Running Head: Inhaler Formulary Change and COPD Outcomes
Funding Support: Research time for KID was supported by the Agency for Healthcare Research and Quality grant K12HS026369 and the Doris Duke Charitable Foundation grant 2021086. None of the funding sources were involved in the design, conduct, or analysis of this project.
Date of Acceptance: October 13, 2023 | Published Online Date: October 31, 2023
Abbreviations: AME=average marginal effects; BMI=body mass index; CDW=Corporate Data Warehouse; CI=confidence interval; COPD=chronic obstructive pulmonary disease; DiD=difference in differences; ICS=inhaled corticosteroid; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; SMD=standardized mean differences; SD=standard deviation; VA=Veterans Health Administration
Citation: Duan KI, Donovan LM, Spece LJ, et al. Inhaler formulary change in COPD and the association with exacerbations, health care utilization, and costs. Chronic Obstr Pulm Dis. 2024; 11(1): 37-46. doi: http://doi.org/10.15326/jcopdf.2023.0425
Online Supplemental Material: Read Online Supplemental Material (325KB)
Background
Inhaler therapy is a cornerstone of management for chronic obstructive pulmonary disease (COPD).1 In the United States, insurer and payer drug formularies are a key factor that affects patient access to inhalers. However, formularies change frequently for multiple reasons, including changes in costs, generic substitution, drug availability, and clinical evidence.2,3 The Office of the Inspector General identified 11,827 formulary changes across 120 Medicare Part D-sponsored formularies in 2008, almost 100 changes per plan per year.2 Stability of formularies may be particularly important in COPD, given the wide array of inhaler delivery devices on the market that are dependent on patient technique.4 If formulary changes force patients to switch inhalers (nonmedical switching), problems with adherence or inhaler technique arising from the switch could harm COPD disease control.5,6
Evidence from other clinical areas, such as rheumatology and cardiology, suggests disease control can worsen after nonmedical switching.7 However, there is currently limited evidence on the effects of nonmedical switching in chronic pulmonary diseases. Among patients with asthma and COPD, a recent systematic review found low-quality studies and heterogeneous clinical outcomes due to nonmedical inhaler switching, with some studies reporting improved disease control, while others reported worsened control.8 Only 2 of these studies evaluated patients with COPD in the United States, and both were limited by the lack of a control group and study designs that limited causal inference.9,10 Other studies identified by Usmani et al, and a more recent study by Bickel et al, were either primarily conducted in the United Kingdom and/or focused on asthma, limiting generalizability to patients in the United States with COPD.8,11 Therefore, we sought to evaluate the clinical and health system effects of nonmedical inhaler switches in COPD by studying a national formulary inhaler change in the Veterans Health Administration (VA) using quasi-experimental methods. Generating higher-quality evidence could inform health system leaders or insurance decision-makers on how to modify formularies without compromising patient outcomes. Given the potential for the formulary change to worsen adherence or inhaler technique, we hypothesized that the formulary change would be associated with a higher risk of COPD exacerbations, and higher utilization and costs.
Methods
Study Design, Cohort Selection, and Data
We conducted a retrospective study to evaluate the effect of the VA national formulary removal of formoterol, a long-acting beta2-agonist (LABA) single inhaler. This formulary change occurred on January 9, 2016, due to manufacturer discontinuation, with the VA then adopting olodaterol as the default LABA single inhaler.12,13 The VA formulary has been nationally standardized since the elimination of regional VA formularies in 2009. The available medications are determined via 2 national-level committees with representation from physicians, pharmacists, and regional pharmacist executives from the VA’s 21 regional networks.14 As a result, any formulary changes affect all Veterans receiving medications through the VA.
Difference-in-differences (DiD) is a quasi-experimental study design used to study the implementation of a specific policy or intervention.15 In our study, the national formulary change serves as a natural experiment, and we compared the pre- and postformulary change periods between a control and an exposure group. DiD is designed to mitigate the effect of unobserved confounders by comparing both longitudinal within-group differences and between-group differences, thereby improving causal inference.
