). The 6MWT distance similarly improved for intervention patients more than for controls, for a DiD at 6 months of 7.4 meters (95% CI -45.1 to 59.8). Self-reported hospitalizations were lower for intervention patients (DiD -9.8% [95% CI -42.3% to 22.8%]) but ED visits were higher (DiD 10.6% [95% CI 17.7% to 38.8%]). Intervention patients used fewer quick reliever doses each week (DiD -9.6 [95% CI -22.5 to 3.3]).
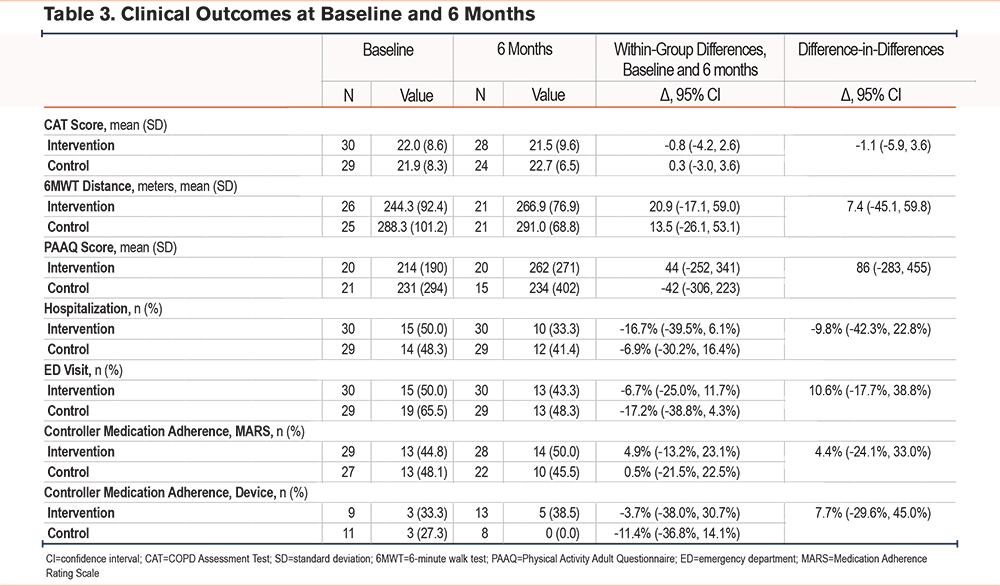
For self-management behaviors, intervention patients had slightly better controller medication adherence by self-report (DiD 4.4% [-24.1%, 33.0%]) and objective inhaler monitor (DiD 7.7% [-29.6%, 45.0%]). They also self-reported more physical activity, as measured by the PAAQ (DiD 86 [95% CI -283 to 455]).
Discussion
This pilot randomized controlled trial of the SaMBA-COPD self-management support program demonstrated that most elements of the intervention were feasible and acceptable to patients and provided preliminary data suggesting efficacy.
The SaMBA-COPD program is distinguished from other COPD self-management support interventions by its comprehensive approach, which involves screening for the full range of contributors to personal challenges with COPD self-management and control, including social determinants of health, and its focus on addressing the identified barriers rather than pursuing a one-size-fits-all approach to patient support. It is thus suitable for supporting individuals, including those who participated in this study, who face significant psychosocial stressors and minoritized groups that have historically experienced high levels of illness burden and poor access to care. Additionally, it includes HBPR and action plans with rescue pack prescriptions to round out the approach to supporting patients with evidence-based strategies to improve COPD outcomes. To support sustainability, the program uses CHWs, which stands in contrast to other COPD self-management support programs involving HBPR that use nurses or respiratory therapists. All the elements of the SaMBA-COPD intervention are, thus, geared toward addressing gaps in health equity for at-risk populations.
The highly tailored approach is a unique feature of SaMBA-COPD. The 29 COPD self-management support intervention studies included in a Cochrane review, as well as individual studies not included in the review, largely focused on general education and or skills training, but with tailoring often limited to a small number of barriers or goals identified by the patient.19,40,41 One study with a particularly large impact on hospital admissions, an 11.4% absolute risk reduction, involved motivational coaching for medication adherence by a respiratory therapist, a written action plan for exacerbations, and brief instructions on home exercise (n=215).13 While this and other interventions13,42 have produced meaningful outcomes, further gains may be achievable through more highly patient-centered and comprehensive approaches.
