Running Head: Dronabinol for Dyspnea and Quality of Life in COPD
Funding Support: This material is the result of work supported with resources and the use of facilities at the VA Loma Linda Healthcare System. The contents of this article do not represent the views of the VA or the United States government.
Date of Acceptance: January 26, 2024 | Publication Online Date: February 2, 2024
Abbreviations: AE=adverse event; BMI=body mass index; CB1/CB2=cannabinoid receptors; CI=confidence interval; COPD=chronic obstructive pulmonary disease; FDA=U. S. Food and Drug Administration; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GDS=geriatric depression scale; GOLD=Global initiative for chronic Obstructive Lung Disease; HCO3=plasma bicarbonate; ISWT=incremental shuttle walk test; PaCO2=partial pressure of arterial carbon dioxide; PCO2=partial pressure of carbon dioxide within arterial or venous blood; PFSDQ=Pulmonary Functional Status and Dyspnea Questionnaire; SAE=severe adverse event; SD=standard deviation; SGRQ=St George’s Respiratory Questionnaire; SpO2=oxygen saturation by pulse oximetry; THC=tetrahydrocannabinol
Citation: Zaid AH, Thapamagar SB, Anholm JD, et al. Effects of dronabinol on dyspnea and quality of life in patients with COPD. Chronic Obstr Pulm Dis. 2024; 11(2): 206-215. doi: http://doi.org/10.15326/jcopdf.2023.0401
Online Supplemental Material: Read Online Supplemental Material (261KB)
Background
Dyspnea is the most common and frequently debilitating symptom in patients with chronic obstructive pulmonary disease (COPD).1 Despite decades of research, the pathophysiology of exertional dyspnea in COPD is yet to be fully understood.2 Several cortical and subcortical central neural pathways play a role in exacerbating dyspnea in COPD patients.3 Exertion appears to result in an imbalance between rising central neural drive and impaired thoracic volume displacement. This imbalance intensifies the sensation of dyspnea on exertion.1 The mismatch between medullary respiratory motor discharge and peripheral mechano-sensor afferent feedback results in a distressing urge to breathe independent of muscular effort.1
Most current therapies aim to improve ventilatory mechanics, however, despite maximal conventional therapy, a significant proportion of patients remain symptomatic. Furthermore, as the disease progresses, these patients experience a downward spiral of physical deconditioning and reduced exercise capacity, compounding the impact of COPD on quality of life.4 This contributes to a high prevalence of depressive disorders in these patients.5 Modulation of the neuronal component of COPD-related dyspnea perception and “air hunger” has the potential to decrease the perception of dyspnea, and improve quality of life and exercise capacity. Such therapies targeting the central neural signaling of dyspnea are limited at present.6-9
In the United States, tetrahydrocannabinol (Δ9-THC) is manufactured as dronabinol in sesame oil-based soft gel capsules.10 It is currently U.S. Food and Drug Administration (FDA)-approved for use in HIV-associated cachexia to stimulate appetite, and in the treatment of chemotherapy-induced nausea and vomiting. It is generally well tolerated in these populations and has been used off-label in palliative care medicine for the relief of “air-hunger.”11 There are currently no large, prospective trials exploring the role of cannabinoids in COPD-related dyspnea. One pilot study suggests a possible overlap between cannabinoid receptors (CB1) and cortical centers responsible for dyspnea perception and demonstrated a reduction in the perception of “air hunger” in COPD patients following the administration of dronabinol.11
While another study involving vaporized cannabis in COPD did not show improvement in dyspnea and exercise endurance,12 our randomized, controlled pilot study tested the hypothesis that dronabinol would improve dyspnea intensity and, thereby, exercise tolerance in patients with COPD.
