Running Head: Pulmonary Rehab for Alpha-1 Antitrypsin Patients
Funding Support: N/A
Date of Acceptance: October 3, 2023 | Published Online Date: October 9, 2023
Abbreviations: %pred=percentage predicted; 6MWT=6-minute walk test; AATD=alpha-1 antitrypsin deficiency; AAT=alpha-1 antitrypsin; BMI=body mass index; CFR=capillary to fibers ratio; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CRP=C-reactive protein; CT=computed tomography; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; HRQoL=health-related quality of life; IL=interleukin; MCID=minimal clinically important difference; PaO2=partial pressure of arterial oxygen; PaCO2=partial pressure of arterial carbon dioxide; PCO2=partial pressure of carbon dioxide within arterial or venous blood; PGC-1α=peroxisome proliferator-activated receptor gamma coactivator1-alpha; PFT=pulmonary function test; PR=pulmonary rehabilitation; PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analysis; PWR=peak work rate; SF36=short form health survey; TFAM=mitochondrial transcription factor; TNFα=tumor necrosis factor alpha
Citation: Alwadani FA, Wheeler K, Pittaway H, Turner AM. Pulmonary rehabilitation for chronic obstructive pulmonary disease patients with underlying alpha-1 antitrypsin deficiency: a systematic review and practical recommendations. Chronic Obstr Pulm Dis. 2024; 11(1): 121-132. doi: http://doi.org/10.15326/jcopdf.2023.0434
Online Supplemental Material: Read Online Supplemental Material (169KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an underdiagnosed genetic disorder characterized by low circulating alpha-1-antitrypsin (AAT) levels and specific disease-associated genotypes. 1,2 AAT, a vital enzyme, shields lung elastic tissue from harmful neutrophil elastase, particularly during lung infections or inflammation. Insufficient AAT has been linked to the early onset of obstructive lung diseases like emphysema and chronic obstructive pulmonary disease (COPD).3,4 Studies reveal that COPD patients with AATD are, on average, 10 years younger and have a significantly shorter history of smoking compared to usual COPD patients.5,6
Despite its rarity (about 1 in 2000 individuals of European descent), AATD is found in 2%–5% of usual COPD patients.3,4,7 These patients experience COPD symptoms, but often with greater dyspnea, rapid exercise-induced oxygen desaturation, and poorer lung function outcomes. 5,8,9 These severe symptoms are linked to accelerated emphysematous changes in lung tissue as AATD progresses.10 Additionally, a high prevalence of liver disease is seen among AATD patients and is indicative of the hepatocytes' inability to release AAT into the serum.9,11,12
Management strategies for these patients often involve regular COPD recommendations, including the use of inhalers, steroids, and antibiotics based on the severity of exacerbation and its underlying cause.13 In addition, select AATD patients receive intravenous infusion of AAT enzyme, commonly referred to as augmentation therapy, with some evidence that suggests a slower rate of emphysema progression on a computed tomography (CT) scan for those augmented with the deficient enzyme.13,14 However, evidence of its clinical efficacy in terms of reducing the number of exacerbations and improving quality of life has been limited.15
AATD patients with chronic lung conditions are also eligible candidates to participate in a pulmonary rehabilitation (PR) program, a multifaceted health care service that includes exercise therapy and educational sessions with each component serving a different purpose.16,17 The clinical effectiveness of PR, particularly its exercise component, in enhancing the functional capacity and quality of life of patients with lung diseases cannot be overlooked.18 However, the integration and potential varying needs of AATD patients in PR programs, considering factors like age, disease stage, educational content, and exercise intensity, are less understood. Notably, one study underlined a difference in exercise response between usual COPD and those with underlying AATD, indicating that the latter group experienced less improvement in slow oxidative muscle fibers after a 3-week exercise intervention.19
This review has 2 principal objectives: (1) extensively assess PR effectiveness in managing COPD patients with AATD, focusing on exercise-related impacts on functionality and quality of life, and (2) compile existing evidence regarding exercise interventions for AATD patients.