We utilized a national inception cohort of all Veterans who were newly diagnosed with COPD and started long-acting inhaler therapy between January 4, 2010, and December 31, 2018. In the cohort, we defined COPD using a pragmatic, health systems-oriented definition using both encounter diagnosis codes and prescribed inhalers.16 For encounters, we required 2or more outpatient COPD visits within a rolling 12-month period based on diagnosis code. For prescribed medications, we required receipt of inhaler therapy, defined as 2 dispensations of a long-acting inhaler (long-acting muscarinic antagonist [LAMA], LABA, or inhaled corticosteroid [ICS]) within a rolling 12-month period. From this larger cohort, we then identified Veterans who received at least 2 dispensations of inhaler therapy in the 6 months prior to the formulary change on January 9, 2016, to be included in our analysis. We required at least 2 dispensations as a sign of active and ongoing prescribing, thereby excluding patients who may receive a one-off inhaler prescription as an empiric trial. Given that the formulary change of interest was the discontinuation of formoterol single inhaler (an early first-line therapy in COPD), our analysis centered around those with mild, low-exacerbation risk COPD. We excluded those on a baseline ICS or those switched to a new ICS after the formulary change due to the evolving recommendations for ICS use over the study period from 2010–2018. During the study period, the 2017 Global Initiative for Chronic Obstructive Lung Disease recommendations changed indications for an ICS.17 In these updated recommendations, airflow limitation severity was removed as an indication for ICSs, instead focusing on exacerbation risk as a key indication. Including incident ICS use could have introduced selection bias based on the severity of illness, pathophysiology, quality of care, or practice norms. We also excluded patients on any combination inhalers to reduce the risk of bias due to severity of illness. Finally, we excluded Veterans who died during the 6-month follow-up period so that all Veterans included in the analysis had equal follow-up duration. Data were extracted from the VA Corporate Data Warehouse (CDW), a national database capturing information on all encounters and dispensed medications across all sites of care. The cohort selection process is outlined in Supplement Figure 1 in the online supplement.
Exposure Group
The exposure group consisted of Veterans with COPD who received 2 prior dispenses of formoterol before the formulary change with no dispenses of formoterol afterward. To isolate the effect of the formulary change, we assumed discontinuations 90 days before or 90 days after January 9, 2016, were related to the formulary change. Each Veteran had formoterol discontinued at different times before or after January 2016, depending on individual prescription end dates, local pharmacy supply, and local pharmacy planning. These Veterans were then switched to either an alternative LABA, LAMA, or no alternative long-acting control inhaler. The index date to define the pre- and postformulary change periods for each Veteran was set as the date of the last dispensation of formoterol, plus the prescription duration in days. In other words, the index date was the day they would have run out of medication when taken as prescribed. The selection of index dates is graphically displayed in Supplement Figure 2 in the online supplement.
Control Group
The control group consisted of Veterans with COPD who were using a LAMA single inhaler at the time of the formulary change, and therefore, unaffected by formoterol inhaler discontinuation. We selected LAMA single inhaler use as the control group since both LAMA and LABA are long-acting bronchodilators recommended as initial therapy for most patients with COPD.1 For the control group, the index date to define pre- and postformulary change was set to the formulary change date of January 9, 2016.
Outcomes
The primary outcome was the total number of inpatient and outpatient COPD exacerbations in the 6-month period after the index date. Inpatient and outpatient exacerbations were defined using a combination of International Classification of Diseases-10th Revision diagnosis codes and prescribed medications, based on established methods of defining exacerbations with administrative data (online supplement). Secondary outcomes were the total number of health care encounters and the total encounter-related costs in the 6-month period after the formulary change. Encounters included in these secondary outcomes were all-cause hospitalizations, emergency and urgent care visits, primary care visits, pulmonary specialty visits, and all-cause telephone visits. Encounters were identified and defined via “stop codes,” a VA system of identifying the type of encounter in administrative data.18 Encounter-related costs for hospitalizations were inclusive of all services provided, including radiology, laboratory, pharmacy, and procedural services. The numbers of COPD exacerbations and total encounters were count variables. Costs were extracted from the VA Managerial Cost Accounting System, an activity-based cost allocation system.19 As a result, all cost analyses were conducted from a VA health system perspective. Costs were adjusted for inflation and measured in 2016 constant U.S. dollars.
