Running Head: Plant-Centered Diets and Emphysema Risk
Funding Support: The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood. This manuscript has been reviewed by CARDIA for scientific content but holds no restrictions regarding publication.
Date of Acceptance: October 27, 2023 │ Publication Online Date: November 6, 2023
Abbreviations: ANOVA=analysis of variance; APDQS=A Priori Diet Quality Scores; BMI= body mass index; CARDIA=Coronary Artery Risk Development in Young Adults; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CRF=cardiorespiratory fitness; CT=computed tomography; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; MV=multivariable; OR=odds ratio; SD=standard deviation
Citation: Jackson MK, Choi Y, Eisenberg E, et al. A plant-centered diet is inversely associated with radiographic emphysema: findings from the CARDIA Lung Study. Chronic Obstr Pulm Dis. 2024; 11(2): 164-173. doi: http://doi.org/10.15326/jcopdf.2023.0437
Online Supplemental Material: Read Online Supplemental Material (218KB)
Introduction
Chronic obstructive pulmonary disease (COPD) affects over 15 million Americans and is a leading cause of death in the United States.1 Emphysema, a key manifestation of chronic lung disease, is characterized by irreversible structural changes to the lung and carries important implications for long-term lung health, even in patients without an established COPD diagnosis. There is increasing recognition that emphysema is an important, independent clinical predictor of worse respiratory outcomes, including respiratory symptoms and quality of life, even in the absence of airflow limitation on spirometry.2 Therefore, emphysema may represent a clinically significant, intermediate phenotype of impaired lung health3 and a critical window for early interventions to intercept future respiratory complications. However, modifiable factors to intercept the development of emphysema have not been identified.
While smoking remains the primary environmental risk factor for the development of emphysema, smoking cessation interventions have resulted in long-term quit success rates in only up to 25% of patients.4,5 These data highlight that these strategies alone are insufficient to optimize respiratory health among smokers and that a simultaneous focus on other treatable traits is greatly needed to mitigate the adverse health effects of tobacco use across the life course. Notably, individual adherence to healthy dietary patterns may serve as a potential strategy for preserving lung health in these exposed populations.
Emerging data provides support for the role of diet in lung health among individuals with competing risk factors such as lifetime tobacco exposure. In one study, smokers without respiratory disease with greater adherence to a Western diet pattern (increased red and cured meat and sweets; low fruits, vegetables, legumes, and fish consumption) were at an increased risk of impaired lung function.6 In addition, we have shown that as early as adolescence, adherence to a higher quality diet is associated with significantly decreased environmental, tobacco-associated respiratory symptoms.7 As respiratory symptoms—including cough/phlegm and wheeze—among young adults have been associated with greater odds of future radiographic emphysema,8 these data emphasize a potential role for diet as an early modifiable risk factor for the lifetime risk of chronic lung disease.
A recent analysis of data from a large, multicenter longitudinal cohort study, the Coronary Artery Risk Development in Young Adults (CARDIA) study, demonstrated that a nutritionally rich, plant-centered diet was protective against cardiovascular-related morbidity and mortality.9 Diets higher in fruits and vegetables have also been associated with improved lung outcomes, including reduced wheeze in children,10 better lung function in adults,11 and lower prevalence of asthma in both adults and children.12 However, few studies have evaluated the effect of dietary patterns on emphysema. In this study, we examined the association between adherence to a nutritionally rich, plant-based diet and emphysema in early to mid-adulthood using the A Priori Diet Quality Score (APDQS), a novel diet quality assessment tool that accurately assesses the intake of the most nutritious, plant-centered foods without excluding animal products. The objective of this analysis was to determine the extent to which adherence to a nutritionally rich, plant-centered diet—via the APDQS—in ever-smoker young adults is associated with future development of radiographic emphysema.