Methods
Search Strategy
We searched 4 databases - Ovid via Embase, Medline via Embase, CINAHL plus via EBSCO, Cochrane Central Register of Controlled Trials via Wiley - and clinicaltrials.gov for relevant studies from September 2022 to October 2022. The search combined MeSH terms related to "alpha-1 antitrypsin deficiency" AND "pulmonary rehabilitation" OR "exercise" and separately "alpha-1 antitrypsin deficiency" AND "muscle morphology". This was done in accordance with our registered protocol at PROSPERO, an international prospective register of systematic reviews (CRD42022367250). Supplementary materials (in the online supplement) provide more details on the search terms and syntaxes used.
Inclusion and Exclusion Criteria
The inclusion criteria for the studies included in the review covered prospective and retrospective trials of PR or other exercise-focused interventions for AATD COPD patients, and case series of over 10 AATD patients, due to the disease's rarity. The search focused on studies of exclusively AATD patients, defined by severe deficiency variant genotypes (SZ and ZZ) or AAT levels below the protective threshold of 0.57 g/L. Comparators were AATD patients on usual care, or usual COPD patients on PR or exercise intervention. Primary outcomes were exercise capacity measures, such as the 6-minute walk test (6MWT), shuttle walking, and quality-of-life assessments. There were no limitations on study outcomes or publication language.
Data Extraction and Analysis
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was implemented for the screening process. Studies identified were screened for their titles and abstracts using the Rayyan tool20 by 2 independent reviewers. Persistent disagreements were resolved through full-text reviews and consultation with a third reviewer. Study quality was assessed independently by the same reviewers using ROBINS-1 for quasi-experimental and retrospective cohort studies, and RoB2 for randomized clinical trials.21 For the quality assessment, a third reviewer was consulted for finalization. Data extraction was initially performed by the primary author and then reviewed and finalized by a second reviewer. Three studies that reported the primary outcome of interest (mean improvement in 6MWT) were pooled in a meta-analysis using a random-effects model.22 The effect size was determined by mean differences with 95% confidence intervals (CIs), considering a P-value <0.05 as statistically significant.
Results
Study Selection
