Running Head: Lung Function Decrease and COPD Outcomes
Funding Support: This study was funded by GSK (study number: CTT116855; NCT02164513). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data and the final responsibility to submit it for publication.
Date of Acceptance: November 22, 2023 │Published Online Date: December 6, 2023
Abbreviations: CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; FF=fluticasone furoate; IMPACT=InforMing the Pathway of COPD Treatment; ITT=intent-to-treat; OR=odds ratio; PROs=patient-reported outcomes; RR=rate ratio; SGRQ=St George’s Respiratory Questionnaire; SD=standard deviation; UMEC=umeclidinium; VI=vilanterol
Citation: Han MK, Criner GJ, Halpin DMG, et al. Any decrease in lung function is associated with worse clinical outcomes: post hoc analysis of the IMPACT interventional trial. Chronic Obstr Pulm Dis. 2024; 11(1): 106-113. doi: http://doi.org/10.15326/jcopdf.2023.0391
Introduction
Clinical trials of pharmacological treatments in chronic obstructive pulmonary disease (COPD) often focus on improvements in forced expiratory volume in 1 second (FEV1).1 Preventing disease progression, including an FEV1 decrease, is an established goal of clinical management.2,3 Worsening lung function is associated with worse patient outcomes and increased risk of hospitalizations and mortality.4-6 A threshold of ≥100mL has commonly been used to define a clinically significant FEV1 decrease.7,8 The relationship between different magnitudes of FEV1 worsening (also previously termed deterioration3), including<100mL/year, and clinical outcomes is not well understood. This post hoc analysis of the InforMing the Pathway of COPD Treatment (IMPACT) trial (CTT116855; NCT02164513)9 investigated the relationship between the magnitude of different FEV1 decreases and clinical outcomes over 1 year, and the effect of fluticasone furoate (FF)/umeclidinium (UMEC)/vilanterol (VI) on FEV1 and other clinical outcomes versus FF/VI or UMEC/VI.
Methods
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the relevant national, regional, or independent ethics committees or institutional review boards. Study details for IMPACT have been previously described.9 IMPACT was a phase 3, double-blind, parallel-group, 52-week, multicenter study with participants randomized 2:2:1 to FF/UMEC/VI 100/62.5/25µg, FF/VI 100/25µg, or UMEC/VI 62.5/25µg once daily via a single inhaler (ELLIPTA, GSK).9 Visits were conducted at screening (baseline), randomization (Day 1), and Weeks 4, 16, 28, 40, and 52. FEV1 decrease at Week 52 was defined as any decrease from baseline in trough FEV1 >0mL. On-treatment moderate/severe exacerbations, defined as those requiring antibiotics and/or oral/systemic corticosteroids (moderate), and events resulting in inpatient hospitalization or death (severe), were compared between participants with an FEV1 increase/no change at 52 weeks versus a decrease at Week 52 using a generalized linear model assuming a negative binomial distribution. Covariates included deterioration, treatment group, sex, exacerbation history (≤1, ≥2 moderate/severe), smoking status (screening), geographical region, postbronchodilator percentage predicted FEV1 (screening), and age. The St George’s Respiratory Questionnaire (SGRQ) total score and the COPD Assessment Test (CAT) score at Week 52 were also compared between participants with an FEV1 increase/no change versus any decrease at Week 52 using a mixed measures model, with covariates of deterioration, treatment group, smoking status (screening), geographical region, baseline SGRQ total score (SGRQ total score analysis only), baseline CAT score (CAT score analysis only), sex, and age. Treatment comparisons for participants with and without an FEV1 decrease in the intent-to-treat (ITT) population were performed using logistic regression with covariates of treatment group, smoking status (screening), geographical region, and baseline trough FEV1. Participants were allocated into quartile subgroups based on their FEV1 decrease (>0mL and <60mL, ≥60mL and <110mL, ≥110mL and <210mL, and ≥210mL). Differences between FEV1-decrease subgroups were evaluated for change from baseline SGRQ total score and CAT score at Week 52, and moderate and/or severe exacerbation rates over 52 weeks. For participants with evaluable data at Week 52, defined as having both baseline and Week 52 trough FEV1, the frequency of the FEV1 decrease at prior visits was evaluated. Evaluable data at Week 52 was selected for evaluation to enable worsening to be analyzed throughout the study duration.