Methods
Study Population and Design
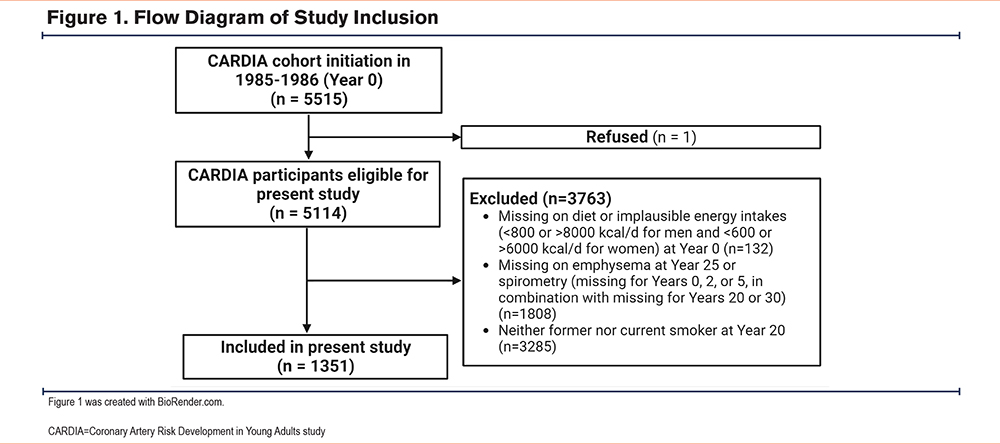
Population: This study is a secondary analysis of the CARDIA study, a multicenter, prospective cohort of 5115 adult Black and White men and women from 4 geographically diverse U.S. cities.13 Participants at baseline were aged 18 to 30 years, and were followed for 30 years with 71% retention at year 30. The study protocol has been published elsewhere.13 For the present study, we included ever-smoker participants (defined as self-reported smoking at any time point prior to year 20) due to the low prevalence of emphysema in never smokers within this population. For the outcome of emphysema, we restricted the population to those who had computed tomography (CT) measurements available at year 25. As additional analyses were conducted as a part of this investigation, those with the absence of spirometry (missing for years 0, 2, or 5, in combination with missing for years 20 or 30) were also excluded (n=42). A flow diagram for participant inclusion is included in Figure 1. All participants provided written informed consent at all examinations, and research protocols were approved by institutional review boards at the CARDIA coordinating center and each field center. The University of Alabama at Birmingham Institutional Review Board (IRB-981106002) reviewed and approved the CARDIA study prior to data collection.
Assessment of Plant-based Diet Quality Score: Diet was assessed at baseline, year 7, and year 20 via the validated CARDIA diet history.14 The APDQS utilized CARDIA’s comprehensive dietary data to generate an evenly weighted score incorporating 46 food groups and was calculated as previously described.9,15 Unlike other measures of diet quality, the APDQS emphasizes greater consumption of plant-based foods with the highest nutritional value over nutritionally poor plant-based foods, while also accounting for animal product quality. Therefore, plant-based foods such as fruits, avocado, green and yellow vegetables, and whole grains contribute to a higher score, while higher intakes of plant-based foods like fried potatoes, grain desserts, margarine, and fruit juice would result in lower scores.9 Scores range from 0 to 132, with higher numbers indicating greater adherence to a high-quality diet.
Assessment of Outcome Variable: The primary outcome wasthe presence of radiographic emphysema. Radiographic emphysema was detected by visual assessment of year 25 CT scans utilizing methods as previously described.8,16 Briefly, all CT scans were reviewed by an initial reader (Reader 1) and classified as having paraseptal emphysema, centrilobular emphysema, both, or neither. All CT scans classified as having emphysema and a random sample of 10% of the remaining CT scans were reviewed by a second reader (Reader 2). Disagreements between Readers 1 and 2 were adjudicated by a third reader (Reader 3).
Other Covariates: Baseline demographic information and clinical data were obtained from the CARDIA database and included sex, age (years), maximal educational attainment (highest grade completed), race (Black, White), smoking and pack-year history, anthropometrics, and energy intake (kcal). Smoking status was assessed every year. Previous studies in this cohort have demonstrated misclassification between self‐reported cigarette smoking and year 0 serum cotinine measurements to be low.17 Spirometry, performed using standard procedures per American Thoracic Society guidelines18 to assess lung function, was reported at baseline. Body mass index (BMI) was calculated as weight/height squared (kg/m2) derived from measurements by trained technicians. Cardiorespiratory fitness (CRF) (treadmill time, seconds), history of asthma, and field center were also recorded.