Methods
Study Sites and Participants
Our study was a prospective, randomized, double-blind, placebo-controlled, crossover pilot study conducted at VA Loma Linda Healthcare System, Loma Linda, California. Potential participants were referred by the pulmonary rehabilitation staff to the study investigators and were screened for eligibility. Eligible participants had a diagnosis of COPD as defined by the American Thoracic Society and European Respiratory Society13 and remained dyspneic despite maximal medical therapy indicated for their level of disease. Participants with COPD were identified using the Crapo et al (1981) spirometry reference equations as the normal value dataset, as this was the standard used in the pulmonary function laboratory at the VA Loma Linda Healthcare System at the time the study was conducted.14 All participants had also completed a pulmonary rehabilitation program (including reconditioning exercise, education, and support group meetings) prior to study enrollment.
Participants were excluded if their pre-enrollment urine drug screen was positive for THC, if they had chronic hypercapnia (partial pressure of arterial carbon dioxide [PaCO2] >45mmHg), or were anemic (hemoglobin < 7g/dL). Participants who were pregnant or had a known allergy to sesame seeds, sesame oil, or dronabinol, and those with uncompensated acute heart failure or a history of neuromuscular disease were also excluded from the study. Additional clinical characteristics of the study participants are available in Table S4 in the online supplement.
The study was approved by the VA Loma Linda Healthcare System Institutional Review Board and all participants gave written, informed consent prior to any study procedures. The study conformed to the amended Declaration of Helsinki, apart from registration in a database. A letter of exemption from an investigational new drug application from the FDA was obtained before the study was started.
Study Design and Interventions
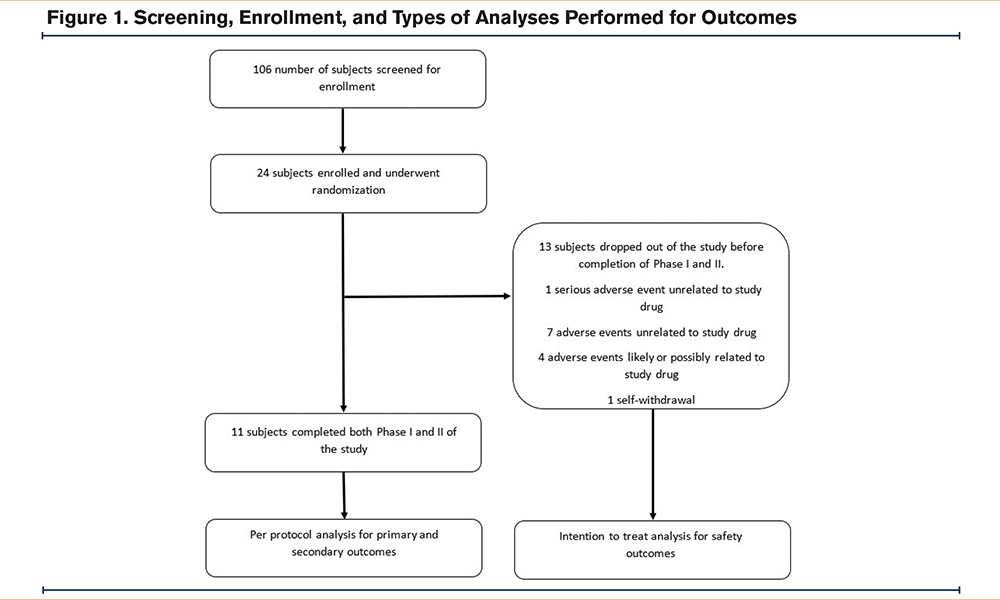
After obtaining informed consent, the enrolled participants underwent an arterial blood gas and a urine drug screen, and a focused history and physical examination were performed. This was followed by randomization. Screening and enrollment are depicted in Figure 1.
This was a randomized double-blind, placebo-controlled pilot study. A computer-generated random number sequence was used for randomization to drug versus placebo. All study personnel, including outcome assessors as well as study participants, were blinded to the study medication allocation. Only the research pharmacist involved in this study had access to study medication allocation and randomization and the study was “unblinded” only after all study procedures and outcomes had been assessed. The pharmacist was not involved in making outcome assessments of the study participants.