Results
Of the ITT population, 7916 participants had evaluable data at Week 52; 3274 (41%) had an FEV1 decrease at Week 52 (FF/UMEC/VI: 1065 [32%], FF/VI: 1555 [51%], UMEC/VI: 654 [44%]). Baseline characteristics were similar across treatment groups for both patients with and without an FEV1 decrease (Table 1).Of the participants with an FEV1 decrease at Week 52, 2873 (88%) also had an FEV1 decrease at previous visits, including 1190 (37%) who experienced a decrease at all previous visits (FF/UMEC/VI: 283 [8%], FF/VI: 709 [23%], UMEC/VI: 198 [13%]), and a total of 325 (10%), 444 (14%), 546 (17%), and 693 (21%) participants experienced a decrease at Week 52 only, Week 52 and one prior visit, Week 52 and 2 prior visits, and Week 52 and 3 prior visits, respectively. Percentages were calculated using the number of participants at Week 52 with an FEV1 decrease (n=3274) with no missing prior visit data.
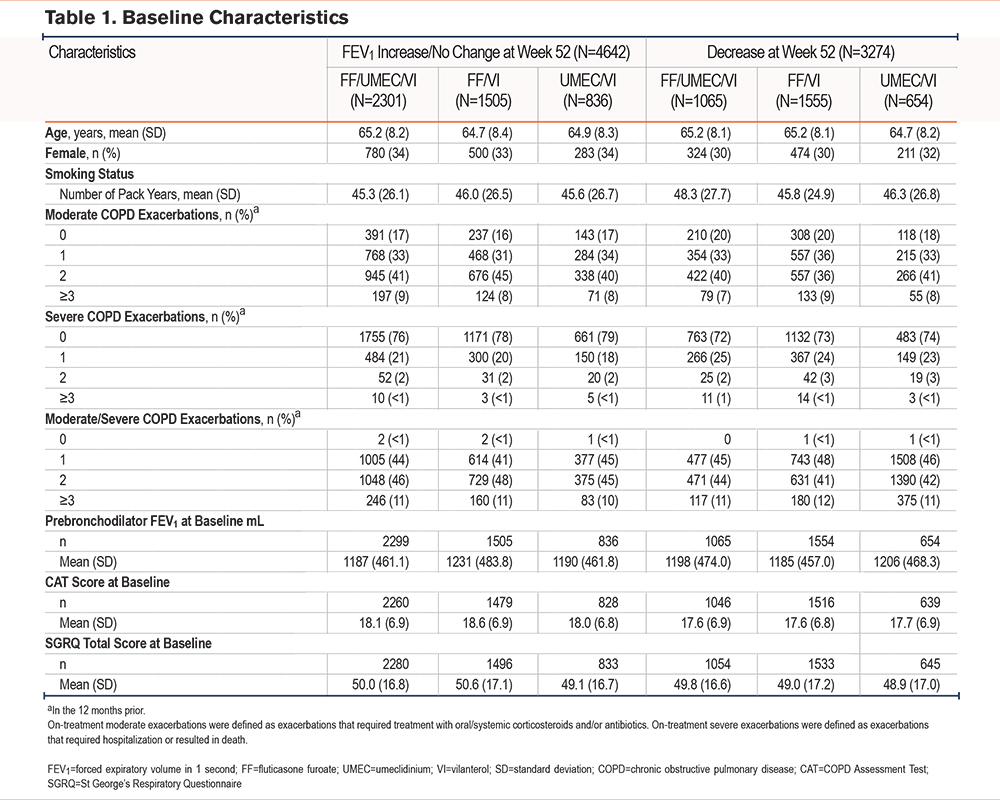
Overall, participants with an increase/no change in FEV1 at Week 52 had a mean (standard deviation [SD]) change from baseline in trough FEV1 of 207mL (212.2) (FF/UMEC/VI: 218mL [212.5], FF/VI: 190mL [216.9], UMEC/VI: 210mL [200.4]). Participants with an FEV1 decrease at Week 52 had an overall mean (SD) change from baseline in trough FEV1 –158mL (159.6) (FF/UMEC/VI: –148mL [186.1], FF/VI: –169mL [145.6], UMEC/VI: –149mL [142.5]). Participants with the greatest FEV1 mL decrease at Week 52 (decrease ≥210mL) showed negligible improvement in SGRQ and CAT (Figure 1) scores. A significant reduction in the odds of having any FEV1 decrease when treated with FF/UMEC/VI was observed at Week 52 versus FF/VI (odds ratio [OR], 0.45; 95% confidence interval [CI] 0.40–0.50; p<0.001) and UMEC/VI (OR, 0.59; 95% CI, 0.52–0.67; p<0.001).