Covariates
While DiD mitigates bias due to unobservable confounders, adjusting for observable confounders remains important to improve precision and further minimize the risk of bias due to time-varying confounders.20,21 Therefore, we adjusted for potential confounders that may affect COPD exacerbations and VA health care utilization based on previous work.22-26 The selected covariates, assessed at cohort entry, were age, gender, race, ethnicity, body mass index (BMI), Charlson comorbidity index, smoking status, marital status, driving distance to primary care, and VA priority group.27 VA priority group is a status based on military service, disability, and a needs assessment that determines copay and access to care.28
Statistical Analysis
For descriptive statistics, we compared the balance of patient characteristics between the exposure and control groups using standardized mean differences (SMDs).29 SMDs greater than 0.1 were considered meaningful. We then performed a 2-group, 2-period DiD analysis, comparing changes in outcomes in the 6 months pre- and postindex date, and between the control and exposure groups. For outcomes that were count variables (COPD exacerbations and encounters), we conducted the DiD analysis using a negative binomial model. For cost outcomes, we conducted the DiD analysis using a generalized linear model with a log link and gamma distribution due to the skewed distribution of cost data.30 We produced both unadjusted univariable and adjusted multivariable estimates. The multivariable models adjusted for potential confounders are noted above in the “Covariates” Section. Since Veterans in the exposure group switched off formoterol at different times, all our adjusted models included fixed effects for the index calendar month. For inference, the DiD estimates of all univariable and multivariable analyses were expressed as average marginal effects (AMEs). AMEs for outcomes that were count variables reflect the difference in the number of encounters in the pre/postformulary change for the exposure group, compared to the same pre /post difference in the control group. Similarly, AMEs for cost outcomes reflect the difference in dollars spent on encounters pre- and postformulary change for the exposure group, compared to that same pre/post difference in the control group. We evaluated for parallel trends in the preformulary change period as a prerequisite specification test prior to performing DiD (see online supplement for details).
Sensitivity Analysis
We conducted 4 sensitivity analyses. First, we restricted the exposure group definition to exclude patients who received no long-acting inhalers after discontinuing formoterol. Restricting the exposure group to those who continued inhalers served 2 purposes. First, it enriched the exposure group with Veterans for whom inhalers are necessary, as the formulary change may have provided an opportunity to reevaluate and discontinue unnecessary inhalers. Second, restricting the exposure group also eliminated potential confounding by medical center quality by excluding Veterans whose medical center may have failed to switch them to an alternative inhaler.
In our second sensitivity analysis, we excluded individuals with comorbid asthma identified by diagnosis code to avoid selection bias. While all patients in the cohort had a physician diagnosis of COPD, a subset also had diagnosis codes for asthma and may have a different chronic airway disease phenotype like asthma-COPD overlap syndrome. By restricting the cohort to exclude asthma, we potentially reduce selection bias if these patients are differentially affected by formulary changes, or if these patients may be exposed to lower quality of care given formoterol monotherapy is not indicated in asthma.31
In our third sensitivity analysis, we included patients switched to a LABA/ICS after the formulary change due to the possibility that some providers may have wanted to continue LABA in some form and instead selected a LABA/ICS combination instead of a LABA single-inhaler therapy.