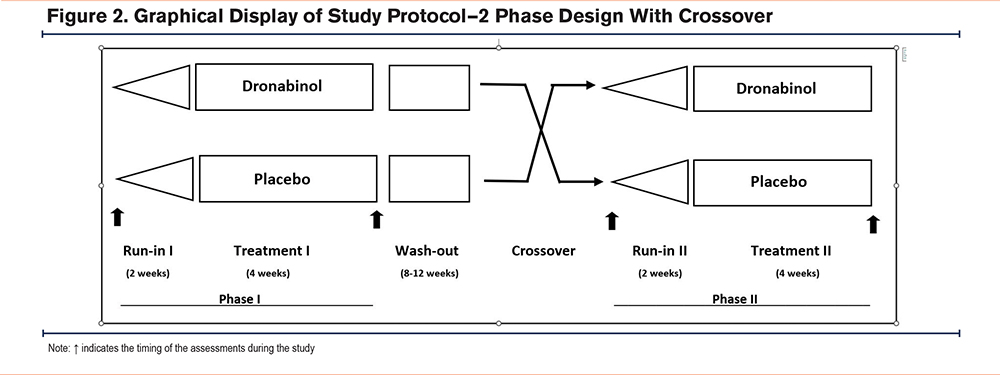
Participants were randomized in a 1:1 fashion to either the study drug or the placebo arm for phase 1 and crossed over to the other arm during phase 2. A random number table was used to generate a list of random numbers, with the odd/even numbers determining the order of administering either the study drug or the placebo enclosed within individual envelopes. These were given to the research pharmacist who opened an envelope each time a new participant was enrolled. The research pharmacist was blinded to all other study procedures and the investigators remained blinded to the randomization until all study procedures were completed. Each study phase consisted of a 2-week run-in period followed by a 4-week treatment period. Dronabinol capsules were overencapsulated in a gelatin capsule filled with cornstarch. A matching gel placebo cornstarch capsule with similar color, consistency, and weight was prepared by the research pharmacist at the Loma Linda VA Medical Center. The study medication (dronabinol or placebo) was dispensed by the research pharmacist, and labeled as an “investigational drug.”
Depending on the arm of the study they were in, participants were started on either the placebo or dronabinol 5mg capsules orally. During the run-in period, study participants were asked to start taking the study drug’s (or matching placebo) 5 mg once daily in the morning for 3 days. If well tolerated, participants gradually increased the frequency to up to 5 mg 4 times daily by day 9. They continued 5 mg 4 times daily for the remainder of the run-in period and the 4-week study period before the drug washout time. At any point during the run-in phase, if the participants reported side effects, they were given the option to reduce the dosing frequency to the previously well-tolerated dosage and to remain at the same frequency for the rest of the run-in and study periods. On completion of the first phase, each patient underwent an 8- to 12-week washout. This was followed by crossover to a similarly structured phase 2 (Figure 2).
We evaluated dyspnea and fatigue, both at rest and postexercise, using the modified Borg scale.15 To evaluate quality of life before and after each phase, we employed the St George’s Respiratory Questionnaire (SGRQ).16 To study changes in functional status before and after the 2 phases, we employed the modified Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ).17 To further assess exercise capacity and exercise-induced dyspnea and fatigue, we used the incremental shuttle walk test (ISWT).18 To determine effects on mood and symptoms of depression, we used the shortened Geriatric Depression Scale (GDS).19 The blinding of treatment order was maintained until all participants had completed the trial.
Study Outcomes
The primary outcomes of this study were changes in Borg dyspnea scale at rest and postexercise, shuttle walk distances before and after phases 1 and 2 of the study, and the PFSDQ scores before and after phases 1 and 2 of the study. The secondary outcomes were changes in Borg fatigue scores at rest and postexercise, the SGRQ scores, and the GDS scores before and after phases 1 and 2. Safety outcomes were the number of adverse events during the study.