Figure 1 shows the result of the screening process following the PRISMA model. Initially, we retrieved 1016 records from the 4 identified databases in addition to 2 other records for ongoing clinical trials from www.clinicaltrials.gov. Of these, 221 were duplicates and were, thus, removed at the beginning of the screening process. After screening the titles and abstracts, 768 records were excluded, while 29 records qualified for full text review. Full text articles were not available for 3 conference abstracts, and 2 ongoing clinical trials, and they were excluded after contacting the authors and confirming their unavailability for full text review. We were also unable to retrieve the full text of 3 other abstracts because they were relatively old, and they were not available on any accessible database. Of the 21 publications which were reviewed in full, only 5 articles were found to be eligible for inclusion, 4 pertaining to PR and one to a single exercise session intervention.
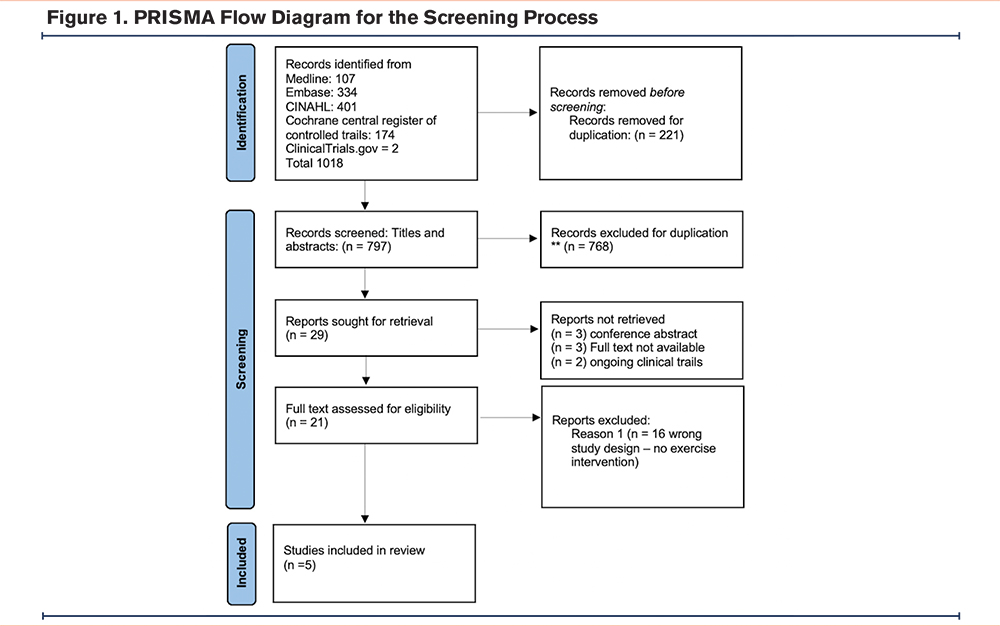
Pulmonary Rehabilitation as the Main Intervention for Alpha-1 Antitrypsin Deficiency Patients
Four studies were identified that explored the impact of PR on AATD patients. The first, a small quasi-experimental study based in Germany,19 involved 2 groups of 9 AATD patients and 10usual COPD patients, matched by age and with comparable baseline characteristics, except for significantly higher partial pressure of arterial carbon dioxide (PaCO2) levels of 43.8 mmHg among the latter. Over 3weeks of inpatient PR, both groups showed an upward trend in exercise capacity, as determined by the 6MWT and peak work rate (PWR). However, improvements in PWR were more pronounced in usual COPD patients (p<0.05). Interestingly, muscle morphology differed between groups. The usual COPD group exhibited a minor increase in type 1 muscle fiber with no observable change in the AATD group. Also, while type II muscle fibers decreased in the usual COPD group, an opposite trend was observed in the AATD group. As for mitochondrial signaling, key markers peroxisome proliferator-activated receptor gamma coactivator 1-alpha ( PGC-1α) and mitochondrial transcription factor A (TFAM) significantly increased in the usual COPD group but not in the AATD group. Capillary to fibers ratio (CFR), however, increased in the AATD group with no marked changes in the usual COPD group (see Table 1).
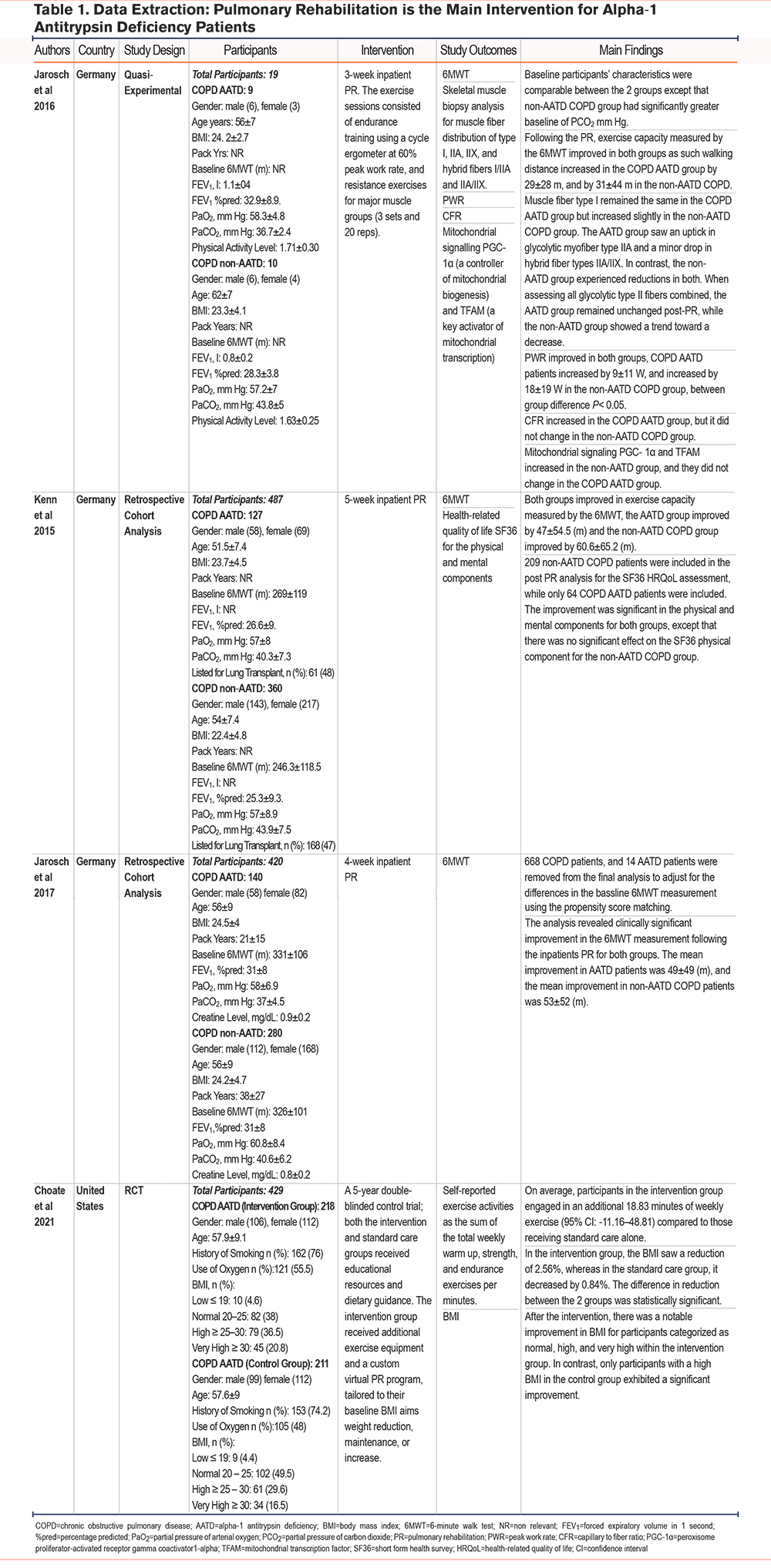