In our fourth sensitivity analysis, we allowed LABA/LAMA combinations and ICS-containing regimens in both the exposure group (i.e., patients switched from formoterol to LABA/LAMA or an ICS-containing regimen) and in the control group (i.e., patients on LABA/LAMA or an ICS-containing regimen serving as controls). While we deliberately restricted these inhaler regimens from the main analysis to select an appropriate control group, this sensitivity analysis provides a broader, all-encompassing analysis of the effect of the formulary change among all patients receiving inhalers in real-world practice.
Analysis was conducted using Stata version 17 (StataCorp, College Station, Texas). This study was approved by the institutional review boards of the Department of Veterans Affairs (#00461) and the University of Washington (#STUDY00013458).
Results
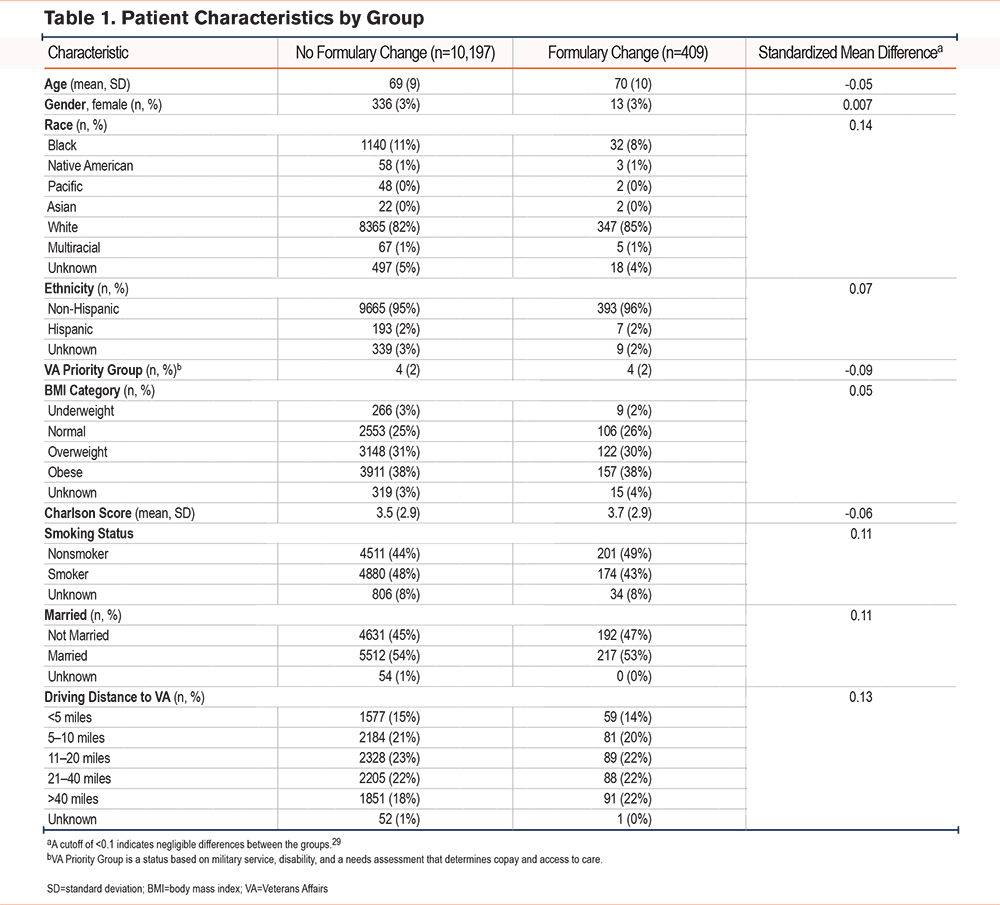
We identified 10,606 Veterans who met our cohort entry criteria, of which 409 were on formoterol and experienced the formulary change (exposure), whereas 10,197 on LAMA did not (control). Among the exposure group (n=409), 290 (71%) switched to LABA, 16 (4%) switched to a new medication class (LAMA), and 103 (25%) did not continue inhaler therapy. Compared to the control group, Veterans in the exposure group were slightly more likely to be nonsmoking (49% versus 44%, SMD=0.11), White (85% versus 82%, SMD=0.14), unmarried (47% versus 45%, SMD=0.11), and live >40 miles from VA primary care (22% versus 18%, SMD 0.13). Baseline characteristics were otherwise well-balanced between exposure and control groups (Table 1).