Statistical Analyses
For the purposes of this analysis, APDQSs assessed at years 0, 7, and 20, for which updated information was available, were cumulatively averaged over follow-up and divided into quintiles. Baseline descriptive statistics were reported according to quintiles of the APDQS, and statistical significance was tested using analysis of variance (ANOVA) for continuous variables and Chi-square tests for categorical variables.
Multivariable logistic regression models for binary outcome were used to evaluate associations between the APDQS quintile of the averaged dietary data and year 25 radiographic emphysema. This averaging approach, previously used in CARDIA cohorts,9 allows for minimization of random within-person error, better reflects the cumulative, long-term dietary effect, and preserves sample size. The primary model was adjusted for age, sex, race (Black and White), field center (Birmingham, Chicago, Minneapolis, and Oakland), maximal educational attainment, baseline height, averaged total energy intake (years 0, 7, 20), averaged BMI (years 0, 2, 5, 7, 10, 15, and 20), and life-time pack years of smoking (years 0, 2, 5, 7, 10, 15, and 20). Maximal educational attainment was used as a surrogate for socioeconomic status, to be consistent with previous CARDIA analyses. As a sensitivity analysis, the model was further adjusted for CRF and asthma as potential mediating factors for emphysema. Potential effect modification by pack years of smoking was evaluated by testing the statistical significance of a multiplicative interaction term of the APDQS as a continuous variable with <10, 10–20, and >20 pack years of smoking. All analysis was conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Study Population