Two other retrospective cohort studies from Germany,23,24 involving significantly larger sample sizes (267 AATD and 640 usual COPD patients), reported similar findings in exercise capacity measured by the 6MWT. Both groups showed improvement after undergoing 4 and 5 weeks of inpatient PR, respectively. However, the AATD group in the 2017 study by Jarosch et al24 had a higher baseline 6MWT, leading to the exclusion of 682 participants via propensity score matching. Additionally, the 2015 study by Kenn et al23 reported incomplete data for quality of life, as assessed by the short form health survey (SF36), for more than half of the participants from both groups. Despite this, both groups demonstrated significant improvement in mental health measurements, but the improvement in the physical domain was significant for the AATD group only.
Finally, a 5-year longitudinal study in the United States by Choate et al25 divided AATD patients into a control group, receiving only educational materials, and an intervention group receiving additional exercise equipment and virtual PR. Participants' baseline characteristics were similar across both groups, with most participants being White (98.3%). However, baseline characteristics for lung function or exercise capacity were not reported. The findings demonstrated that the intervention group engaged in significantly more exercise during the study (18.8 additional minutes of weekly exercise) and experienced a greater reduction in body mass index (BMI): 2.56% reduction as compared to 0.84% in the control group.
Proinflammatory Cytokines in Alpha-1 Antitrypsin Deficiency Patients During Exercise Intervention
In the 2014 study by Olfert et al,26 differences in proinflammatory cytokines during a single moderate-intensity exercise session were explored among various groups: AATD patients receiving augmentation therapy, AATD patients not on therapy, usual COPD patients, a healthy control group, and an AATD group with normal lung function. The groups were age and BMI-matched, with COPD patients with underlying AATD presenting significantly poorer lung function, as illustrated by lower forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio. Researchers evaluated circulating inflammatory cytokines (C-reactive protein [CRP], interleukin [IL]-6, IL-1 beta, and tumor necrosis factor alpha [TNFα]) in arterial and venous blood during and post-exercise, which involved a 1-hour, moderate-intensity single knee extension. Additionally, pre and postexercise muscle biopsies from the vastus lateralis were examined to determine skeletal muscle cytokine expression (Table 2).