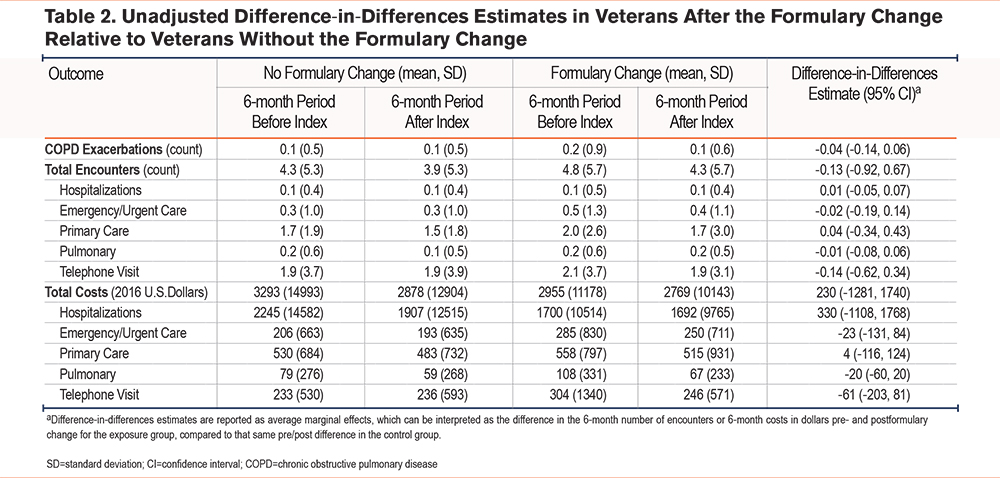
The outcomes during the preformulary change baseline period were similar between exposure and control groups. The exposure group had a baseline mean of 0.2 COPD exacerbations over 6 months (standard deviation [SD]=0.9), 4.8 encounters over 6 months (SD=5.7), and $2955 in encounter-related costs over 6 months (SD=11,178). In comparison, the control group had a baseline mean of 0.1 COPD exacerbations over 6 months (SD=0.5), 4.3 encounters over 6 months (SD=5.3), and $3293 in encounter-related costs over 6 months (SD=14,993) (Table 2). In the postformulary change period, the exposure group had a mean of 0.1 COPD exacerbations over 6 months (SD=0.6), 4.3 encounters over 6 months (SD=5.7), and $2769 in encounter-related costs over 6 months (SD=10,143). The control group had a mean of 0.1 COPD exacerbations over 6 months (SD=0.5), 3.9 encounters over 6 months (SD=5.3), and $2878 in encounter-related costs over 6 months (SD=12,904).