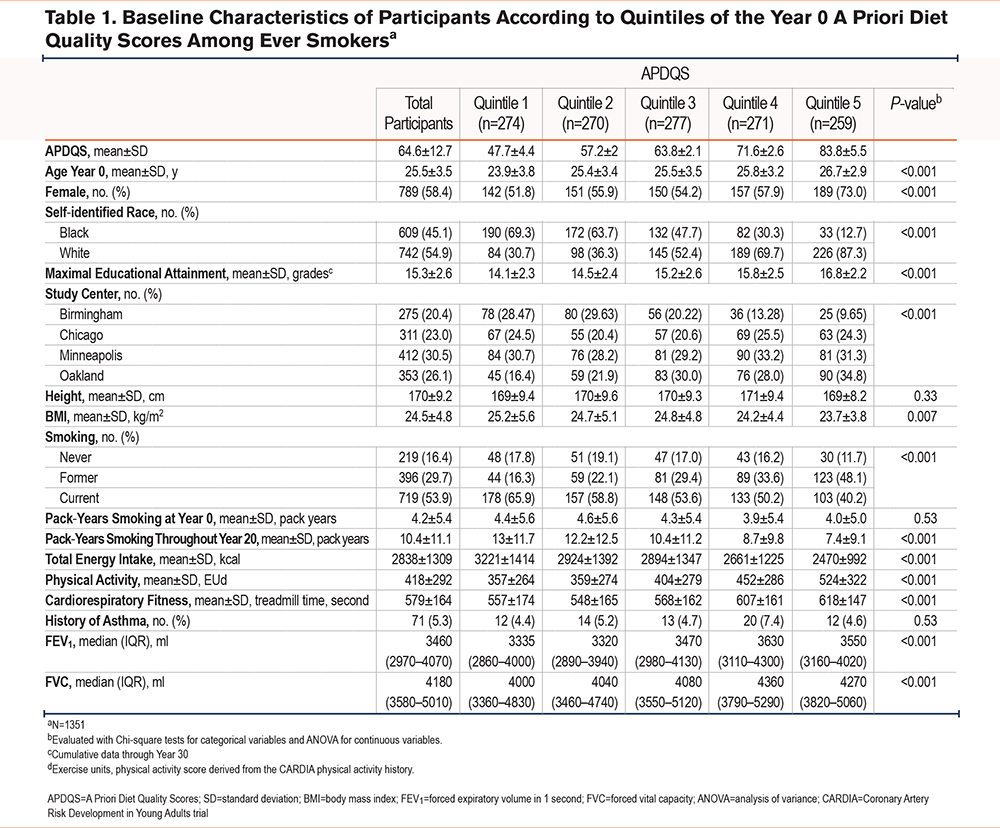
Of the original CARDIA cohort, 1351 ever-smokers (ever reported current or former smoking at any exam between year 0 and year 20) were available for analysis. The baseline mean (standard deviation [SD]) APDQS in this analytic population was 64.6 ± 12.7. Among ever smokers, 999 (73.9%) had dietary information at all 3 measures, 310 (22.9%) had 2 measurements, and 42 (3.1%) had only 1 measurement. Dietary intake consistently tracked over time. For example, Year 0 APDQSs had a correlation of about 0.62 and 0.58 with year 7 and year 20 APDQSs, respectively. The correlation between year 7 and year 20 was 0.60. Table 1 shows baseline (year 0) characteristics of study participants according to quintiles of the baseline APDQSs. Smoking history at enrollment (never, former, current) was similar across APDQS quintiles. Those with a higher APDQS were more likely to be older, be female, self-identify as White, have obtained a higher educational level, and have higher activity levels and CRF. Participants with a higher APDQS also had a lower BMI, lower total energy intake, fewer mean pack years of smoking over 20 years, and a higher forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC).
A Priori Diet Quality Scores and Radiographic Emphysema
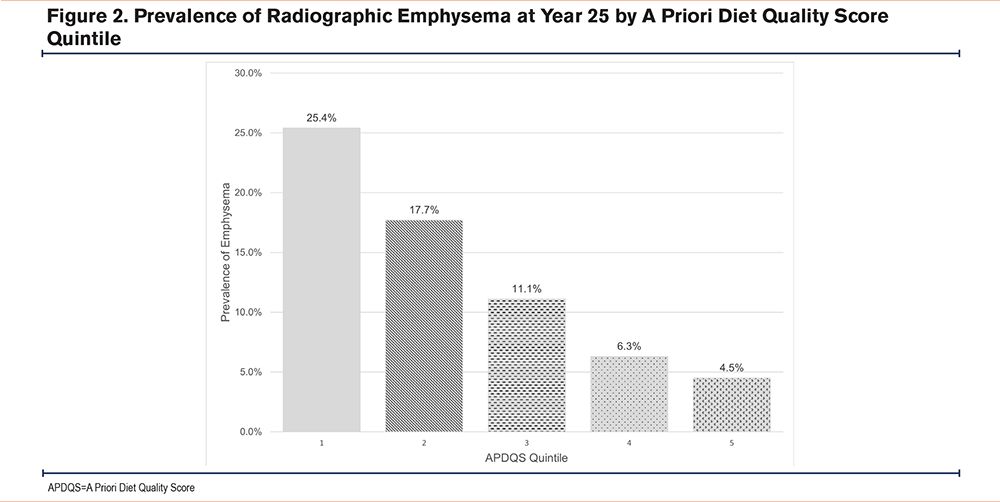
Of the 1351 ever-smokers who completed year 25 CT scans, emphysema was observed in 13.0% (n=175). The mean age of those with emphysema was 50.4 ± 3.5 years. The prevalence of emphysema was 4.5% in the highest APDQS quintile, compared with 25.4% in the lowest quintile (Figure 2).