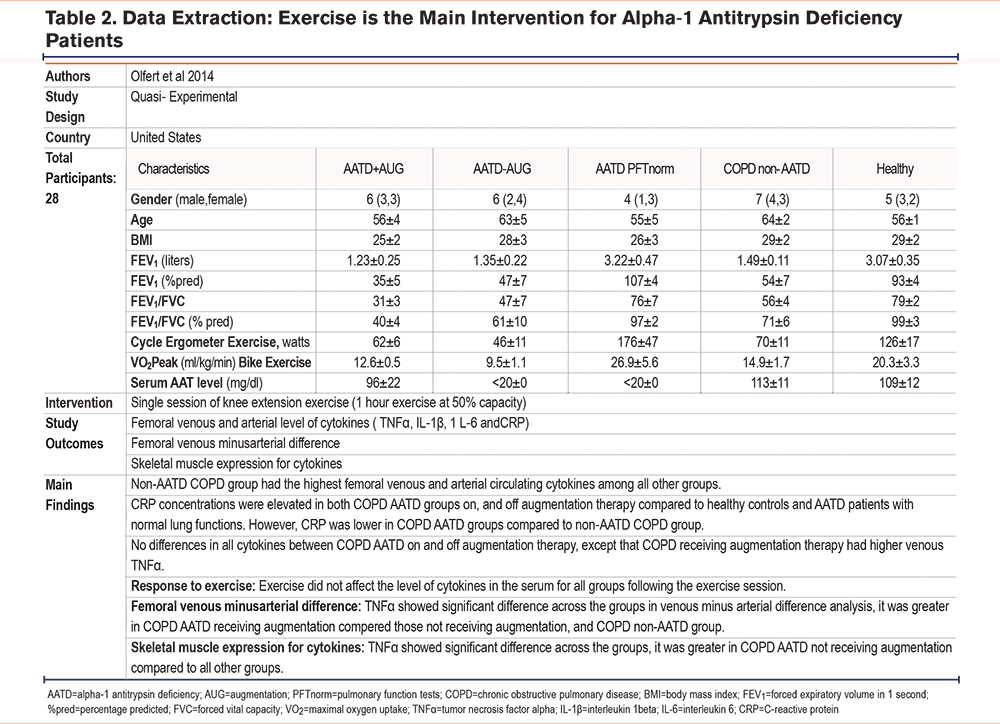
The study's findings revealed that usual COPD patients had the highest circulating cytokine levels. While COPD patients with underlying AATD had elevated CRP levels compared to the healthy group, these were less than those found in usual COPD patients. Interestingly, the exercise session did not significantly alter serum cytokine levels in any of the groups postexercise. However, muscle biopsies showed a higher expression of TNFα in AATD COPD patients not receiving augmentation therapy compared to all other groups.
Changes in the 6-Minute Walk Test After Pulmonary Rehabilitation
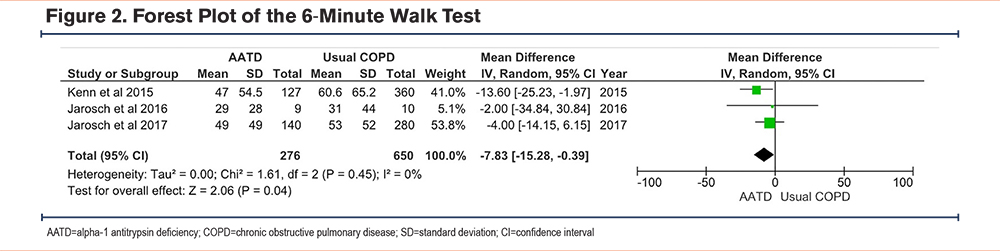
In 3 individual studies,19,23,24 both the AATD and the usual COPD groups showed significant improvement in the 6MWT following PR. However, the usual COPD patients demonstrated slightly better mean improvement compared to the AATD group, and these differences were not significant in any of the studies (see Table 1). Interestingly, the quantitative meta-analysis revealed a significant difference in the 6MWT distance gain between the AATD and usual COPD groups. AATD patients exhibited a lower gain with a Z-score of 2.06, p-value of 0.04, and I2 of 0, with a CI of -7.83m to -0.39m (see Figure 2). It is worth noting that while the numerical difference is lower, it falls within a range that is less than the minimal clinically important difference (MCID) for the 6MWT, making its clinical significance debatable. 21
Quality Appraisals of the Included Studies
In the assessment using the ROBINS-I tool, Jarosch et al (2017 and 2016)19,23 demonstrated serious risk of bias, with areas of missing information further complicating their evaluation. Kenn et al23 and Olfert et al26 both exhibited a moderate risk, though the latter had fewer areas of missing data. The study by Choate et al,25 assessed using the RoB tool for randomized trials, showed a predominantly low risk across categories, but there were some concerns regarding the selection of reported results. Overall, while some studies presented robust methodologies, several had gaps that introduced uncertainty and potential biases. Further details are available in the supplementary materials (see Table 2 and Table 3 in the online supplement respectively).