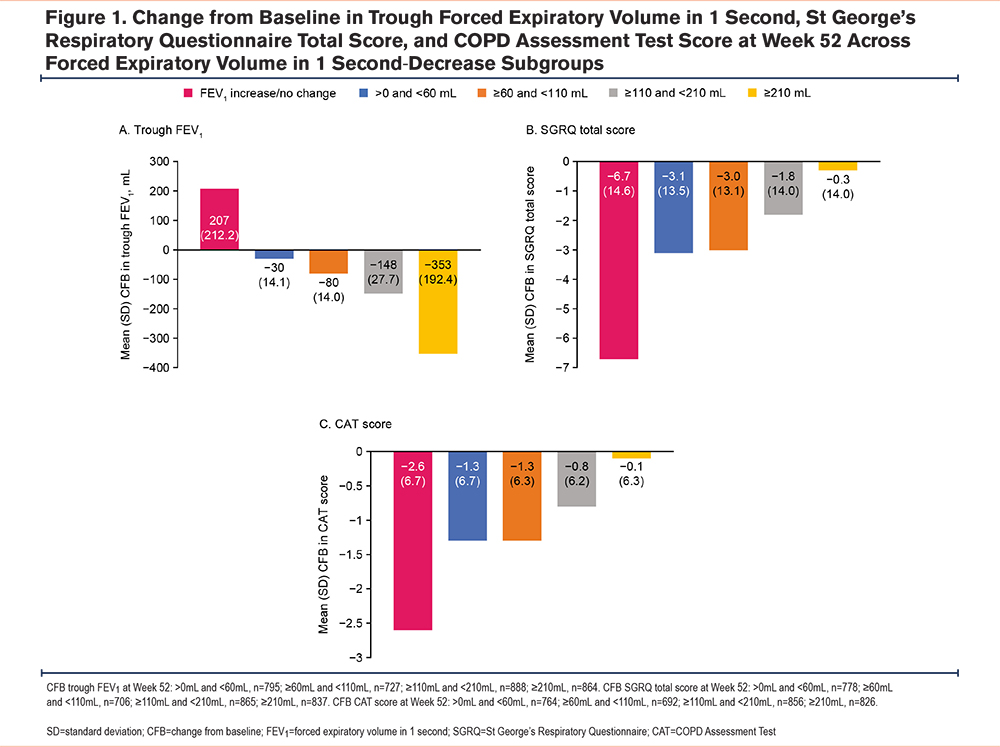
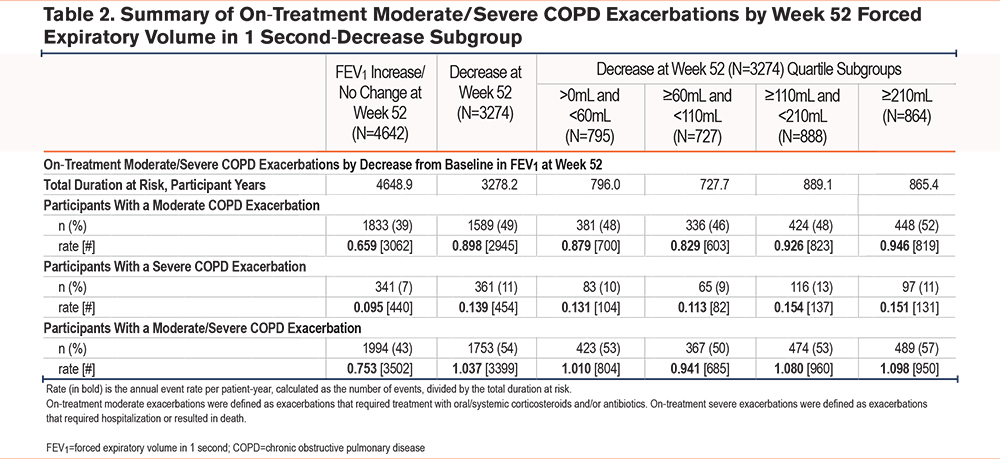
On-treatment moderate/severe exacerbation rates were significantly lower among participants with an FEV1 increase/no change at Week 52, compared with those with a decrease (rate ratio [RR] 0.74; 95% CI 0.70, 0.79; p<0.001) (Figure 2). Participants with an FEV1 increase/no change had an annual exacerbation rate of 0.72 (95% CI 0.69, 0.75), compared with 0.97 (95% CI 0.93, 1.02) for participants with a decrease. In all Week 52 FEV1-decrease subgroups (where a decrease in lung function ranged from >0mL to ≥210 mL), a higher percentage of participants experienced moderate (49%), severe (11%), and moderate/severe (54%) exacerbations versus those with no lung function decrease (39%, 7%, and 43%, respectively) (Table 2). Annual exacerbation rates were highest in the FEV1-decrease ≥210mL subgroup compared with the increase/no change subgroup (moderate/severe, 1.098 versus 0.753 per patient-year [950 events among 864 participants versus 3502 events among 4642 participants]) (Table 2). Overall exacerbation rates in all decrease subgroups were similar.
SGRQ total score was significantly better among participants with an FEV1 increase/no change at Week 52 compared with those with an FEV1 decrease, with a mean change (95% CI) from baseline –6.5 (–6.9, –6.1) and –2.3 (–2.8, –1.8), respectively; difference: –4.2 (–4.9, –3.6; p<0.001) (Figure 2). In participants with any FEV1 decrease at Week 52, the mean change from baseline in SGRQ total score at Week 52 ranged from −0.3 to −3.1, versus −6.7 in the subgroup of participants with an FEV1 increase/no change at Week 52 (Figure 1, Table 1).
CAT scores were significantly better among participants with an FEV1 increase/no change at Week 52 compared with those with an FEV1 decrease, with a mean change (95% CI) from baseline –2.5 (–2.7, –2.3) and –1.0 (–1.2, –0.8), respectively; difference: –1.5 (–1.8, –1.3; p<0.001) (Figure 2). In participants with any FEV1 decrease at Week 52, the mean change from baseline in CAT score at Week 52 ranged from −0.1 to −1.3, versus −2.6 in participants with an FEV1 increase/no change at Week 52 (Figure 1, Table 1).