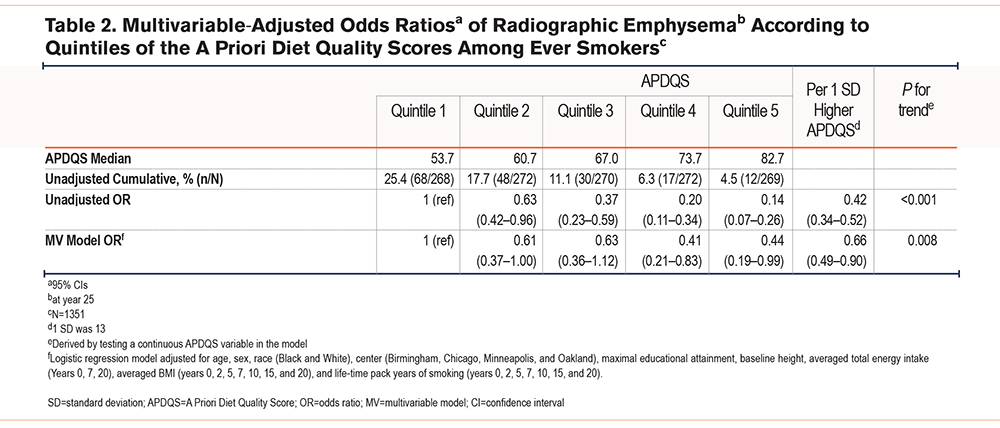
In unadjusted analyses of highest versus lowest quintile, ADPQS was inversely associated with radiographic emphysema (odds ratio [OR]: 0.14, 95% confidence interval [CI] 0.07–0.26). After adjusting for covariables, this inverse relationship persisted (OR 0.44, 95% CI 0.19–0.99 for the highest versus lowest quintile). For each one SD higher APDQS, there was a 34% (OR 0.66, 95% CI: 0.49−0.90) lower odds of emphysema (Table 2). Further adjustment for CRF or current asthma status, both individually and together, did not considerably alter the findings (data not shown).
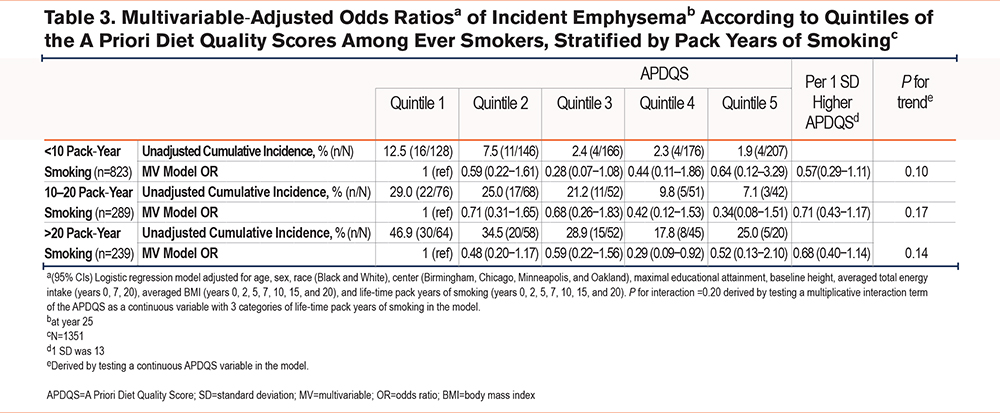
There was no significant interaction between APDQS and category of pack-year smoking history (<10 pack years, 10−20 pack years, and >20 pack years) in the development of incident emphysema (p for interaction=0.20), with no significant differences between the diet quintiles within each strata (Table 3).
Discussion
In this longitudinal study of young adult ever-smokers, we found that long-term consumption of a nutritionally rich, plant-centered diet was associated with a lower risk of future radiographic emphysema, independent of an influence of smoking history. Participants with the highest adherence to a plant-centered diet had a 56% lower risk of emphysema, compared with those with the lowest adherence. These associations were evident even after adjustment for established demographic, comorbid, and lifestyle factors that contribute to lung health. Notably, our study captured a population undergoing a critical transition from young to middle adulthood, for whom adherence to a plant-centered dietary pattern may represent an important early modifiable factor for the lifetime risk of chronic lung disease.
Emphysema is characterized by irreversible structural change to the lung, emphasizing the importance of preventing its development.19 The presence of emphysema among smokers, even in the absence of obstruction on spirometry, is an important, independent clinical predictor of worse respiratory outcomes.2 However, given the dependence on CT imaging to establish the presence of emphysema, very few prior studies have examined modifiable risk factors impacting the development and progression of this disease among a high-risk smoking population. Prior studies have relied primarily on self-report and have usually combined emphysema and chronic bronchitis to create a composite outcome of COPD. In contrast, our study’s unique availability of CTs in middle age allowed for the ability to detect associations between diet and radiographically-demonstrated structural changes in the lung.