Discussion
This review has confirmed there is a paucity of good quality evidence regarding PR in AATD. The key findings of the 4 studies where PR is the main intervention19,23,24,25showed AATD patients improved in exercise capacity, quality of life, and weekly exercise routine. Although the evidence presented suggests that undergoing PR is effective for AATD patients, just as in any other group of patients with chronic lung conditions there were suggestions in the studies that further optimization or personalization of PR for AATD patients may be valuable.
COPD Alpha-1 Antitrypsin Deficiency Patients’ Characteristics
Cohort studies from Germany included AATD and usual COPD patients who were of similar age and exhibited similar lung function before undergoing a PR program.23,24 However, conflicting results emerged from other studies, indicating that COPD patients with AATD displayed poorer lung function when compared to their counterparts with usual COPD.26 This aligns with previous research indicating that AATD patients are generally younger by a decade and suffer from more severe lung dysfunction.11,26-29
Notably, despite their poorer lung function, AATD patients demonstrated superior baseline exercise capacity, as evidenced by better performance in the 6MWT.24 This echoes a recent finding where AATD patients could walk longer distances than usual COPD patients, albeit with more frequent exercise desaturations.28 However, AATD patients endure twice as many yearly exacerbations, which also last longer, than usual COPD patients. These frequent exacerbations, linked to higher hospitalization rates and reduced quality of life,30-33 combined with more pronounced dyspnea, may impact AATD patients’ engagement in PR programs.34,35
Pulmonary Rehabilitation: The Exercise Component
Exercise training as part of PR is beneficial for patients with lung diseases, including those with AATD. These patients demonstrate improvement in both exercise capacity and quality of life, albeit less than those with usual COPD (Table 1). However, the implementation of PR in the included studies varied, and the program duration, exercise intensity, and supervision level often differed from what is recommended by some health systems.16 Three studies from Germany implemented inpatient PR programs for AATD patients that were shorter in duration (3–5 weeks) but higher in frequency (daily exercise).19,24,36 These studies justified this approach as cost-effective and equally beneficial as conventional outpatient PR.37 Yet, this format may limit patients' day-to-day activities and make it challenging for them to maintain an exercise routine postdischarge. Moreover, it might not be as cost-effective or even possible in countries with fewer hospital beds per capita. Home-based virtual PR presents a viable alternative. The Choate et al study reported the effectiveness of virtual PR among AATD patients, demonstrating an increase in exercise activity and a reduction in BMI over a 5-year follow-up period.25 However, this study did not examine other potential benefits of such an intervention, like the impact on patients' quality of life and exercise capacity, reported elsewhere for usual COPD patients.38-40
Exercise is beneficial for muscles, particularly in reversing the muscle dysfunction characteristics linked to aging and the progression of COPD. Advanced COPD patients often exhibit reduced muscle size, an increased proportion of type II glycolytic muscle fibers, a decreased proportion of oxidative type I muscle fibers, diminished mitochondrial signaling, and a reduced capillary-to-fiber ratio.41,42
One study in this review found distinct differences in muscle adaptation between AATD patients and usual COPD patients following 3 weeks of PR.19 In AATD patients, there was no improvement in oxidative muscle fiber type I, a notable increase in glycolytic type II muscle fibers, no enhancement in mitochondrial signaling, and an increased fiber to capillary ratio. Although these findings suggest AATD patients may have a different muscle response to exercise, the study was limited by a small participant pool and the specific design of the exercise intervention.
Interestingly, there was a difference in muscle composition between the groups at baseline, which made it harder to draw conclusions from the changes between groups after intervention.19 Furthermore, their intervention method was suboptimal for promoting changes in muscle fiber distribution, as evidenced by other studies.42,43 The authors reported using moderate intensity exercise,19 but a recent review concluded that combining resistance exercise with high intensity interval training optimally shifted muscle fiber distribution towards an increase in type I fibers, which consequently improved exercise capacity in usual COPD patients.41 These findings raise questions about the most beneficial exercise modality for AATD patients to optimally improve muscle morphology and functionality. Different types of muscle fibers are specialized for specific functions during exercise or daily activities. Type I oxidative fibers are resistant to fatigue and are recruited during endurance exercise, while type II glycolytic fibers generate power and are used during resistance exercise.44 This underscores the importance of understanding muscle composition when considering exercise interventions for individuals with AATD, noting awaited results from 2 clinical trials investigating the effect of moderate versus high intensity exercise for AATD patients [NCT03802357] [NCT02915614].