Discussion
This analysis of the IMPACT study demonstrates that 41% of participants experienced FEV1 worsening (FEV1 decrease >0mL) at Week 52, with most (88%) experiencing FEV1 worsening at an earlier visit, and 37% experiencing FEV1 worsening at all prior study visits. The clinical relevance of FEV1 worsening was highlighted by the significantly higher rate of exacerbations and significantly worse patient-reported outcome scores (SGRQ and CAT) for participants who experienced any FEV1 decrease compared with an FEV1 increase/no change. The IMPACT population included patients who were ≥40 years of age with symptomatic COPD and with either an FEV1 <50% of predicted normal values and ≥1 moderate or severe exacerbation in the previous year, or an FEV1 50%–80% of predicted normal values and ≥2 moderate or ≥1 severe exacerbation(s) in the previous year.9 Subsequently, findings may differ in younger patients or those with milder disease. Studies in the general population have shown an association with more rapid lung function decrease over 3–4 years and increasing risk of COPD hospitalizations and mortality.4,5 Guidance and previous studies have used either a range of 100 mL to 140 mL as clinically important, a change between 5% and 10% from baseline,10 or a decrease ≥100mL as the definition for an FEV1 decrease.7,8 While a trend towards greatest clinical worsening in the ≥210mL FEV1‑decrease subgroup was seen, there was little distinguishing the other FEV1-decrease subgroups, with all displaying similarly worse clinical outcomes. These results suggest all levels of FEV1 decrease are associated with worse clinical outcomes in terms of exacerbations and quality of life, with no clear “minimal clinically important difference” threshold. It is important to mention that while CAT and SGRQ scores were significantly worse for those with a decreased FEV1, there was a trend towards improved patient-reported outcomes (PROs) for all patients. Notably, improvements in PROs over time are common in interventional studies, even those that are placebo-controlled, and the data presented here may be influenced by this. As such, this warrants further investigation in other datasets.
Participants were less likely to have an FEV1 decrease at Week 52 and at earlier visits if they received FF/UMEC/VI rather than FF/VI or UMEC/VI, suggesting that triple therapy provides significantly greater preventive effects for exacerbations and lung function decrease compared with dual therapies. Such preventative effects conferred by FF/UMEC/VI may decrease the clinical and economic burden associated with COPD, as exacerbations and low FEV1 are associated with high medical costs and health care resource utilization.11-15 However, almost a third of patients who received FF/UMEC/VI still experienced a decrease in FEV1 at Week 52, suggesting that further investigation of this patient population is warranted to determine other potential factors (e.g., emphysema, secondary pulmonary hypertension, bronchiectasis), that may be contributing to this decrease.
The strength of this study is the large population size, and while participants with the worst FEV1 decrease may have dropped out of the study, potentially leading to underestimation of participants with FEV1 worsening, a sensitivity analysis showed that imputing missing data for the odds of having a >0mL FEV1 decrease when treated with FF/UMEC/VI versus dual therapy provided similar results (data not shown). This was a post hoc analysis, therefore, inferences of causality between FEV1 decrease and changes in symptoms or exacerbations cannot be performed. Further, the relationships between FEV1 decrease and outcomes are associations and not predictions. Additionally, this analysis did not account for the temporality of exacerbations with respect to whether patients experienced a decrease in FEV1 and then experienced an exacerbation, or vice versa. However, as patients with an FEV1 decrease had worse outcomes on the CAT and SGRQ, this indicates that exacerbation was not the sole outcome affected by the FEV1 decrease. This post hoc analysis used absolute changes in FEV1 to assess the effect of lung function decline. While relative change has been suggested as a more meaningful measure in patients with more severe airflow limitation,16 and as this analysis focuses on the clinical outcomes in subgroups based on lung function change, absolute and relative change offer comparable clinical relevance in this case. Finally, IMPACT was an interventional study, and further validation from real-world evidence is needed, particularly in younger populations and in those with milder disease.
This post hoc analysis of IMPACT demonstrated that any FEV1 decrease is associated with worse clinical and patient-reported outcomes, however, no threshold for minimal clinically important differences for FEV1 deterioration was apparent. Results also indicate that symptomatic patients with prior exacerbations treated with FF/UMEC/VI are less likely to experience FEV1 worsening than patients treated with dual therapy.
Acknowledgements
Author contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis. All authors were involved in data analysis and interpretation of the data. DA Lipson contributed to the concept and design of the trial. DMG Halpin, EM Kerwin, and GJ Criner contributed to the acquisition of data. All authors contributed to data analysis and interpretation.