Our data supports a potentially protective role of diet specifically for emphysema and is unique compared to prior studies that have examined diet and COPD without accounting for structural changes in the lung.20,21 Notably, very few studies have had the opportunity to leverage objective CT measurements of lung structure in relation to nutrition. One study found that intake of low-fat dairy products was associated with less severe measures of CT-defined emphysema in 3271 participants enrolled in the Multi-Ethnic Study of Atherosclerosis trial (P=0.02 and 0.01 for α, a measure of emphysema, and apical versus basilar distribution of emphysema, respectively),22 but to our knowledge dietary patterns and objective measures of emphysema have not been evaluated. Therefore, our findings fill an important gap in the literature and support diet patterns as a strategy for preventing emphysema development.
Although exact mechanisms for the association between the consumption of a nutritionally-rich, plant-centered diet and emphysema are unknown, recent animal studies have shown diets high in fiber (a key characteristic of plant-centered diets) attenuate pathological changes associated with emphysema progression and inflammatory response in cigarette-exposed mice.23 In particular, fiber supplementation modulated the diversity of the gut microbiome and increased the production of anti-inflammatory metabolites including short-chain fatty acids, which are known to significantly influence systemic inflammation24 through activation of G-protein receptors, inhibiting histone deacetylase, and serving as energy substrates for immune-regulating cells.25 In another study of mice exposed to cigarette smoke, an intervention of fecal microbiota transplantation and a high-fiber diet resulted in protective effects on the lung. The high-fiber diet significantly decreased macrophages and lymphocytes in bronchoalveolar lavage fluid (BAL), and interleukin-6 and interferon-gamma were decreased in BAL and serum. Both the fecal microbiota transplant and the high-fiber diet attenuated the development of emphysema and protected against alveolar destruction and cellular apoptosis.26 Similarly, human studies have demonstrated that a high-fiber diet is associated with reduced blood-based inflammatory biomarkers27; however, a greater understanding of specific mechanisms by which consumption of a plant-centered diet may protect against emphysema is needed.
We found it notable that 13% of all participants already had emphysema noted on CT at a relatively young age (range 42–56 years old), concurring with previous findings of respiratory impairments in smokers without spirometric COPD.28 Further research on the specific critical windows—during which dietary exposures have the greatest impact on lung health—is essential to develop public health dietary recommendations for children and young adults with the goal of preventing future adverse respiratory outcomes. Since structural outcomes of emphysema and physiologically apparent outcomes (such as lung function decline or airflow obstruction) are often clinically discordant and may represent divergent pathways of pathology, this will be an important area of future investigation.
This study has several key strengths. To our knowledge, this is the first prospective study to assess the relationship between a healthy diet pattern and radiographically-demonstrated emphysema. In addition, we had a prolonged follow-up period with high retention of participants, which provides insight into treatable traits for smoking-related respiratory outcomes during the critical transition of young to middle adulthood—a stage where smoking-related respiratory diseases typically begin to manifest. Importantly, the APDQS provides multiple real-world achievable pathways to healthy eating. Another defining feature included the rigorous assessment of smoking status, repeated annually, with previous evaluations demonstrating a strong relationship between baseline smoking reports and cotinine levels.17,29 Finally, the utilization of CT imaging provided objective outcomes and avoided potential misclassification associated with a self-reported diagnosis of emphysema.