Statistical Analysis
Participants who completed both phases of the study were included in per-protocol analysis for primary and secondary outcomes. All participants who received at least a single dose of the study drug or placebo were included in safety outcomes. Outcome measures were assessed for normality using the Shapiro-Wilk test. For normally distributed variables, the values on the dronabinol phase were compared to those on placebo using the paired t-test. Nonnormally distributed data was analyzed with the Wilcoxon signed rank test. All statistical procedures were carried out using Stata (version 15; StataCorp LLC, College Station, Texas). Values are listed as means ± standard deviation. A p-value of less than 0.05 was considered statistically significant.
Results
A total of 24 individuals were enrolled in the study. Of those, only 11 individuals completed both phases of the study (“completers"). Participants were predominantly male and White. A total of 13 participants dropped out of the study before completion (“dropouts") after at least one dose of study medication. The demographics and clinical characteristics of the participants at baseline are listed in Table 1. Comorbidities and treatment of medical conditions including COPD are summarized in the supplemental Table S2 in the online supplement.
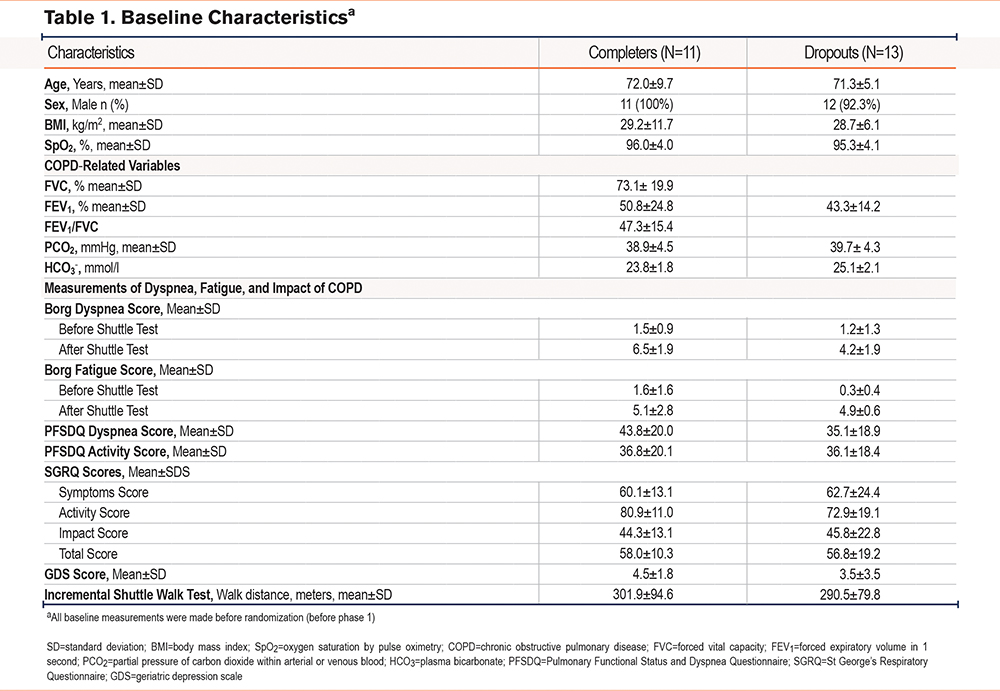
Notably, individuals who dropped out had a numerically higher mean predicted percentage of forced expiratory volume in 1 second (FEV1) but also had a higher prevalence of diabetes, osteoarthritis, and anxiety. Among the 11 participants who completed the study, 4 had an FEV1 <50 % of predicted (COPD Global initiative for chronic Obstructive Lung Disease [GOLD]20 stages 3 and 4) while 7 had an FEV1 >50% of predicted (COPD GOLD stages 1 and 2).