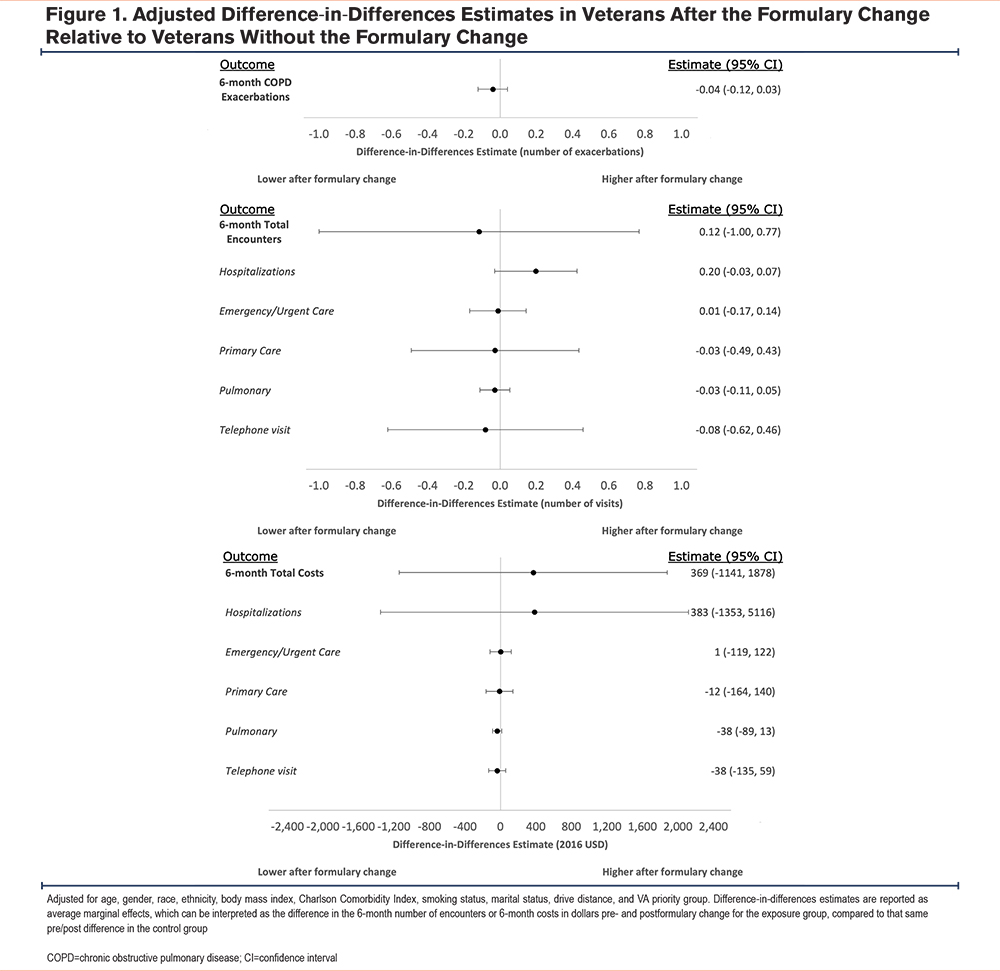
In unadjusted DiD analysis, we found that the formulary change was not associated with a change in the number of COPD exacerbations among Veterans who switched medications as compared to controls (DiD estimate -0.04, 95% confidence interval [CI] -0.14, 0.06)(see Table 2). Similarly, for our secondary outcomes, we observed no change in either 6-month total encounters (DiD estimate -0.13, 95% CI -0.92, 0.67) or 6-month encounter-related costs (DiD estimate $230, 95% CI -$1281, $1740) compared to the control group. In adjusted DiD analysis, we observed that the formulary change was not associated with a change in either 6-month COPD exacerbations (DiD estimate -0.04, 95% CI -0.12, 0.03), 6-month number of encounters (DiD estimate -0.12, 95% CI -1.00, 0.77), or 6-month encounter-related costs (DiD estimate $369, 95% CI -$1141, $1878), compared to the control group (Figure 1). Subcategorizing our secondary outcomes by type of encounter, we observed no change in the 6-month number of encounters or 6-month encounter-related costs.
Sensitivity Analysis
In sensitivity analyses, we identified 306 Veterans who met the restricted exposure group definition of transition onto alternative long-acting bronchodilators. The control group remained unchanged with 10,197 Veterans. In adjusted analysis, we observed no change in the 6-month number of COPD exacerbations (DiD estimate -0.04, 95% CI -0.11, 0.03), the 6-month total number of encounters (DiD estimate 0.11, 95% CI -1.00, 1.21), or the 6-month encounter-related costs (DiD estimate $904, 95% CI -$566, $2374) associated with the formulary change (Supplement Table 3 in the online supplement).