A few limitations are worth noting. The observational study design hinders arriving at any causal conclusions; replication in an independent cohort would strengthen causal inferences. Diet was self-reported and is subject to recall bias. While we used a subset of the CARDIA cohort and our baseline APDQSs were similar to previous examinations of the larger CARDIA cohort,9 there is limited use of the APDQS outside the CARDIA cohort30,31 and is an area of future examination. In addition, despite adjustment for multiple covariates (including smoking), residual confounding cannot be excluded. While adjusted results remained significant, a wide confidence interval indicates a lack of precision in our findings, warranting future prospective studies with a larger sample size to ensure the reliability, strength of association, and generalizability of our findings. While the contribution of smoking, the strongest known risk factor for emphysema, was accounted for in both the adjusted model and by testing for interaction, other key factors, such as air pollution and other social determinants of health, should be accounted for in future studies. Furthermore, the dose-response relationship between diet and decreased incidence of emphysema was maintained, suggesting a true relationship. Additionally, the CARDIA cohort is composed of Black and White participants, thus, the generalizability of the results to other races/ethnicities is limited. We limited our analyses to current and past smokers, limiting the generalizability to all populations, yet capturing an appropriate at-risk population, as prevalence of emphysema is very low among nonsmokers. Lastly, though the scope of this work was limited to radiographic outcomes, there is value to explore additional respiratory-related outcomes (e.g., lung function trajectories, mortality, etc.) in this cohort.
In summary, there is a substantial need for identifying treatable traits for patients with a history of smoking to improve lung health outcomes. In this study, we demonstrated that adherence to a nutritionally rich, plant-centered diet during early and middle adulthood was significantly associated with decreased odds of radiographic emphysema later in the life course. Adherence to a nutritionally rich plant-centered dietary pattern may represent a potential early target to influence the lifetime risk of chronic lung disease.
Acknowledgements
Author contributions: SB is the guarantor of the content of the manuscript, having had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CH, MKJ, EE, RK, YC, and SB contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. DRJ, AW, JGW, GRW, SA, RSJE, GL, JS, LMS, and RW contributed substantially to the data analysis and manuscript review. All authors read and approved the final manuscript.
Data Sharing: CARDIA data described in the manuscript, code book, and analytic code are available upon reasonable request from the CARDIA Coordinating Center. CARDIA investigators are eager to collaborate with investigators interested in using CARDIA data. Please see the CARDIA website (https://www.cardia.dopm.uab.edu) for publications policies and for a list of CARDIA investigators. CARDIA data are also publicly available on the National Institutes of Health (NIH)-supported BioLINCC and dbGaP platforms.
Declaration of Interests
Mariah K. Jackson, Yuni Choi, Elliot Eisenberg, Corrine Hanson, Ann Wang, Gabrielle Liu, Robert Wharton, and David R. Jacobs have no interests to disclose. Jing Gennie Wang has received funding from the American Lung Association Early Career Investigator Award. George R. Washko reports serving on an advisory committee for Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, and Vertex; personal fees from Boehringer Ingelheim, CSL Behring, Janssen, Novartis, PulmonX, and Vertex; serving on a data safety and monitoring board for PulmonX; receiving research support from Boehringer Ingelheim, BTG and Janssen, all outside the submitted work; ownership and investment interest in Quantitative Image Solutions; and his spouse is an employee of Biogen. Samuel Ash has received funding from the NIH (K08HL145118) and has stock/ownership related to Quantitative Imaging Solutions. Raul San Jose Estepar has received funding from the National Heart, Lung, and Blood Institute and contracts with Lung Biotechnology, Insmed, and Boehringer Ingelheim. Dr. San Jose Estepar has received royalties from Imbio, consulting fees from Leuko Labs, speaker fees from Chiesi, and has pending patents related to lung cancer risk technology, and stock/ownership in Quantitative Imaging Solutions. James Shikany is the principal investigator of the contract for the CARDIA study Coordinating Center at the University of Alabama at Birmingham. Lyn M. Steffen has received grant funding from the University of Minnesota. Ravi Kalhan reports receiving grant support, consulting fees, and lecture fees from Boehringer Ingelheim and GlaxoSmithKline; grant support from PneumRx/BTG and Spiration; grant support and consulting fees from Astra-Zeneca; and consulting fees from CVS Caremark, Aptus Health, Boston Scientific, and Boston Consulting Group. Sonali Bose has received funding from the NIH. She also receives research support from 4D Medical and the American Lung Association.