Two individuals were withdrawn before administration of the study medications for meeting exclusion criteria after enrollment. One had hypercapnia (PaCO2 >45) and the other had an uncontrolled psychiatric illness. A total of 22 patients (study dropouts and completers) were included in safety outcomes. Among 11 completers, 6 had the placebo and 5 had the active drug as the lead treatment. Among 11 dropouts (who received at least 1 treatment), 6 had the placebo and 5 had the active drug as the lead treatment. Baseline characteristics of participants who completed the study compared to participants who dropped out were not significantly different.
Primary outcomes are displayed in Figure 3. Dronabinol did not improve dyspnea measured by Borg dyspnea score at rest or after exercise. The change in Borg dyspnea scale at rest was 0.27 (95% confidence interval [CI], -0.6 to 1.1) points following treatment with dronabinol compared to 0.23 (95% CI -0.7 to 1.1) points following placebo (mean difference -0.04, 95% CI -1.4 to 1.2, p=0.94). Following ISWT, the Borg dyspnea scale changed by 0.8 (-.06 to 2.2) points on dronabinol compared to 0.4 (0.1 to 2.8) on placebo (mean difference -0.4, 95% CI -3.7 to 0.6, p=0.69). Similarly, the PFSDQ dyspnea score marginally improved on both dronabinol (0.6 points, 95% CI -3.9 to 5.2) and placebo (5.0 points, 95% CI -6.3 to 16.3). Overall, dyspnea measured by PFSDQ did not improve on dronabinol as compared to placebo (mean difference -4.4 points, 95% CI -14.0 to 5.2).
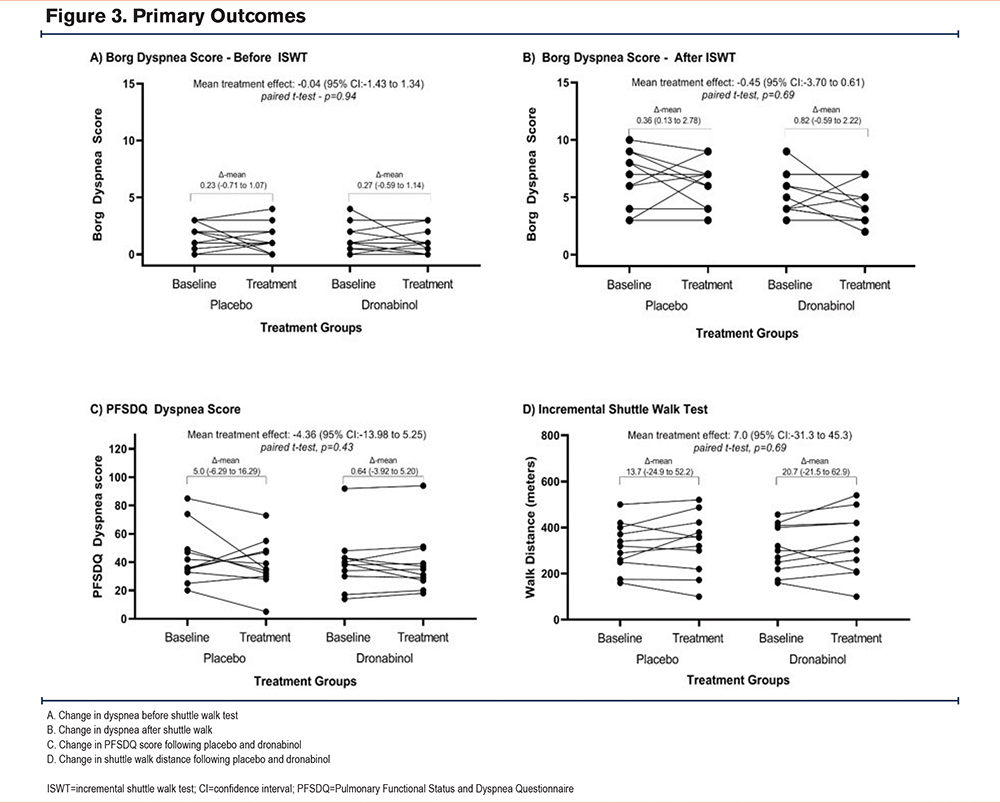
The mean improvement in shuttle walk distance was 20.7m (95% CI -21.5 to 62.9) on dronabinol compared with 13.7m following placebo (mean improvement 7.0m, 95% CI -31.3 to 45.3, p=0.69). We examined changes in shuttle walk distance among participants with severe to very severe COPD (GOLD stages 3 and 4)20 as compared to mild to moderate COPD (GOLD stages 1 and 2) while taking either placebo or dronabinol. There was no significant difference in the change in shuttle walk distance following either placebo or study drug based on COPD severity (Wilcoxon rank-sum test, p=1.0 and p=0.09 respectively). Additionally, there was no significant difference between shuttle walk distance before and after the placebo or before and after the study drug based on COPD severity (Wilcoxon signed-rank test, p=0.58 and p=0.55 respectively).