In the second sensitivity analysis, we excluded 1200 Veterans with a comorbid asthma diagnosis, of which 88 were in the exposure group (22% of the exposure group) and 1112 were in the control group (11% of the control group). In adjusted analysis, we observed no change in the 6-month number of COPD exacerbations (DiD estimate -0.03, 95% CI -0.10, 0.04), the 6-month total number of encounters (DiD estimate -0.15, 95% CI -0.98, 0.68), or the 6-month encounter-related costs (DiD estimate $875, 95% CI -$298, $2048) associated with the formulary change (Supplement Table 4 in the online supplement).
In the third sensitivity analysis, by including patients who were switched to LABA/ICS, the exposure group was 556 Veterans, with an unchanged control group of 10,197. In adjusted analysis, we again observed no change in the 6-month number of COPD exacerbations (DiD estimate -0.02, 95% CI -0.10, 0.07), the 6-month total number of encounters (DiD estimate 0.19, 95% CI -0.52, 0.90), or the 6-month encounter-related costs (DiD estimate $935, 95% CI -$562, $2432) associated with the formulary change (Supplement Table 5 in the online supplement).
Finally, in the fourth sensitivity analysis, including LAMA/LABA and ICS-containing regimens in both groups yielded an exposure group of 1048 Veterans and a control group of 78,153. Again, in adjusted analysis, we observed no change in the 6-month number of COPD exacerbations (DiD estimate -0.05, 95% CI -0.11, 0.02), the 6-month total number of encounters (DiD estimate -0.11, 95% CI -0.59, 0.38), or the 6-month encounter-related costs (DiD estimate -$260, 95% CI -$1,149, $628) associated with the formulary change (Supplement Table 6 in the online supplement).
Discussion
Among a cohort of Veterans with COPD on a single inhaler, we found no change in COPD exacerbations, total encounters, or encounter-related costs associated with nonmedical inhaler switches due to discontinuation of formoterol from the national VA formulary. Our results were robust to multiple sensitivity analyses. This study addresses current knowledge gaps around formulary management in the United States among patients with COPD using a quasi-experimental study design to enhance causal inference.
Nonmedical inhaler switches due to formulary changes have the potential to negatively impact patients’ COPD control in multiple ways, including transitioning to a suboptimal alternative inhaler, reduced adherence, or improper technique using the new inhaler. However, we did not observe any negative impacts on our studied outcomes, despite 103 of the 409 patients in the formulary change group discontinuing long-acting inhalers altogether. Several mechanisms could explain our findings. First, it is possible that the VA’s centralized formulary management and coordination by local VA pharmacies provided enough anticipatory guidance and support services to successfully transition patients off formoterol and onto correct alternative treatment regimens without negative outcomes.32 Since this formulary change was not restrictive and was due to a manufacturer discontinuation, appropriate alternatives were readily available on the VA formulary with no difference in patient copay. For the patients who did not continue any inhalers, it is possible that the formulary change provided an opportunity to reevaluate patients and appropriately discontinue inhalers for those without an indication. Second, an alternative explanation for the lack of association between formoterol discontinuation and outcomes may be suboptimal inhaler adherence in COPD. Full inhaler adherence is estimated to be only 20%–30% among Veterans with COPD.33 Therefore, switching an inhaler due to a formulary change may not meaningfully change outcomes when baseline adherence is already poor. Importantly, the only formulary change that occurred during our study period was formoterol discontinuation. As a result, we restricted our evaluation to patients on single bronchodilator therapy, which in turn enriched the study population to those with less symptomatic, low-exacerbation risk COPD. While formulary changes may not negatively affect those with milder COPD, those with severe COPD could experience negative outcomes. Further work is needed to evaluate whether such differential effects exist.