Editorial support in the form of preparation of the first draft based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, was provided by Eloise Morecroft, PhD, and Alexandra Berry, PhD, of Fishawack Indicia Ltd, UK, part of Avalere Health, and was funded by GSK. ELLIPTA is owned by or licensed to the GSK group of companies.
Dave Singh is supported by the National Institute for Health Research Manchester Biomedical Research Centre.
Data sharing statement: Anonymized individual participant data and study documents can be requested for further research from https://www.gsk-studyregister.com/en/
Declaration of Interests
All authors report other and nonfinancial support from GSK (funding the study and funding medical writing support by Alexandra Berry, PhD, and Eloise Morecroft, PhD, of Fishawack Indicia Ltd, UK). MKH reports personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, United Therapeutics, Altesa Biopharma, UpToDate, Medscape, Integrity, and Amgen. She has received either in-kind research support or funds paid to the institution from the National Institutes of Health (NIH), Novartis, Sunovion, Nuvaira, Sanofi, AstraZeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association. She has participated in data safety monitoring boards for Novartis and Medtronic with funds paid to the institution. She has received stock options from Meissa Vaccines and Altesa Biopharma. GJC has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, CSA Medical, Eolo, GSK, HGE Technologies, Novartis, Nuvaira, Olympus, Pulmonx, and Verona. DMGH has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, and Pfizer, and nonfinancial support from Boehringer Ingelheim and Novartis. EMK is an employee of Altitude Clinical Consulting; has served on advisory boards, and speaker panels, or received travel reimbursement from Amphastar, AstraZeneca, Cipla, Chiesi, Connect Biopharma, GSK, Mylan, Novartis, Sunovion, Teva, and Theravance; and has also conducted multicenter clinical research trials as principal investigator for Abbott, Abbvie, Adare, Alk Abello, Amgen, Amneal, Amphastar, AnaptysBio, AOBiome, Arcutis, AstraZeneca, Avillion, Cara Therapeutics, Chiesi, Cipla, Dermira, DS Biopharma, Galderma, Genentech (Roche), GSK, Glenmark, Gossamer, Incyte, Johnson & Johnson (Janssen), Knopp Biosciences, Leo Pharma, Lupin, Merck, Moderna, Mylan, Novartis, Pearl Therapeutics (AstraZeneca), Pfizer, Romark, resTORbio, Sanofi-Aventis (Sanofi), Sunovion, Teva, Theravance, and Vanda. FJM is editor-in-chief of the American Journal of Respiratory and Critical Care Medicine and reports receiving consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Gala, GSK, Novartis, Polarean, Pulmonx, Sanofi/Regeneron, Sunovion, Teva, Theravance/Viatris, and Verona; grant support from AstraZeneca, Chiesi, GSK, and Sanofi/Regeneron; payment or honoraria from UpToDate for participation in COPD continuing medical education activities; and participated in an event adjudication committee for MedTronic. RAW has received personal fees from AstraZeneca, Boehringer Ingelheim, Contrafect, Roche-Genentech, Bristol Myers Squibb, Merck, Verona, Theravance, AbbVie, GSK, Chemerx, Kiniksa, Savara, Galderma, Kamada, Pulmonx, Kinevant, Vaxart, Polarean, Chiesi, 4D Pharma, and Puretech, and grant support from AstraZeneca, Sanofi, Verona, Genentech, Boehringer Ingelheim, and 4DX imaging. He has received payment for expert testimony from the U. S. government and Genentech; and support for attending meetings and/or travel from AstraZeneca. Additionally, he has received editorial support from GSK, AstraZeneca, Boehringer Ingelheim, and the Merck Foundation; and has served on the Board of Directors and the Medical and Scientific Advisory Committee for the COPD Foundation, and on a Scientific Advisory Board for the American Lung Association. DS declares consulting fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, Glenmark, GSK, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Synairgen, Teva, Theravance, and Verona. LT is a consultant for Veramed and a director for Precise Approach Ltd, London; he was contracted by GSK to conduct the statistical analysis for this study but received no payment for manuscript development.. DAL is an employee of GSK and holds GSK stocks/shares