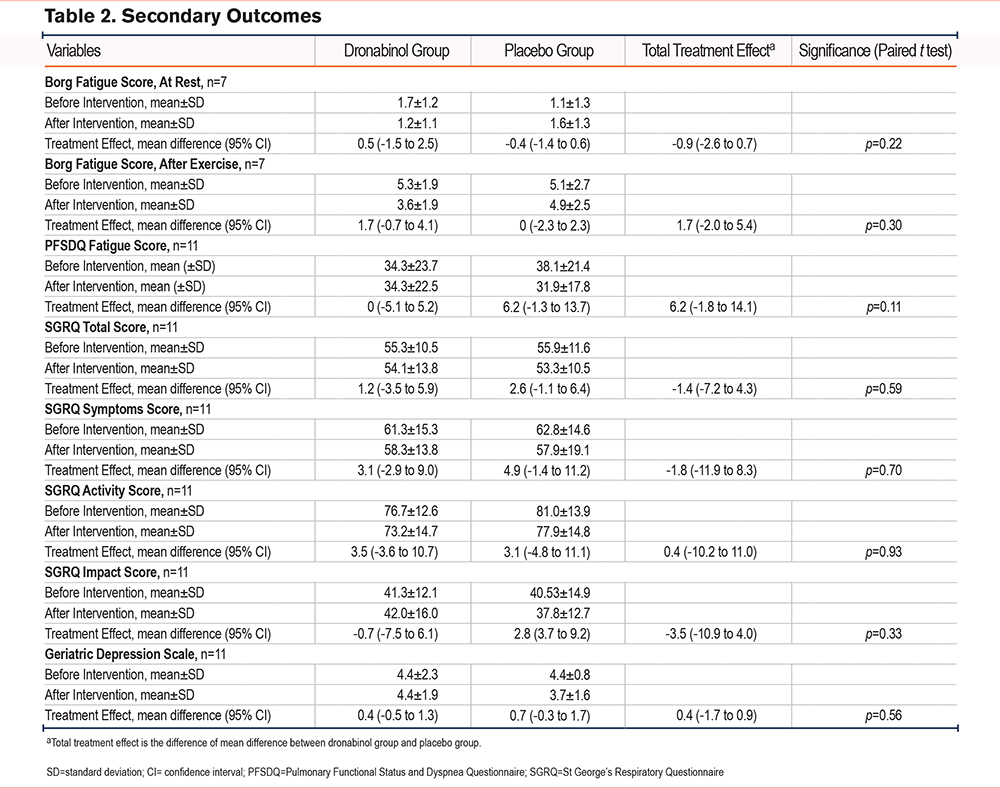
The effect of dronabinol on fatigue and SGRQ scores is summarized in Table 2. Overall, dronabinol did not improve fatigue or respiratory symptoms. The total treatment effect of dronabinol on fatigue before and after ISWT was -0.9 (95% CI -2.6 to 0.7, p=0.22) and 1.7 (95% CI -2.0 to 5.4, p=0.30). The effect on the PFSDQ fatigue score was 6.2 (95% CI -1.8 to 14.1, p=011) indicating a nonsignificant worsening in functional status and symptom control. Changes in SGRQ scores following dronabinol compared with placebo suggested no improvement in quality of life (Table 2). The total treatment effect of dronabinol on the GDS scale was 0.4 points (95% CI -1.7 to 0.9, p=0.56) indicating no improvement in depressive symptoms.