Irrespective of the underlying mechanisms, our study has potential formulary management and policy implications. While most formulary changes involve new medications being added, around 36% involve cost-containment strategies such as generic substitution, adding prior authorization, or utilization controls.2 The rationale for cost-related formulary changes is that multiple stakeholder groups benefit, through reduced out-of-pocket costs for patients and lower expenditures for integrated health systems and payers. However, formulary changes have been met with resistance, with professional and patient groups pointing to a body of evidence that nonmedical switching leads to worsened disease control.7,34 Legislation has been enacted in several states to govern how and when formulary changes can occur.35 Payers and formulary managers must balance the potential negative clinical consequences of nonmedical switching with the potential cost savings. Our findings provide a preliminary, but important, safety data point for formulary managers and policymakers, suggesting that formulary changes may not have negative effects in COPD among patients on a single inhaler and milder disease. However, our work should be replicated among patients with more severe COPD, with different inhaler classes, and in other health care systems before drawing more definitive conclusions.
Our study has several important limitations. First, due to limitations in available data and to enhance causal inference, we limited our analysis to patients on single inhalers and no ICSs. While this was a deliberate restriction, by definition, we selected for less symptomatic patients without frequent exacerbations. As noted above, our study findings cannot be extrapolated to patients with more severe disease, for whom formulary changes may have a different impact. Second, we believe our results are most applicable to integrated health care systems like the VA that have a centrally managed formulary and a care delivery system that can safely coordinate the transition of patients onto alternative medications. Our results should be applied cautiously outside of integrated health systems where formulary changes occur more frequently and may involve less coordination with care providers.13,36,37 Additional investigation must be done to evaluate whether our findings can be replicated in non-VA settings. Third, LABA single inhalers are less frequently prescribed in COPD compared to LAMAs.38,39 There may be selection bias in either the Veterans receiving LABAs or the providers prescribing LABAs, though the DiD design mitigates the effect of such biases. Other COPD inhaler formulary changes beyond LABA single inhalers should be studied to see if our results hold for other medication classes like LAMAs or ICSs. Our dataset did not span the years of any other inhaler changes in the VA formulary, precluding such evaluation in this study. Fourth, we did not have access to prescription or encounter data outside of the VA. It is possible that Veterans obtained medications or suffered exacerbations outside of the VA which could bias our results. However, due to cost-sharing arrangements and other factors, Veterans are generally incentivized to receive their care and medications within the VA system, mitigating this potential bias.40-42 Fifth, our study was a secondary analysis of an inhaler inception cohort, and so does not include patients with several years of prevalent inhaler use. The effect of formulary changes may be different for long-term, prevalent users. Sixth, spirometry results were not available in the CDW for the entire cohort, so we were unable to conduct a sensitivity analysis among patients with confirmed airflow obstruction to ensure our results remained robust in that group. Seventh, while we excluded patients who died during follow-up to ensure a full 6-month period of observed outcomes, this may have introduced bias. Finally, other important COPD outcomes, such as symptom burden or functional status that can be readily measured using tools like the COPD Assessment Test, were unavailable for evaluation using administrative and electronic health record data.
Our study suggests that among patients with COPD on a single inhaler, formulary changes that result in nonmedical inhaler switching from LABA single inhalers are not associated with higher COPD exacerbations, encounters, or encounter-related costs. Further research is needed in patients with more severe COPD and using other inhaler classes to evaluate whether our findings persist.
Acknowledgements
Author contributions: KID, LMD, ESW, LJS, LCF, KC, and DHA contributed substantially to the study conception and design. KID drafted the manuscript. All authors contributed substantially to data acquisition, data analysis and interpretation, and the critical revision of this manuscript. All authors approved this final version and agree to be held accountable.
The authors thank Ethan Nguyen, PharmD, for his assistance identifying previous changes to the VA inhaler formulary.
Disclaimer: The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Declaration of Interests
LCF and KC serve as associate editors of the Annals of the American Thoracic Society. LCF receives consultant fees from the U.S. National Committee for Quality Assurance, the American Thoracic Society, and the Society of Hospital Medicine. DHA serves as deputy editor at the Annals of the American Thoracic Society and served on an advisory board for Boehringer Ingelheim on gaps in quality of care and medication adherence. No other disclosures are reported.