Pulmonary Rehabilitation: The Education Component
Education serves as a crucial element of PR programs, especially for patients coping with chronic lung diseases such as COPD.17,45 This aspect aims to provide a deep understanding to patients of their medical condition and offers valuable insights on how to manage their day-to-day activities effectively. Traditional educational components typically cover a wide range of topics including the anatomy and physiology of the respiratory system, the functioning of lungs, correct use of inhalers, oxygen therapy, energy conservation, welfare benefits, and the importance of maintaining regular exercise routines postprogram.46,47
However, in the PR programs scrutinized in this review, the educational component was either overlooked or merely briefly touched upon.23 Instead, these were described as standard education sessions intended for COPD patients. This highlights an area of potential improvement since patients with AATD, despite having lung disease, have distinct educational requirements due to the unique genetic underpinning of their condition.48 For instance, one area of particular interest among AATD patients is augmentation therapy. Although this treatment option may not be available in every country,49 it often piques the interest of these patients. Therefore, tailored education sessions exploring such topics could be highly beneficial for these patients, enabling them to better understand their treatment options and potential outcomes.
Furthermore, it's worth noting that AATD is a known predisposing factor for liver disease. This occurs because of the accumulation of AAT polymers in the liver, leading to endoplasmic reticulum stress and ultimately causing liver fibrosis and cirrhosis.50,51 Despite the severity of this potential complication, education about liver disease and its implications is largely absent from current PR programs. Nonetheless, some self-management interventions, such as AlphaNet, a patient support system in the United States, have ventured into this area. They have developed a self-administered alpha-1 disease management program that includes comprehensive education about the underlying risk of liver disease for AATD patients. Patients who engaged with this program reported an increased understanding of their health condition and a heightened motivation to adopt preventative measures, thus, improving their overall wellbeing.49
Lastly, there is a noticeable gap in evidence regarding the logistics of PR for AATD patients. The question of how these patients can be integrated into usual COPD groups is a complex one. Usual COPD patients are often older and may grapple with different challenges associated with work, family life, and aging. Given these factors, more thought should be put into how PR programs are tailored and implemented to accommodate the unique needs of AATD patients.
Systemic Inflammatory Cytokines in Usual COPD Patients Versus Alpha-1 Antitrypsin Deficiency Patients
In typical COPD patients, elevated proinflammatory cytokines correlate with frequent exacerbations and reduced lung function.52-54 Yet, a study by Olfert et al26 revealed that AATD patients, irrespective of their augmentation therapy status, had lower serum levels of these cytokines. This might be surprising, given that systemic inflammation indicators, such as elevated CRP, would be anticipated to be greater in AATD patients due to their inferior lung function. This deviation is speculated to be due to potential hepatocyte malproduction of CRP, a phenomenon possibly linked to AATD digenesis.26 Another notable observation was the elevated TNFα post exercise in muscle biopsies of AATD patients. This irregularity could make them more susceptible to cachexia, emphasizing the need for more research into the disease progression pathophysiology and related exercise outcomes in the AATD population.55
Moreover, the study's exercise regimen didn't alter baseline proinflammatory cytokines for any group, contrasting with the typical response in healthy individuals where exercise boosts proinflammatory cytokine levels, balanced by postexercise anti-inflammatory responses.56 While the results align with some prior research,57,58 the literature remains ambiguous, with conflicting studies reporting an increase in inflammatory cytokines following exercise in usual COPD patients.59,60 Once seen as additional inflammatory stress, regular exercise is now viewed as beneficial for its long-term anti-inflammatory effects and possible contribution to improved longevity.61,62
Conclusion
The available evidence on the effectiveness of PR for AATD patients remains limited, necessitating further research in this area. Key areas of interest for investigation include identifying the most beneficial type of exercise, determining the optimal approach to education, and finding the best method for delivering PR services to individuals with AATD.
Acknowledgements
Author contributions: FA drafted the initial manuscript and contributed to the screening process and data extraction. AT provided valuable supervision, guidance, and edited the manuscript. KW and HP were involved in article screening, data extraction, and conducting quality assessments. All authors critically reviewed and provided input for the final draft of the manuscript, ensuring its accuracy and coherence. All authors approved the manuscript.
Declaration of Interests
All authors declare no conflict of interest.