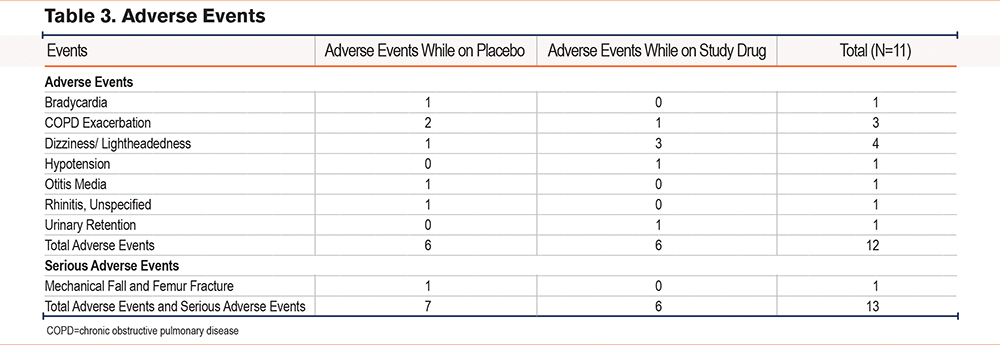
There were 12 adverse events (AEs) and 1 serious adverse event (SAE). There was profound reluctance on the part of patients who experienced an AE to continue the study drug. Eight participants had at least one AE (including 1 SAE) and 7 of these 8 participants dropped out of the study before completion of both arms of the protocol. Only 1 of the patients who experienced an adverse event was able to continue taking the study drug at a lower dose. Overall, adverse events were similar between the dronabinol arm and the placebo arm. Investigators attributed 4 adverse reactions to the study drug.
All 4 AEs deemed possibly related to or likely related to the study medication occurred in participants who were on dronabinol at the time of the AE. A total of 7 AEs and the 1 SAE (mechanical fall) were deemed unlikely to be related to the study drug. Dizziness and lightheadedness were the most common AEs and were likely related to the study drug. The majority of adverse events were moderate in intensity and all AEs in participants who dropped out were resolved after the study drug was discontinued. A breakdown of all adverse events is shown in Table 3 and supplemental Table S3 in the online supplement.
Discussion
Contrary to our hypothesis, in this randomized, double-blind, placebo-controlled, crossover pilot study, dronabinol, when compared to placebo, did not improve dyspnea as measured by the Borg scale at rest or after the shuttle walk. Dronabinol compared to placebo did not improve functional status or activity-associated dyspnea as measured by the PFSDQ questionnaire in participants with COPD.
Some of the earliest references to the use of the Cannabis sativa plant date back as far as 4000 B.C. in China.21 Over the millennia, the plant has been consumed in various forms for recreational and, less commonly, for medicinal purposes.22 In 1974, the most active and clinically relevant component, THC in C. sativa extracts was identified.23 Subsequent discovery and cloning of the 2 cannabinoid receptors, CB1 and CB2, have led to expanded pharmacological research.24-26 Of particular interest for the perception of and response to dyspnea, is the presence of CB1 receptors in the frontal cortex, hypothalamus, and the brainstem – areas involved in regulating dyspnea.2,27-29 Although initially showing some promise,6-9 other psychoactive agents such as opioids, benzodiazepines, and selective serotonin reuptake inhibitors have not been shown to alleviate dyspnea in COPD in larger, randomized trials.30-32 We hypothesized that by acting on the central CB1 receptors, dronabinol would improve exercise capacity and dyspnea scores. However, our randomized pilot study did not support this hypothesis.