Our study is also notable for its use of CHWs. CHWs are lay persons trained to provide chronic illness self-management support. Typically, residents of the communities in which they work, CHWs develop trusting relationships with patients, social networks, and other community members and organizations, enabling them to serve as health care liaisons in the community.43 The National Academy of Medicine has endorsed the employment of CHWs based on their success with chronic illnesses like type 2 diabetes and asthma.44 Although also recommended for COPD patients,45 CHW programs for COPD patients have not been described until the present study.
One unique feature of the current study is the use of CHWs to guide patients through HBPR. PR interventions consisting of graduated aerobic exercise and upper extremity resistance training to date have typically involved respiratory therapists or nurses.46 In a move toward sustainability, others have tested less formal home exercise interventions that involve remote coaching and monitoring to support patients.42,47,48 Such programs have not resulted in the same level of improvements in physical activity and symptom reduction as the more intensive, nurse or respiratory therapist driven interventions. For example, a health coach-directed intervention that promoted 30 minutes of daily exercise (n=305) did not improve respiratory symptoms or 6MWT distance for COPD patients, although it did reduce sedentary time and lung-related health care utilization.48 A more recent study of video-guided home exercises with supportive and motivational telephonic health coaching (n=375) showed somewhat better quality of life and physical activity gains but was still short of the impact achieved in formal PR programs.42
Using CHWs to guide patients through HBPR during home visits could fill a gap between the low-intensity approach of remote patient-health coach encounters and the higher-intensity services provided by licensed respiratory therapists or nurses remotely or in person. Ultimately, the most effective self-management support programs may be those that adjust their modality and intensity of service, including place of encounter, frequency and duration, and professional delivering it. Given the plethora of research describing different COPD self-management support programs, the next critical challenge for researchers in this field may be identifying which patients benefit most from different modalities and intensities of treatment.
One notable shortcoming of the intervention was the low rate of referrals to and visits by patients with the pharmacist to discuss action plans and rescue pack prescribing. Future iterations of the intervention will need to focus on identifying and developing strategies to address barriers to engaging patients with this service.
The findings of this study are notable for the rapid conversion of the intervention from an in-person format to all remote delivery through video conferencing and telephone, or telephone alone. This change was made shortly before recruitment was intended to begin because of the COVID-19 pandemic, which ensued about 6 months after the project was launched. The SAMBA-COPD intervention is highly complex, involving a number of activities that may benefit most from in-person encounters, such as building a strong patient-coach rapport, home exercise training, review of inhaler technique and training, and review of the safety of the home environment. Given this and the advanced ages and frailty of study participants, SAMBA-COPD may not be suited to fully remote delivery.
Study Limitations
This study was designed as a pilot for feasibility assessment and was not powered for the outcomes we measured. A fully powered randomized trial is needed to rigorously assess the clinical efficacy of the intervention. Some outcomes were assessed by self-report, including the use of acute care services for COPD exacerbations, which may be subject to recall and other biases. The intervention was designed for in-person delivery but required a rapid, and untested change to remote delivery because of the COVID-19 pandemic, which may have diminished its impact. Finally, the study was not designed to identify the features of the intervention that were effective and those that were not. A fully powered study would provide an opportunity to do so.
Conclusion
In conclusion, SaMBA-COPD is a novel COPD self-management support intervention that was well received by patients, performed well on several other feasibility measures, improved patient outcomes, and produced no adverse events. A fully powered randomized trial of the intervention is warranted to test its efficacy.
Acknowledgements
Author contributions: ADF takes full responsibility for the article, including for the accuracy and appropriateness of the reference list. ADF, RO, MSW, and JPW conceived the study. ADF, RO, MSW, PL, MM, and JPW were involved in study design, protocol development, researching literature, and gaining ethical approval. All authors contributed substantially to data analysis and interpretation, and the writing of the manuscript. All authors approved the version of the manuscript submitted for publication.
Clinicaltrials.gov registration # NCT04533412.
Declaration of Interests
Dr. Wisnivesky received consulting honoraria from Sanofi, PPD, Banook, and Prospero and research grants from Sanofi, Regeneron, Axella, and Arnold Consultants. All other authors have nothing to declare.