The crossover design of this study yielded an efficient comparison of treatment to placebo while eliminating the effects of confounding covariates. Our study evaluated patient-centered outcomes in COPD such as degree of dyspnea, exercise capacity, overall quality of life, and mental well-being. Eligibility criteria were stringent and further minimized the influence of other factors such as anemia, hypercapnia, musculoskeletal disease, or uncontrolled cardiac disease on dyspnea and exercise tolerance.
Our study has several limitations. This was a single-center study comprised of mostly White, male patients within the Veteran population. Our sample size was small. The confidence limits around the improvement in shuttle walk distances in our study included the “minimum important difference” of the test in COPD patients.33 However, given the small sample size, a clinically significant treatment effect on shuttle walk distances could not be ruled out. Additionally, we did not measure physiological parameters such as lung volumes, flow rates, or minute ventilation changes during exercise in response to the intervention. These measures could have provided valuable mechanistic insights into the effect of study medication on dyspnea in COPD patients. Furthermore, assessments were made after participants had exercised to the point of symptomatic limitation, likely blunting the determination of the study drug efficacy. Isotime symptom score responses recorded at specific time intervals during fixed-intensity exercise testing may be better suited for this purpose.34
The stringent eligibility criteria, arguably a strength of the study, also resulted in the exclusion of most patients screened. This may have also resulted in a disproportionate recruitment of participants with milder COPD (see Table 1). The inclusion of more severe COPD patients may have altered our outcomes. In addition, we experienced a higher than anticipated dropout rate. Of the 24 enrolled participants, 13 dropped out of the study before completion. All patients who had AEs possibly related to or likely related to study medication were on dronabinol at the time of the AE.
Another potential limitation was the long (8–12 weeks) wash-out period. This was done to ensure complete elimination of the study drug as determined by a urine drug screen. This may have led to a higher rate of attrition from the study. Dronabinol is a synthetic THC compound and is known to have certain psychoactive side effects such as dizziness, drowsiness, and cognitive impairment. Unfortunately, our study did not systematically assess or track these effects of the study drugs. Hence, we could not determine if there was any blinded treatment preference amongst the participants.
Serial measurements of circulating cannabinoid concentrations would have determined if a steady state blood level had been achieved over the 4-week intervention period. Unfortunately, we did not have the laboratory capabilities to carry out such measurements. Finally, although the study participants’ weight and body mass index were collected at the time of enrollment in the study, we did not assess the effects of the drug on body mass at other points during the study. A change in body mass could have influenced the participants’ exercise performance.
Conclusions
In our small clinical pilot study involving individuals with COPD and dyspnea on exertion, dronabinol did not show significant improvement in dyspnea, fatigue, shuttle walk distance, quality of life, and depression when compared to placebo. Larger randomized trials are needed to definitively assess the impact of dronabinol and related THC compounds in COPD.
Acknowledgements
Author contributions: AHZ, ST, JDA, LW-C, LMD, and LS were involved in designing the study, analysis and interpretation of data, and drafting the manuscript.All authors have given final approval of the version to be published and have agreed to be accountable for all aspects of the work.
The authors would like to thank Monica Gertz, MD, Siavash Farshidpanah, MD, Kathleen Ellstrom, PhD, ACNS-BC, Katie Gutierrez, CPhT, and Michelle Chon, PharmD, for their contributions that helped make this study possible. We would also like to thank Aimee Rodriguez, BS, RRT, for administrative help with the manuscript and data analysis.
Declaration of Interests
All authors have no conflicts of interest to declare.